1. Introduction
The Pacific White Shrimp (
L.
vannamei) is the most commercially valuable species in global aquaculture, accounting for over 80% of total farmed shrimp production and representing an industry valued at over
$10 billion [
1,
2]. This species has dominated global markets since large-scale farming commenced in the 1970s, due to its biological advantages, including euryhalinity (5–40‰ salinity tolerance), high-density tolerance (60–150 individuals/m
2), and a short growth cycle (reaching market size within 100 days) [
3]. In recent decades, significant efforts have been made to enhance its economic traits, such as growth rate, disease resistance, and tolerance to extreme temperature and salinity conditions [
4,
5,
6]. Among these traits, growth rate is one of the most critical economic factors in aquaculture, closely linked to profitability, which makes its enhancement a primary focus of shrimp breeding programs.
To date, omics technologies including Quantitative Trait Locus (QTL) mapping and Genome-Wide Association Study (GWAS) have identified numerous candidate genes associated with growth traits in
L. vannamei [
7], such as
AMY2,
CTSL [
8],
STEAP4 [
9], and
BAMBI [
10]. These findings reveal the complex regulatory mechanisms underlying muscle development and energy metabolism. However, growth traits result from intricate genetic networks influenced by multiple factors, and research on growth-related genes and their regulation in crustaceans remains limited.
The unique intermittent growth pattern of crustaceans, which relies on periodic molting for somatic expansion, is closely linked to the dynamic balance of chitin metabolic pathways [
11]. Chitinases, the key enzymes responsible for chitin degradation, exhibit significant functional diversity across different species [
12]. While research on insect chitinases has achieved considerable depth, encompassing gene cloning, molting cycle dynamics, RNA knockdown (RNAi) functional analyses [
13], endogenous hormonal regulation, environmental pollutant impacts, heterologous expression, and disease control [
14,
15], studies on chitinases in aquatic crustaceans remain in their infancy and lack systematic frameworks.
In crustaceans, chitinases, which are members of glycoside hydrolase family 18 (GH18), not only degrade the old exoskeleton but also indirectly enhance growth performance by modulating gut microbiota homeostasis and immune defenses [
16]. Notably, Group 2 chitinases within the GH18 family exhibit tissue-specific expression dynamics during molting cycles. For instance, in the mud crab (
Scylla paramamosain), the expression of
SpChia2 is relatively low during the D1 stage of the molting cycle but declines sharply after the D2 stage (pre-molt phase) [
17]. Similarly, expression profiling of
PmChia2 in the black tiger shrimp (
Penaeus monodon) revealed peak expression during the pre-molt phase [
18]. These stage-specific expression patterns align with the high demand for chitin degradation in the old exoskeleton, suggesting that Group 2 chitinases spatiotemporally regulate the enzymatic hydrolysis of the old cuticle to facilitate subsequent epidermal remodeling. Remarkably, RNAi-mediated silencing of
LsChia2 in the salmon louse (
Lepeophtheirus salmonis) significantly suppresses gene expression during the larval stage, leading to axial developmental defects, impaired locomotion, and reduced host infectivity [
19,
20]. Collectively, these findings highlight the evolutionarily conserved role of Group 2 chitinases as core effectors in crustacean molting, driving the programmed degradation of the old exoskeleton. However, their potential for genetic improvement of growth traits in
L. vannamei remains underexplored.
Transcriptome sequencing analysis, a widely utilized method for studying non-model organisms, facilitates a systematic investigation of gene expression under specific conditions and the elucidation of regulatory networks [
21]. In this study, we analyzed previously generated transcriptomic data [
22] to screen for and identify the growth-related gene
Chia2 in
L. vannamei with divergent growth rates. Following the cloning and characterization of its gene structure, phylogenetic relationships, and expression patterns, we employed RNAi [
13] to knock down
LvChia2 expression and observed associated growth phenotypes and transcriptional changes. Our results suggest that
LvChia2 may regulate shrimp growth through pathways involving lipid and glucose metabolism. This study provides insights into the molecular mechanisms underlying
LvChia2-mediated growth regulation in
L. vannamei and offers valuable genetic information for developing molecular breeding strategies in shrimp aquaculture.
2. Materials and Methods
2.1. Ethics Statement
All procedures for animal breeding, handling, and sampling in this study received approval from the Animal Care and Ethics Committee of Guangxi Academy of Fishery Sciences (Animal ethics approval number: 2025GAFS09), thereby affirming the trustworthiness and ethical conduct of our research.
2.2. Experimental Animals and Aquaculture Management
Specific pathogen-free (SPF) post-larvae of L. vannamei were obtained from the Guangxi Academy of Fishery Sciences (Nanning, China). A total of 150 healthy shrimp, with a uniform body weight of 1.22 ± 0.08 g, were randomly distributed into three circular polyethylene tanks (dimensions: 97 × 81 × 73 cm), at a density of 50 shrimp per tank. Prior to start of the experiment, the shrimp underwent a 5 d acclimation time, During this time, they were maintained under carefully controlled environmental conditions: salinity was set at 25‰ (achieved by diluting brine, followed by 24 h sterilization, 24 h dechlorination, and 24 h aeration), water temperature was maintained between 26 and 28 °C, pH levels were regulated within the range of 7.8–8.2, dissolved oxygen (DO) was ensured to be no less than 6 mg/L, ammonia nitrogen concentration was kept below 0.2 mg/L, and nitrite concentration was maintained at less than 0.01 mg/L. The shrimp were fed commercial feed (nutritional composition: 38% crude protein, 4% crude fat, 8% crude fiber, 16% ash and 11% moisture, supplied by Fujian Tianma Technology Group Corporation, Fujian, China) at a rate of 5% of their body weight, administered three times daily (at 10:00, 16:00, and 22:00), with adjustments made based on their feeding response. To maintain water quality, one-third of the water volume in each tank was replaced daily. For the RNAi experiment, after the acclimation phase, shrimp exhibiting uniform size and weight were selected. Their initial body weight and length were measured precisely using an analytical balance (precision: 0.1 mg) and a digital caliper (precision: 0.1 mm), respectively. These measurements were repeated every 5-day throughout a 20-day trial period to monitor growth dynamics.
2.3. Sample Collection and Analysis
Following a 24 h starvation period to standardize the physiological state of the shrimp, several growth parameters were calculated, including survival rate (SR), weight gain rate (WGR), condition factor (CF), and specific growth rate (SGR), using the established formulas outlined below:
Tissue samples, including muscle, hepatopancreas, and intestinal tissues, were collected from 12 shrimp per group. These samples were immediately flash-frozen in liquid nitrogen and stored at −80 °C for subsequent analyses. Muscle tissues were reserved for transcriptomic sequencing and proximate composition analysis, while hepatopancreas and intestinal tissues were used for enzymatic activity assays.
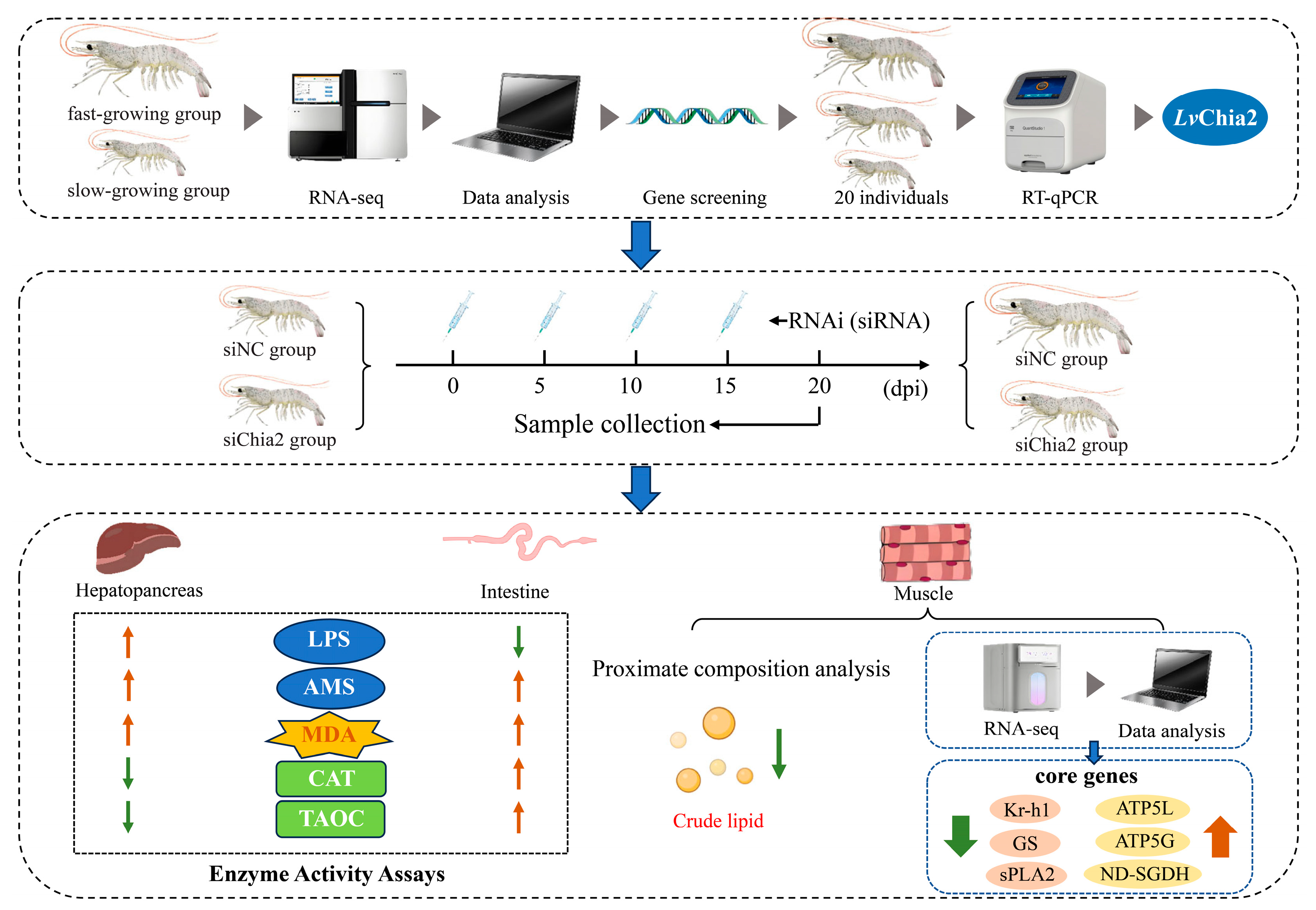
2.4. Transcriptomic Screening of Growth-Related Genes
Muscle tissues were collected from 30 fast-growing and 30 slow-growing individuals of
L. vannamei, selected based on significant differences in both body weight and length. Total RNA extraction was performed using TRIzol reagent, followed by quality assessment through 1% agarose gel electrophoresis and NanoDrop 2000 spectrophotometry (Thermofisher, MA, USA), ensuring an OD260/280 ratio within the range of 1.8–2.2. Sequencing libraries were constructed utilizing the Illumina TruSeq RNA Library Prep Kit and subjected to paired-end sequencing, generating 150 bp reads on the Illumina HiSeq™ 2500 platform (IIlumina, San Diego, CA, USA). Raw sequencing reads underwent rigorous preprocessing with Trimmomatic to remove adapter sequences, low-quality bases (Phred score < 20), and ambiguous reads. High-quality clean reads were then aligned to the
L. vannamei reference genome (ASM378908v1) using HISAT2 [
23]. Transcript assembly and quantification were conducted via StringTie, and DEG were identified using DESeq2, with thresholds set at |log
2(fold change)| ≥ 1 and adjusted
p < 0.05. Functional annotation of DEGs was performed against multiple databases, including Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Onthology (GO), Pfam, and Swiss-Prot [
22].
Nine candidate genes exhibiting consistent upregulation (fold change ≥ 2) in fast-growing shrimp were prioritized for validation. Gene-specific primers, designed using Primer Premier 6.0, were synthesized with amplicon lengths ranging from 150 to 300 bp and annealing temperatures set between 55 and 60 °C. Quantitative PCR (RT-qPCR) assays were performed on 20 muscle tissue samples, with β-actin serving as the reference gene. Linear regression models were employed to establish correlations between gene expression levels and body weight, leading to the identification of LvChia2 as the most robust candidate gene associated with growth traits.
2.5. Molecular Cloning and Characterization of LvChia2
Total RNA extraction from muscle tissues was performed using TRIzol reagent (Takara, Kusatsu, Japan). Briefly, 100 mg of tissue was homogenized in liquid nitrogen, lysed in RNAiso Plus (Takar, Kusatsu, Japan), and treated with chloroform. RNA precipitation was achieved using isopropanol, followed by washing with 75% ethanol and dissolving the RNA in RNase-free water. RNA integrity was confirmed via electrophoresis, and concentration was measured using a NanoDrop 2000 spectrophotometer. cDNA synthesis was conducted using the PrimeScript™ FAST RT Reagent Kit (Takara, Kusatsu, Japan), which included genomic DNA removal with gDNA Eraser (42 °C, 2 min) and reverse transcription (37 °C, 10 min). The LvChia2 open reading frame (ORF) was amplified using primers designed based on transcriptomic data. PCR conditions were optimized as follows: initial denaturation at 95 °C for 3 min; 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min; followed by a final extension step at 72 °C for 10 min. PCR products were gel-purified using a DiaSpin DNA Gel Extraction Kit (Sangon Biotech, Shanghai, China), ligated into the pMD19-T vector, and transformed into Escherichia coli (DH5α) competent cells. Positive clones were verified through colony PCR and Sanger sequencing.
A comprehensive analysis of the LvChia2 nucleotide sequence and the deduced amino acid sequence was conducted using ORF Finder (NCBI) and ExPASy Translate. Protein domain prediction was performed using the SMART tool, and phylogenetic trees were constructed with MEGA 12.0, employing the Neighbor-Joining method with 1000 bootstrap replicates (Institute of Molecular Evolutionary Genetics, State College, PA, USA). Alignment maps of conserved amino acid sites were generated using DNAMAN 9.0 software to further characterize the evolutionary conservation and structural features of LvChia2.
2.6. Developmental Expression Profiling
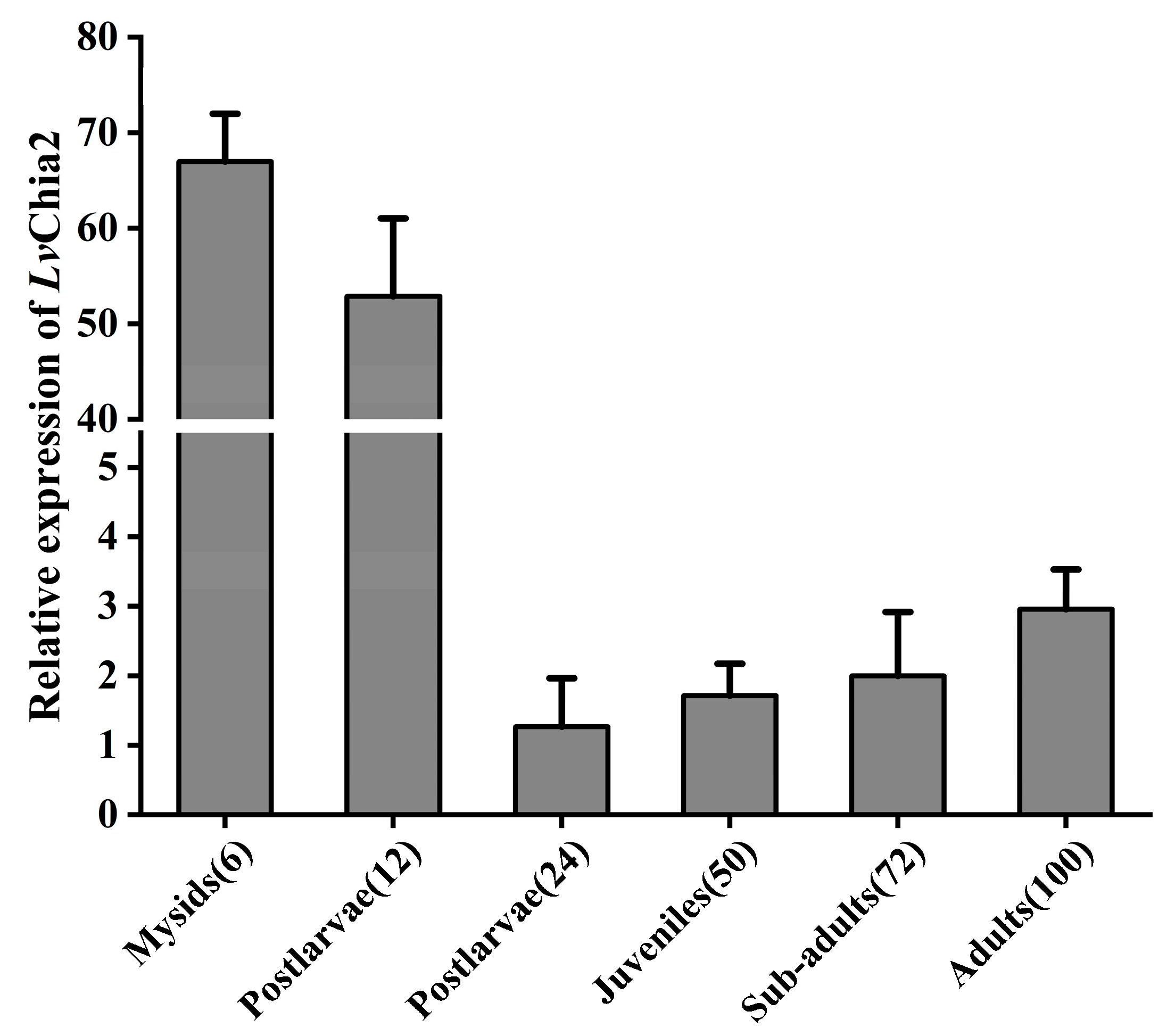
The expression profile of LvChia2 was evaluated across six distinct developmental stages of L. vannamei: mysis larvae (6 d post-hatching, DPH), post-larvae (12 DPH), juveniles (24 DPH), subadults (50 DPH), pre-adults (72 DPH), and adults (100 DPH). Muscle tissues from three biological replicates at each stage were collected and analyzed to ensure robust and representative data across the entire developmental spectrum of the shrimp.
2.7. RNAi and Functional Analysis
Two small knockdown RNA (siRNA) duplexes targeting
LvChia2 (GenBank: LOC113830447) were meticulously designed using the siDirect 2.0 and DSIR algorithms, with rigorous measures implemented to minimize off-target effects, as confirmed by NCBI BLAST analysis (
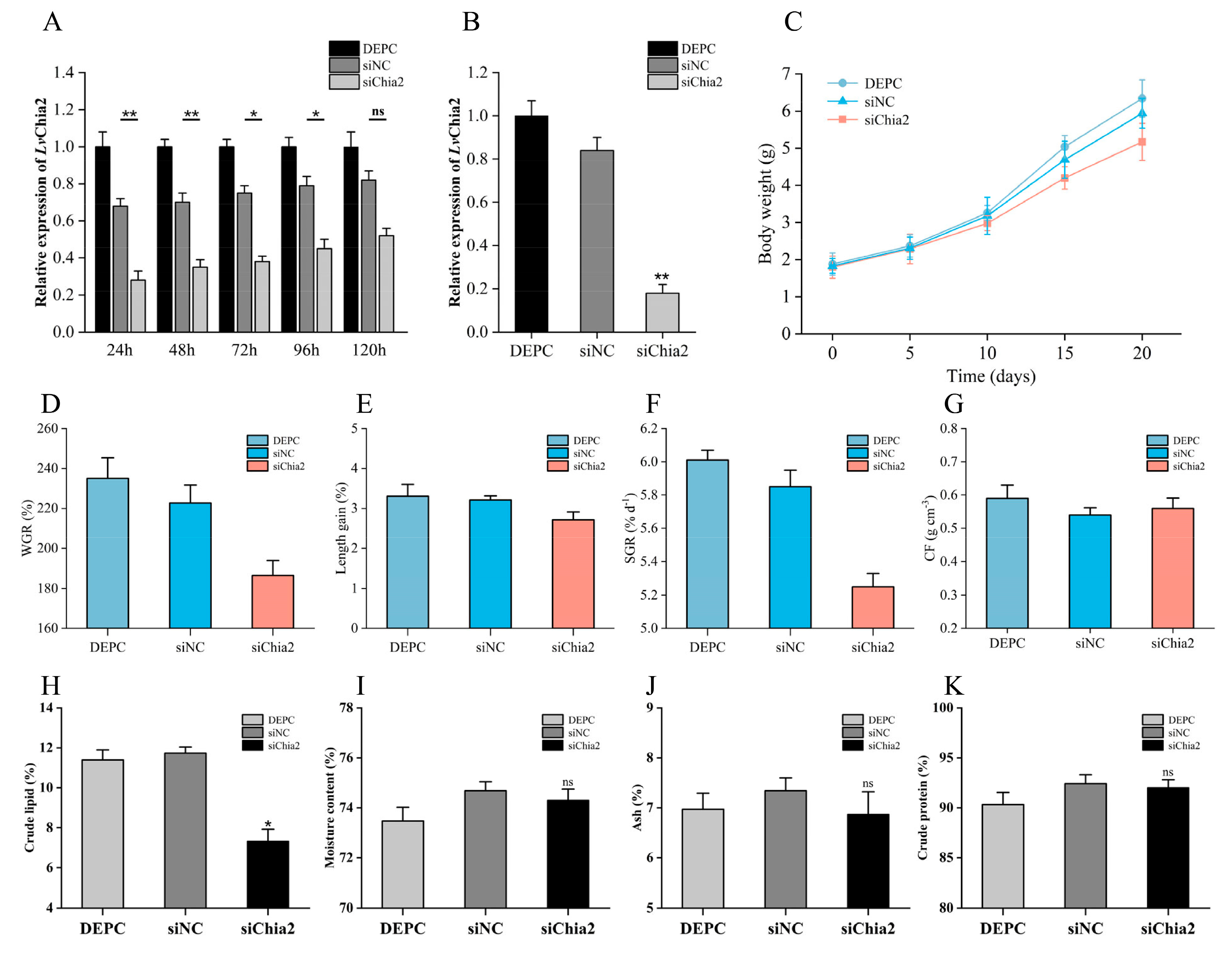
https://blast.ncbi.nlm.nih.gov/Blast.cgi) (accessed on 10 May 2025). These siRNA sequences were chemically synthesized by Sangon Biotech and included dTdT overhangs and phosphorothioate modifications to enhance stability and efficacy. A scrambled siRNA (siNC) was synthesized as a negative control to account for non-specific effects. Eighty-four shrimps were randomly allocated into three groups: the siChia2 group (experimental), the siNC group (negative control), and the DEPC-water group (blank control). The siRNA solution (0.1 μg/mL) was administered via intramuscular injection into the second abdominal segment of the shrimp at a dosage of 5 μg/g of body weight, with injections repeated every 5 days for a total duration of 20 days. Tissue samples, including muscle, hepatopancreas, and intestinal tissues, were collected at multiple time points post-injection (24, 48, 72, 96, and 120 h) for RNA extraction and RT-qPCR validation to assess knockdown efficiency. Additionally, samples were collected 20 days post-injection to verify the subsequent functional impacts.
2.8. Quantification of mRNA by RT-qPCR
Total RNA was extracted from the shrimp muscle using TRIzol
® Reagent (Ambion, Austin, TX, USA). cDNA was synthesized using the FastKing one-step genome-removing method (TianGen, Beijing, China) and used as the template for RT-qPCR. The RT-qPCR was performed with TB Green
® Premix Ex Taq (Takara, Kusatsu, Japan) to quantify mRNA levels. The relative fold change in mRNA expression was calculated by the 2
−ΔΔCt method with β-actin serving as an internal control. Each sample was tested in triplicate. The RT-qPCR procedure consisted of one cycle at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 20 s, concluding with a melting curve analysis from 65 °C to 95 °C. All primers are listed in
Table 1.
2.9. Proximate Composition and Enzyme Activity Assays
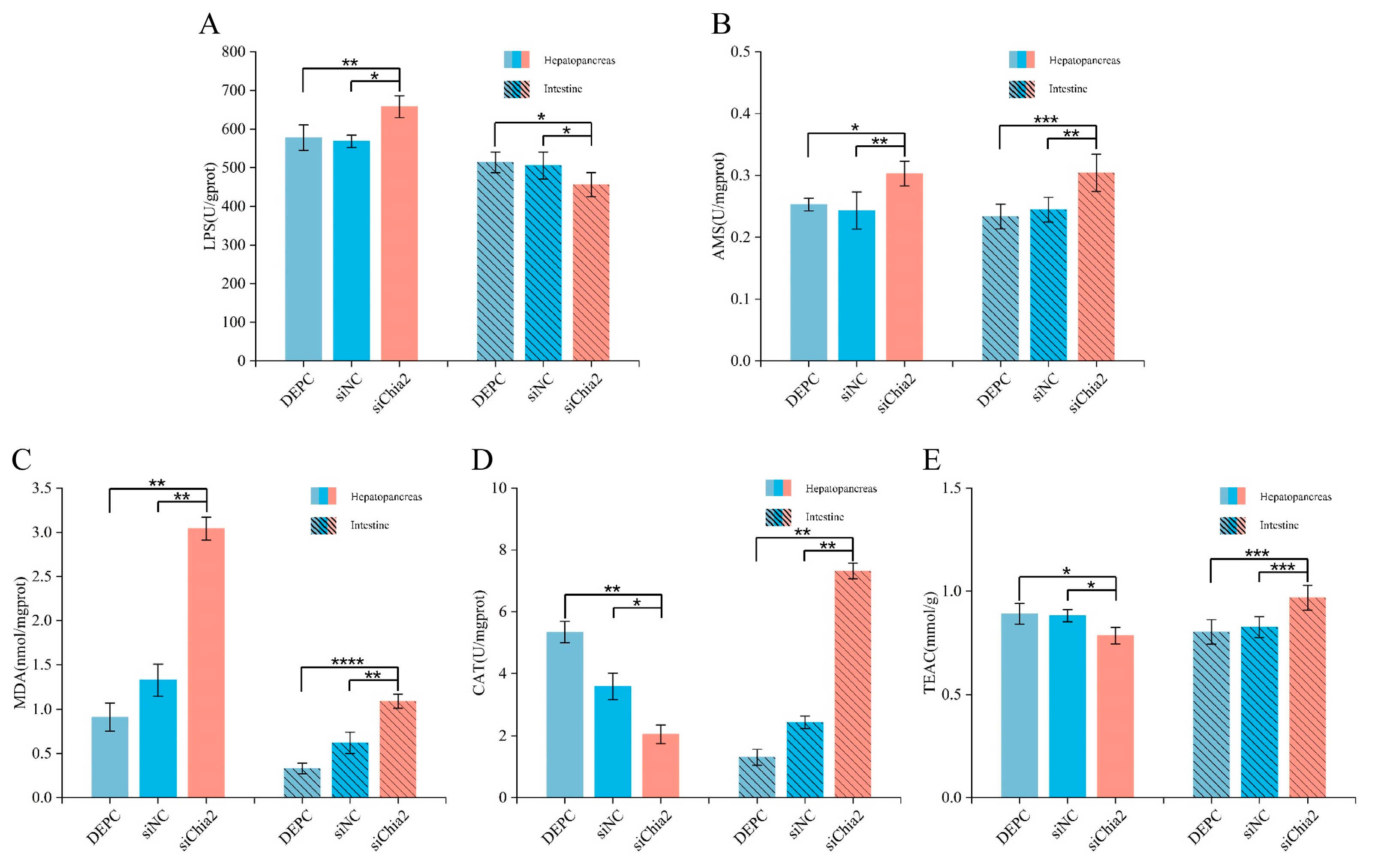
Proximate composition analysis of muscle tissues was conducted to determine the contents of crude protein, lipids, ash, and moisture, following the established Association of Official Analytical Chemists (AOAC) (2005) protocols. Additionally, enzymatic activity assays were performed for lipase (LPS), amylase (AMS), malondialdehyde (MDA), catalase (CAT), and total antioxidant capacity (TEAC), utilizing commercial kits provided by the Nanjing Jiancheng Bioengineering Institute. These analyses aimed to elucidate the physiological and biochemical changes associated with the knockdown of LvChia2.
2.10. RNA-Seq Analysis
Total RNA was extracted from
LvChia2-knockdown (siChia2) and control (siNC) shrimp, with only samples exhibiting a knockdown efficiency of ≥70% selected for sequencing. Sequencing was performed on the NovaSeq X Plus and DNBSEQ-T7 platforms to ensure high-throughput and high-quality data generation. Libraries were constructed using the NEBNext Ultra II RNA Library Prep Kit (NEB, Lpswich, MA, USA), which included steps such as mRNA enrichment (via Oligo dT beads), fragmentation (target size: 300 bp), cDNA synthesis, adapter ligation, and PCR amplification. Raw reads were filtered using Trimmomatic to remove low-quality sequences and contaminants. Subsequent alignment to the
L. vannamei genome (RefSeq: GCF_003789085.1) was conducted using HISAT2 [
23]. DEGs were identified using DESeq2, with thresholds set at |log
2FC| ≥ 1 and
p < 0.05. Functional annotation of DEGs was performed using the KEGG and GO databases to gain insights into the biological pathways and processes affected by
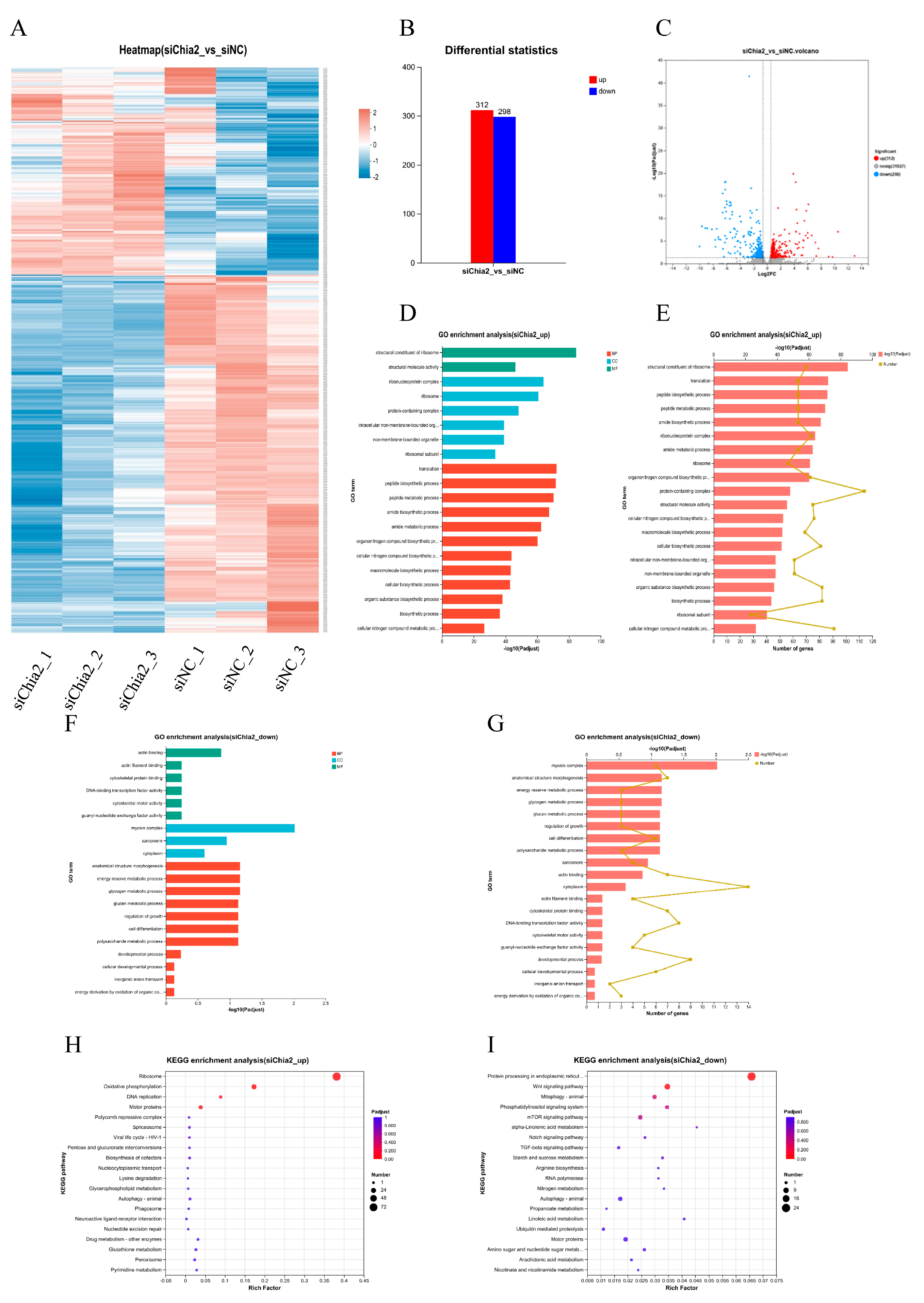
LvChia2 knockdown. Three significantly upregulated and three downregulated DEGs were selected, and their expression was validated via quantitative real-time PCR (RT-qPCR) to elucidate the role of candidate genes in growth rate.
2.11. Statistical Analysis
All data are presented as means ± standard deviations (SD) to provide a clear representation of the variability within the dataset. Statistical significance between groups was determined using a one-way ANOVA, conducted with SPSS version 26.0 software. Significance thresholds were set at p < 0.05 (*) and p < 0.01 (**), ensuring a rigorous evaluation of the experimental results and providing a solid foundation for the conclusions drawn from the data analysis.
4. Discussion
In this study, we identified a chitin metabolism-related gene,
LvChia2, from the transcriptome of
L. vannamei through weighted gene co-expression network analysis of molting-stage-specific expression profiles, along with validation across different developmental stages. The expression patterns of the chitinase 2 (
Chia2) gene in various tissues and molting stages suggest that it may play significant roles in the molting process. For example, the
Chia2 homolog in
Eriocheir sinensis (
EsChia2) exhibited approximately a 12-fold upregulation in the epidermis and hepatopancreas during the pre-molt stage (D-stage) compared to the intermolt phase (C-phase) [
24]. Similarly, the
Chia2 gene in
Portunus trituberculatus (
PtChia2) displayed peak expression in the hepatopancreas during the pre-molt stage (C-stage) and in the intestine during the post-molt stage (E-stage), indicating its dual role in immune defense against pathogens and in the degradation of the peritrophic membrane during post-molt remodeling [
16]. Consistent with these findings, our study revealed stage-dependent expression dynamics of
LvChia2 in
L. vannamei, with significantly elevated transcript levels during the mysis larval stage compared to other developmental phases. This expression pattern strongly suggests that
LvChia2 may regulate metamorphic development in shrimp larvae, potentially coordinating cuticle remodeling and developmental transitions through the regulation of chitinolytic activity.
RNA knockdown experiments confirmed that inhibition of
LvChia2 expression resulted in a significant decrease in body weight, body length, and specific growth rate in shrimp, thereby directly verifying its crucial regulatory role in growth performance. Proximate composition analysis revealed that the crude lipid content in the muscle of the knockdown group (siChia2) was significantly reduced, while no significant changes were observed in water content, ash content, or crude protein content. This indicates that the
LvChia2 knockdown primarily affects growth phenotype through targeted regulation of lipid metabolism. In addition, enzyme activity analysis revealed an increase in LPS content in the hepatopancreas and a decrease in LPS content in the intestine. This suggests that shrimp may compensate the reduction in intestinal fat absorption efficiency by activating the lipolysis pathway in the hepatopancreas, such as fatty acid β-oxidation, to break down stored fat, which is consistent with previous research [
25]. Furthermore, the analysis indicated that the upregulation of α-amylase in both the hepatopancreas and intestine may partially alleviate lipid metabolism imbalances by enhancing glycolytic energy production, aligning with the “synergistic compensation of metabolic pathways” hypothesis [
26]. These findings further confirm the role of
LvChia2 in lipid metabolism. Furthermore, oxidative stress results from an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense system. Studies have shown that high-fat diets induce lipid peroxidation and hepatopancreatic apoptosis in shrimp, thereby inhibiting their growth and development [
27]. The alterations in oxidative stress status caused by
LvChia2 knockdown—such as MDA accumulation in the hepatopancreas and decreased levels of CAT and total antioxidant capacity—suggest that this gene may play a crucial role in maintaining intracellular REDOX homeostasis by regulating the antioxidant system. Conversely, the changes in antioxidant indicators observed in the intestine (including increased CAT and total antioxidant capacity) imply the presence of tissue-specific stress response mechanisms, which may be linked to the interaction of intestinal microbiota or the activation of immune defenses [
28]. These findings not only elucidate a novel role for
LvChia2 in the regulation of oxidative stress in crustaceans but also provide critical insights into the interorgan coordination mechanisms that occur during metabolic disturbances in decapods. Understanding these mechanisms will aid in the development of effective antioxidant strategies to enhance productivity and stress resistance in shrimp aquaculture.
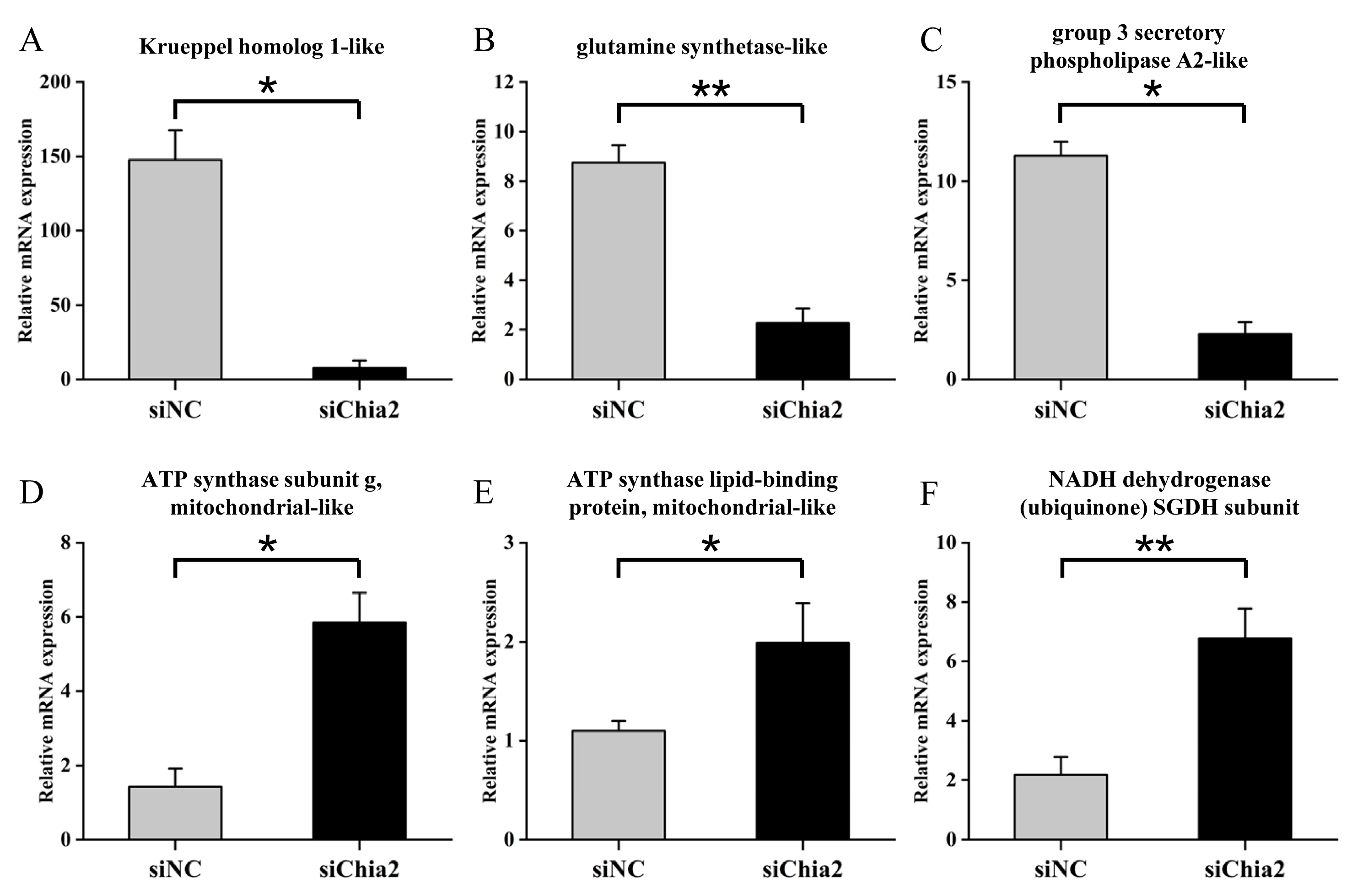
Further transcriptome analysis revealed that
LvChia2 influences a wide range of biological processes, including peptide synthesis, glycogen metabolism, cytoskeletal dynamics, and actin binding, by regulating 610 DEGs. Among these, KEGG-enriched metabolic and genetic information processing pathways, such as ATP synthesis and ammonia metabolism, are strongly associated with growth phenotypes. The six core genes identified through screening, including
ATP5L,
ATP5G,
ND-SGDH,
GS,
sPLA2 and
Kr-h1, exhibited significant expression differences. Their functional networks suggest that
LvChia2 may regulate growth through several mechanisms. Firstly, energy metabolism regulation plays a crucial role. By upregulating the co-expression of ATP synthase subunits and NADH dehydrogenase, the efficiency of mitochondrial oxidative phosphorylation is enhanced, providing a vital energy foundation for growth. Secondly, ammonia detoxification and nitrogen metabolism are significant processes. By inhibiting the expression of genes like glutamine synthetase, the capacity for ammonia assimilation is limited, resulting in an imbalance in nitrogen metabolism. This imbalance corresponds with the observed phenotype of elevated MDA levels in the hepatopancreas. Finally, cytoskeletal remodeling is another important factor. By downregulating secretory phospholipase A2, membrane phospholipid metabolism is affected, which interferes with cytoskeletal dynamics and subsequently inhibits muscle tissue proliferation. This phenomenon is associated with the observed decrease in weight and body length in the knockdown group. These results closely align with the stress-structural remodeling co-growth model of shrimp [
29], further confirming the central role of
LvChia2 in metabolic reprogramming and tissue development.
In conclusion, this study elucidates the pivotal role of the
LvChia2 gene in the growth and development of shrimp (
L. vannamei) by regulating pathways such as lipid metabolism, oxidative balance, and cytoskeletal dynamics. The specific reduction in crude lipid content resulting from its knockdown underscores the significance of this gene in lipid storage regulation. The six key genes (
ATP5L,
ATP5G,
ND-SGDH,
GS,
sPLA2 and
Kr-h1) identified in this research provide a novel perspective for analyzing the growth regulatory network of crustaceans. These findings not only enhance our understanding of chitinase function in shrimp but also establish a theoretical foundation for gene-editing approaches in aquaculture (
Figure 7).