Relating Stool Microbial Metabolite Levels, Inflammatory Markers and Dietary Behaviors to Screening Colonoscopy Findings in a Racially/Ethnically Diverse Patient Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Comprehensive Stool Analysis
2.3. Dietary Intake Analysis
2.4. Colonoscopy Findings
2.5. Statistical Evaluation
3. Results
3.1. Participants
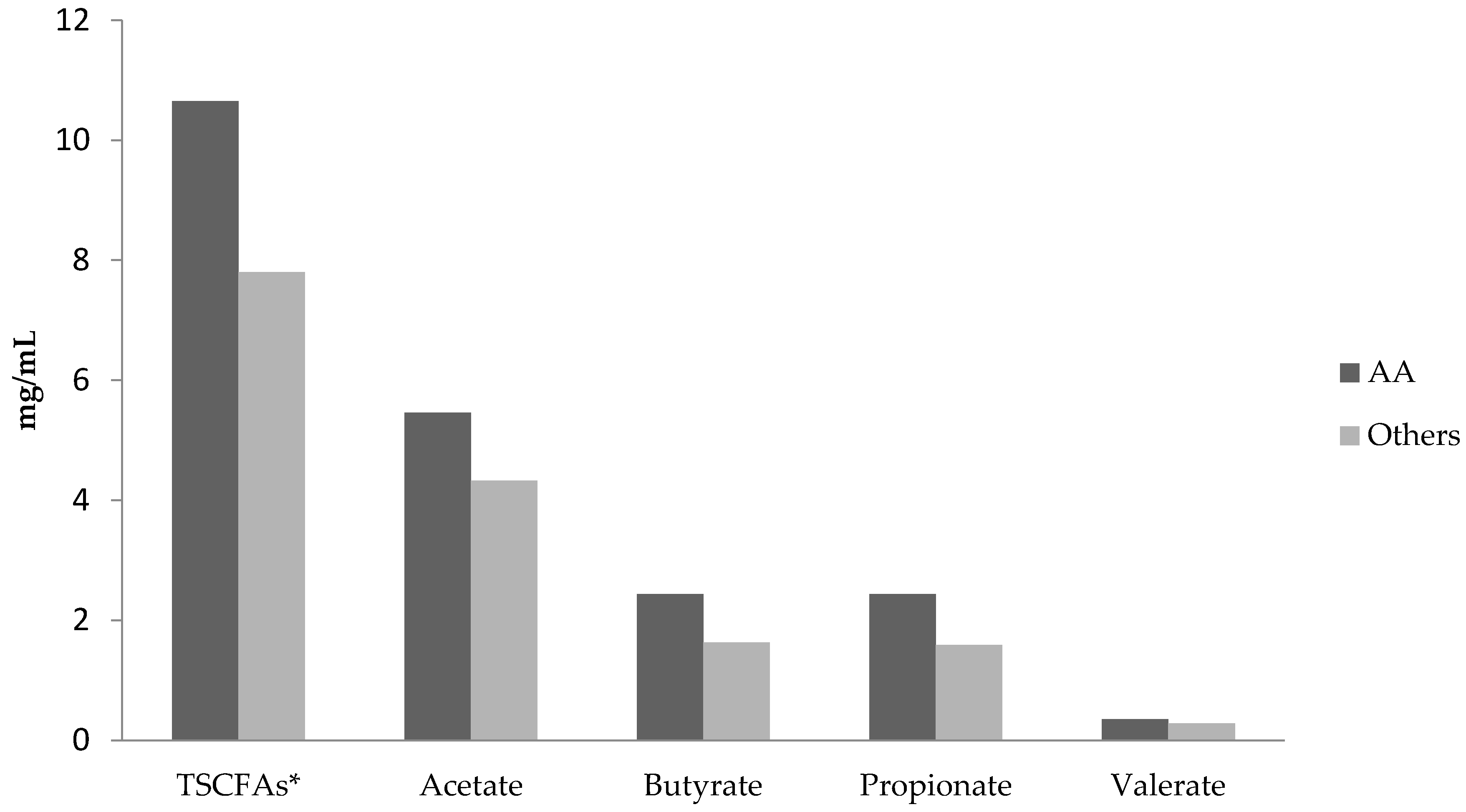
3.2. Comparisons of Short Chain Fatty Acid Levels and Diets in African-Americans versus Others
3.3. Analysis of the Effects of Fruit and Vegetable Consumption on Total Short Chain Fatty Acids
3.4. Correlations between Inflammatory Markers, Short Chain Fatty Acid Levels, Diet Variables and Race/Ethnicity
3.5. Comparison of Abnormal Colonoscopy Results in African-Americans versus Other Race/Ethnicities
3.6. Analysis of the Relationship between Abnormal Colonoscopy Results and Race/Ethnicity, Short Chain Fatty Acids, Inflammatory Markers and Diet
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S.; Blumenthal, D.S.; Seay, S.J.; Smith, S.A. Toward the elimination of colorectal cancer disparities among African Americans. J. Racial. Ethn Health Dispar. 2016, 3, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Dutilh, B.E.; Hall, N.; Peters, W.H.; Roelofs, R.; Boleij, A.; Tjalsma, H. Towards the human colorectal cancer microbiome. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Tran Van Nhieu, J.; Furet, J.P. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, O.; Takahashi, T.; Asahara, T.; Nomoto, K.; Onodera, H. Changes of the intestinal microbiota, short chain fatty acids and fecal ph in patients with colorectal cancer. Dig. Dis. Sci. 2013, 58, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Nikotian, G.; Koutsovasilis, A.; Bramis, J.; Manouras, A.; Mystrioti, D.; Katergiannakis, V. Patients with colorectal cancer are characterized by increased concentration of fecal hb-hp complex, myeloperoxidase and secretory IgA. Am. J. Clin. Oncol. 2011, 34, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J. Diet, microbiota and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Rogers, M.A.M.; Ruffin, M.T.; Schloss, P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Hester, C.M.; Jala, V.R.; Langille, M.G.; Umar, S.; Greiner, K.A.; Haribabu, B. Fecal microbes, short chain fatty acids and colorectal cancer across racial/ethnic groups. World J. Gastroenterol. 2015, 21, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Brim, H.; Yooseph, S.; Lee, E.; Sherif, Z.; Abbas, M.; Laiyemo, A.O.; Varma, S.; Torralba, M.; Dowd, S.E.; Nelson, K.E.; et al. A microbiomic analysis in African Americans with colonic lesions reveals Streptococcus sp.Vt162 as a marker of neoplastic transformation. Genes 2017, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Huycke, M.M.; Gaskins, H.R. Commensal bacteria, redox stress and colorectal cancer: Mechanisms and models. Exp. Biol. Med. 2004, 229, 586–597. [Google Scholar] [CrossRef]
- O’Keefe, S.J. Diet, microorganisms and their metabolites and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Uronis, J.M.; Muhlbauer, M.; Herfarth, H.H.; Rubinas, T.C.; Jones, G.S.; Jobin, C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events and colorectal cancer. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.C.; Jobin, C. The struggle within: Microbial influences on colorectal cancer. Inflamm. Bowel. Dis. 2011, 17, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, R.A. Faecal markers of gastrointestinal inflammation. J. Clin. Pathol. 2012, 65, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R. The role of the gastrointestinal microbiota in colorectal cancer. Curr. Pharm. Des. 2009, 15, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Rafter, J.J. Scientific basis of biomarkers and benefits of functional foods for reduction of disease risk: Cancer. Br. J. Nutr. 2002, 88 (Suppl. 2), S219–S224. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Hoshimoto, M.; Saito, O.; Kayazawa, M.; Nishikawa, T.; Murano, M.; Toshina, K.; Wang, F.Y.; Matsuse, R. Usefulness of fecal lactoferrin and hemoglobin in diagnosis of colorectal diseases. World J. Gastroenterol. 2007, 13, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Boets, E.; Deroover, L.; Houben, E.; Vermeulen, K.; Gomand, S.V.; Delcour, J.A.; Verbeke, K. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients 2015, 7, 8916–8929. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.A.; Wolever, T.M.S. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J. Nutr. 2003, 133, 3145–3148. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M.S. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Kupfer, S.S.; Brim, H.; Carethers, J.M. Racial disparity in gastrointestinal cancer risk. Gastroenterology 2017, 153, 910–923. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Kidd, M.; Espitalier-Noel, G.; Owira, P. Rarity of colon cancer in Africans is associated with low animal product consumption, not fiber. Am. J. Gastroenterol. 1999, 94, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Chung, D.; Mahmoud, N.; Sepulveda, A.R.; Manafe, M.; Arch, J.; Adada, H.; van der Merwe, T. Why do African Americans get more colon cancer than native Africans? J. Nutr. 2007, 137, 175S–182S. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Halpern, M.; Schrag, N.; Cokkinides, V.; DeSantis, C.; Bandi, P.; Siegel, R.; Stewart, A.; Jemal, A. Association of insurance with cancer care utilization and outcomes. CA Cancer J. Clin. 2008, 58, 9–31. [Google Scholar] [CrossRef] [PubMed]
- DeNavas-Walt, C.; Proctor, B.D.; Smith, J.C. Income and Poverty in the United States: 2013; U.S. Census Bureau: Washington, DC, USA, 2014.
- Byrd, D.A.; Agurs-Collins, T.; Berrigan, D.; Lee, R.; Thompson, F.E. Racial and ethnic differences in dietary intake, physical activity and body mass index (BMI) among cancer survivors: 2005 and 2010 national health interview surveys (NHIS). J. Racial. Ethn Health Dispar. 2017, 4, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lazarova, D.L.; Bordonaro, M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol. 2014, 6, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Claycombe, K.J.; Reindl, K.M. Butyrate and deoxycholic acid play common and distinct roles in HCT116 human colon cell proliferation. J. Nutr. Biochem. 2015, 26, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Taussig, D.P.; Cheng, W.H.; Johnson, L.K.; Hakkak, R. Butyrate inhibits cancerous HCT116 colon cell proliferation but to a lesser extent in noncancerous NCM460 colon cells. Nutrients 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.F.; Wang, M.; Qing, Y.; Lin, Y.M.; Wu, D. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World J. Gastroenterol. 2016, 22, 7058–7068. [Google Scholar] [CrossRef] [PubMed]
- Streckfus, C.F.; Welsh, S.; Strahl, R.C. Diminution of parotid IgA secretion in an elderly black population taking antihypertension medications. Oral. Surg. Oral. Med. Oral. Pathol. 1991, 71, 50–54. [Google Scholar] [CrossRef]
- Johnson, R.B. Racial differences in salivary sIgA concentrations in postmenopausal women. Spec. Care Dentist. 2005, 25, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Crowley-Nowick, P.A.; Bull, R.; van den Wall Bake, A.W.; Kulhavy, L.; Julian, B.A.; Jackson, S. Immunological studies of IgA nephropathy in blacks reveal elevations of serum IgA2 as well as IgA1. Nephrol. Dial. Transp. 1994, 9, 1324–1329. [Google Scholar]
| Characteristic | AA (n = 17) | Other (n = 31) | Overall |
|---|---|---|---|
| Age, mean (range) a | 53.5 (50–67) | 58.7 (49–71) | 56.9 (49–71) |
| BMI, mean ± SD b | 33.5 ± 8.6 | 30.1 ± 7.0 | 31.3 ± 7.7 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic AA | 17 (100) | 0 | 17 (35) |
| Non-Hispanic White | 0 | 24 (77) | 24 (50) |
| Hispanic | 0 | 7 (23) | 7 (15) |
| Sex, n (%) | |||
| Male | 9 (53) | 14 (45) | 23 (48) |
| Female | 8 (47) | 17 (55) | 25 (52) |
| Smoking status, n (%) | |||
| Currently smokes | 6 (35) | 6 (19) | 12 (25) |
| Used to smoke | 2 (12) | 7 (23) | 9 (19) |
| Never smoked | 9 (53) | 18 (58) | 27 (56) |
| Marital status, n (%) c | |||
| Married/living with partner | 7 (44) | 16 (52) | 23 (49) |
| Divorced or separated | 5 (31) | 8 (26) | 13 (28) |
| Widowed or never married | 4 (25) | 7 (22) | 11 (23) |
| Educational attainment, n (%) c,d | |||
| ≤High school | 8 (50) | 5 (16) | 13 (28) |
| ≥College | 8 (50) | 26 (84) | 34 (72) |
| Diet Variable a | AA (n = 14) b | Other (n = 29) b |
|---|---|---|
| mean (95% CI) | mean (95% CI) | |
| Fiber, g/d | 25.26 (11.39, 39.13) | 23.08 (19.50, 26.66) |
| Fruit, c/d | 2.21 (0.88, 3.55) | 1.52 (1.03, 2.00) |
| Total vegetable, c/d | 1.65 (0.96, 2.34) | 2.39 (1.75, 3.03) |
| Non-starchy vegetable, c/d * | 1.11 (0.62, 1.61) | 2.00 (1.42, 2.58) |
| Absence of Adenomatous Polyps | Presence of Adenomatous Polyps | Overall | |
|---|---|---|---|
| Race/ethnicity, n (%) | n = 29 | n = 14 | n = 43 |
| Non-Hispanic AA | 9 (31) | 4 (29) | 13 (30) |
| Non-Hispanic White | 15 (52) | 8 (57) | 23 (53) |
| Hispanic | 5 (17) | 2 (14) | 7 (16) |
| SCFA, mean (95% CI) c | n = 26 | n = 13 | n = 39 |
| Total, mg/mL | 8.90 (7.11, 10.69) | 8.58 (6.74, 10.42) | 8.79 (7.50, 10.09) |
| Acetate, mg/mL | 4.75 (3.97, 5.53) | 4.40 (3.57, 5.23) | 4.63 (4.06, 5.20) |
| Butyrate, mg/mL | 2.04 (1.41, 2.67) | 1.87 (1.28, 2.46) | 1.98 (1.54, 2.43) |
| Propionate, mg/mL | 1.82 (1.33, 2.32) | 2.03 (1.37, 2.69) | 1.89 (1.51, 2.27) |
| Valerate, mg/mL | 0.29 (0.25, 0.35) | 0.30 (0.21, 0.38) | 0.30 (0.25, 0.34) |
| Dietary intake, mean (95% CI) d,e | n = 27 | n = 13 | n = 40 |
| Total fiber g/d | 27.1 (20.4, 33.8) | 20.9 (14.9, 26.9) | 25.1 (20.2, 30.0) |
| Total fruit c/d | 2.0 (1.3, 2.8) | 1.4 (0.6, 2.2) | 1.8 (1.3, 2.4) |
| Total vegetable c/d | 2.3 (1.6, 2.9) | 2.3 (1.3, 3.2) | 2.3 (1.8, 2.8) |
| Non-starchy vegetable c/d | 1.9 (1.3, 2.5) | 1.7 (1.0, 2.4) | 1.8 (1.4, 2.3) |
| Inflammatory markers, mean (95% CI) f | n = 26 | n = 13 | n = 39 |
| Lysozyme, ng/mL g | 249.2 (164.1, 334.2) | 255.1 (149.3, 360.8) | 251.2 (187.3, 315.1) |
| Lactoferrin, µg/mL | 5.1 (1.7, 8.5) | 2.4 (0.13, 4.7) | 4.2 (1.9, 6.5) |
| sIgA, mg/dL | 160.3 (103.0, 217.7) | 307.2 (98.3, 516.2) | 209.3 (132.6, 286.0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bridges, K.M.; Diaz, F.J.; Wang, Z.; Ahmed, I.; Sullivan, D.K.; Umar, S.; Buckles, D.C.; Greiner, K.A.; Hester, C.M. Relating Stool Microbial Metabolite Levels, Inflammatory Markers and Dietary Behaviors to Screening Colonoscopy Findings in a Racially/Ethnically Diverse Patient Population. Genes 2018, 9, 119. https://doi.org/10.3390/genes9030119
Bridges KM, Diaz FJ, Wang Z, Ahmed I, Sullivan DK, Umar S, Buckles DC, Greiner KA, Hester CM. Relating Stool Microbial Metabolite Levels, Inflammatory Markers and Dietary Behaviors to Screening Colonoscopy Findings in a Racially/Ethnically Diverse Patient Population. Genes. 2018; 9(3):119. https://doi.org/10.3390/genes9030119
Chicago/Turabian StyleBridges, Kristina M., Francisco J. Diaz, Zhiwen Wang, Ishfaq Ahmed, Debra K. Sullivan, Shahid Umar, Daniel C. Buckles, K. Allen Greiner, and Christina M. Hester. 2018. "Relating Stool Microbial Metabolite Levels, Inflammatory Markers and Dietary Behaviors to Screening Colonoscopy Findings in a Racially/Ethnically Diverse Patient Population" Genes 9, no. 3: 119. https://doi.org/10.3390/genes9030119
APA StyleBridges, K. M., Diaz, F. J., Wang, Z., Ahmed, I., Sullivan, D. K., Umar, S., Buckles, D. C., Greiner, K. A., & Hester, C. M. (2018). Relating Stool Microbial Metabolite Levels, Inflammatory Markers and Dietary Behaviors to Screening Colonoscopy Findings in a Racially/Ethnically Diverse Patient Population. Genes, 9(3), 119. https://doi.org/10.3390/genes9030119




