Genomic Alterations and Microbiota Crosstalk in Hepatic Cancers: The Gut–Liver Axis in Tumorigenesis and Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
- Published in English in peer-reviewed journals.
- Focused on hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), or general hepatic cancers.
- Addressed the relationship between gut microbiota and hepatic cancer pathogenesis, diagnosis, prognosis, or treatment.
- Included original research studies, clinical trials, systematic reviews, and meta-analyses.
- Non-peer-reviewed materials (e.g., conference abstracts, editorials, or letters).
- Studies focusing on unrelated gastrointestinal malignancies or non-microbial factors without clear relevance to the gut–liver axis.
- Animal studies lacking translational relevance or mechanistic insight applicable to humans.
2.3. Data Extraction and Synthesis
2.4. Limitations
3. Results
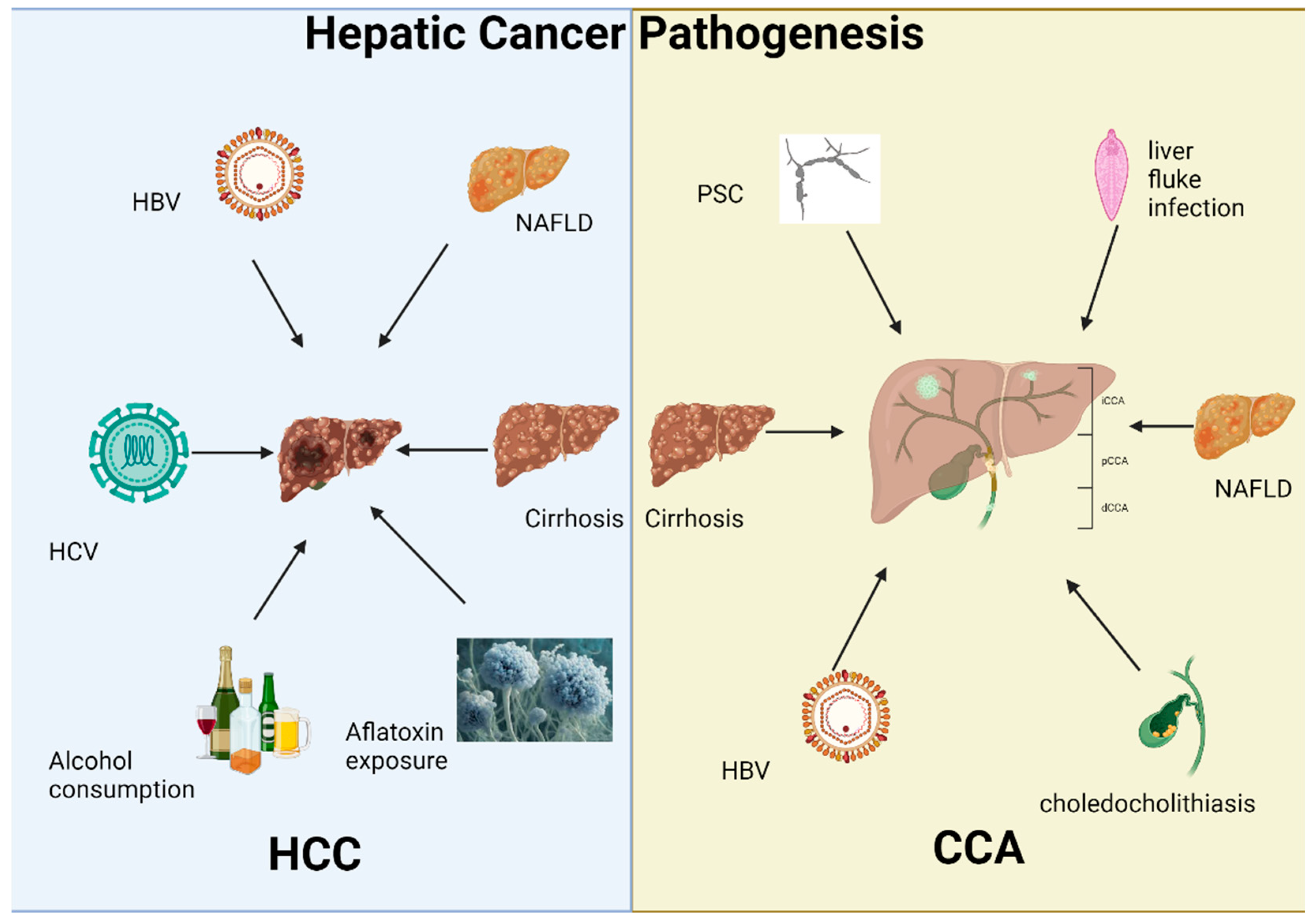
3.1. Hepatic Cancer Pathogenesis
3.1.1. Hepatocellular Carcinoma
Genetic and Genomic Alterations in Hepatocellular Carcinoma
3.1.2. Cholangiocarcinoma
Genetic and Genomic Alterations in Cholangiocarcinoma
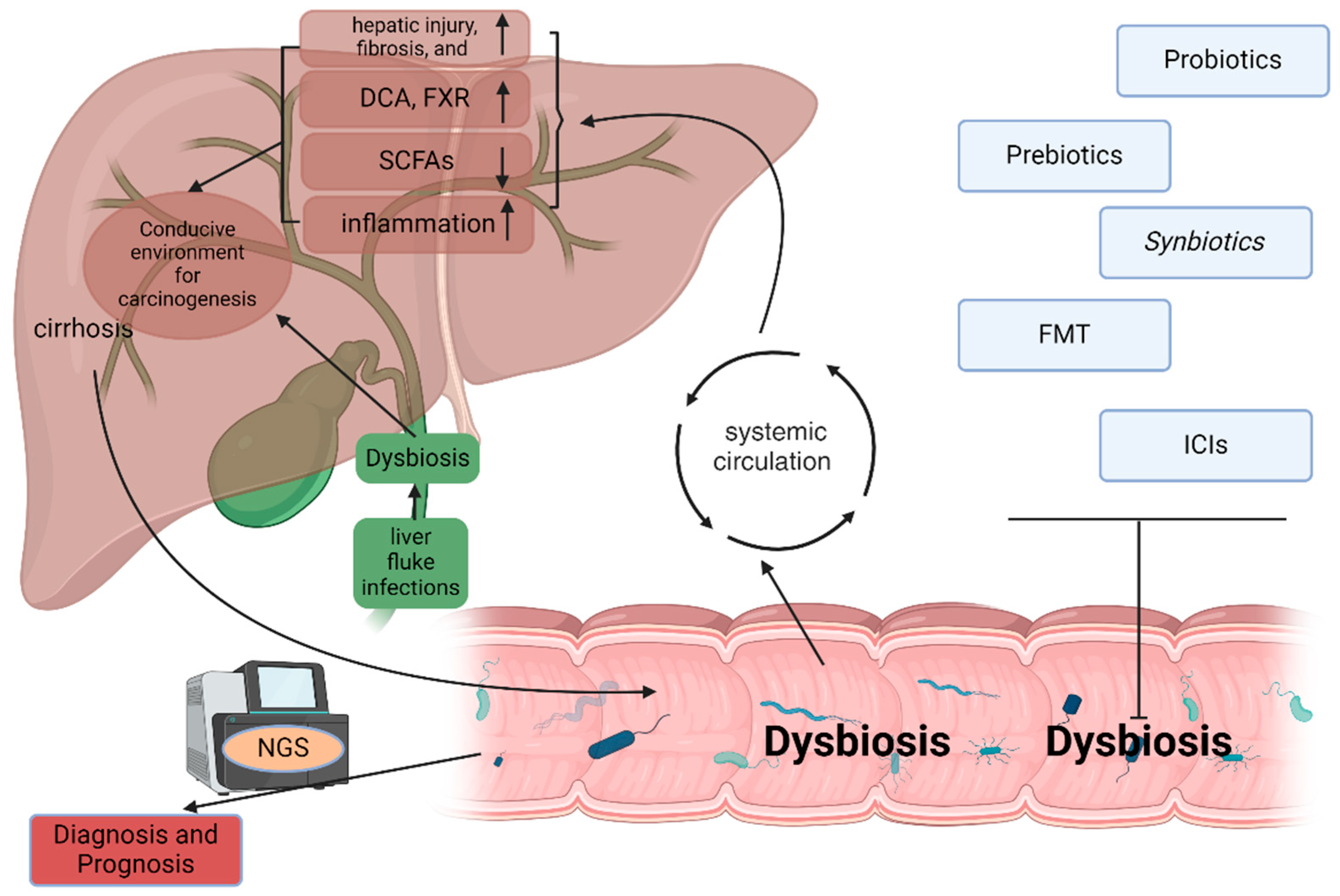
3.2. The Role of Microbiota in Hepatic Cancer Development
3.2.1. Microbiota and Hepatocellular Carcinoma
Dysbiosis and Cirrhosis in HCC Development
Metabolites and Cancer Promotion
Chronic Inflammation and Immune Modulation
3.2.2. Microbiota and Cholangiocarcinoma
Biliary Dysbiosis
3.2.3. Host Genetic Variation and Microbiota Interactions in Hepatic Carcinogenesis
3.3. Microbiota as a Diagnostic and Prognostic Tool in Hepatic Cancers
3.3.1. Microbiota Profiling for Diagnosis
3.3.2. Microbiota and Cancer Prognosis
3.4. Therapeutic Potential of Targeting Microbiota in Hepatic Cancers
3.4.1. Probiotics, Prebiotics, and Synbiotics
3.4.2. Fecal Microbiota Transplantation
3.4.3. Microbiota and Immune Checkpoint Inhibitors
3.5. Future Directions and Research Gaps
3.5.1. Mechanistic Studies
3.5.2. Longitudinal Studies
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gans, J.H.; Lipman, J.; Golowa, Y.; Kinkhabwala, M.; Kaubisch, A. Hepatic Cancers Overview: Surgical and Chemotherapeutic Options, How Do Y-90 Microspheres Fit in? Semin. Nucl. Med. 2019, 49, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, W.; Ge, X.; Lu, J.; Xu, X.; Gao, J.; Wang, Q.; Song, C.; Zhang, Q.; Yu, C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. Nutrients 2022, 14, 5335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mranda, G.M.; Xiang, Z.P.; Liu, J.J.; Wei, T.; Ding, Y. Advances in prognostic and therapeutic targets for hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The hippo signaling pathway. Front. Oncol. 2022, 12, 937957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Sarcognato, S.; Sacchi, D.; Fassan, M.; Fabris, L.; Cadamuro, M.; Zanus, G.; Cataldo, I.; Capelli, P.; Baciorri, F.; Cacciatore, M.; et al. Cholangiocarcinoma. Pathologica 2021, 113, 158–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Diwany, R.; Pawlik, T.M.; Ejaz, A. Intrahepatic Cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2019, 28, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 315–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fletcher, N.F.; Humphreys, E.; Jennings, E.; Osburn, W.; Lissauer, S.; Wilson, G.K.; van IJzendoorn, S.C.D.; Baumert, T.F.; Balfe, P.; Afford, S.; et al. Hepatitis C virus infection of cholangiocarcinoma cell lines. J. Gen. Virol. 2015, 96 Pt 6, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Dai, M.G.; Lu, W.F.; Wang, D.D.; Ye, T.W.; Xu, F.Q.; Liu, S.Y.; Liang, L.; Feng, D.J. Preoperative prediction model for microvascular invasion in HBV-related intrahepatic cholangiocarcinoma. BMC Surg. 2023, 23, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahani, D.; Prasad, S.R.; Tannabe, K.K.; Hahn, P.F.; Mueller, P.R.; Saini, S. Thorotrast-induced cholangiocarcinoma: Case report. Abdom. Imaging 2003, 28, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Tavolari, S. Asbestos and Intrahepatic Cholangiocarcinoma. Cells 2020, 9, 421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathema, V.B.; Na-Bangchang, K. Current insights on cholangiocarcinoma research: A brief review. Asian Pac. J. Cancer Prev. 2015, 16, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Sas, Z.; Cendrowicz, E.; Weinhäuser, I.; Rygiel, T.P. Tumor Microenvironment of Hepatocellular Carcinoma: Challenges and Opportunities for New Treatment Options. Int. J. Mol. Sci. 2022, 23, 3778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. S1), 75–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brunner, S.F.; Roberts, N.D.; Wylie, L.A.; Moore, L.; Aitken, S.J.; Davies, S.E.; Sanders, M.A.; Ellis, P.; Alder, C.; Hooks, Y.; et al. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature 2019, 574, 538–542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mejia, J.C.; Pasko, J. Primary Liver Cancers: Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Surg. Clin. N. Am. 2020, 100, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Schnabl, B. The gut–liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, L. Analysis of hepatocellular carcinoma associated with hepatitis B virus. J. Cell. Mol. Med. 2023, 27, 2271–2277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Péneau, C.; Imbeaud, S.; La Bella, T.; Hirsch, T.Z.; Caruso, S.; Calderaro, J.; Paradis, V.; Blanc, J.F.; Letouzé, E.; Nault, J.C.; et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut 2022, 71, 616–626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khatun, M.; Ray, R.; Ray, R.B. Hepatitis C virus associated hepatocellular carcinoma. Adv. Cancer Res. 2021, 149, 103–142. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Hoshida, Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 105–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Song, C.; Suo, C.; Fan, H.; Zhang, T.; Jin, L.; Chen, X. Alcohol consumption and hepatocellular carcinoma: Novel insights from a prospective cohort study and nonlinear Mendelian randomization analysis. BMC Med. 2022, 20, 413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki-Tanaka, R.; Ray, R.; Moriyama, M.; Ray, R.B.; Kanda, T. Molecular Changes in Relation to Alcohol Consumption and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 9679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garrido, A.; Djouder, N. Cirrhosis: A Questioned Risk Factor for Hepatocellular Carcinoma. Trends Cancer 2021, 7, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Seki, E. Hepatic Stellate Cell–Macrophage Crosstalk in Liver Fibrosis and Carcinogenesis. Semin. Liver Dis. 2020, 40, 307–320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Wang, W.; Morgan, M.P.; Robson, T.; Annett, S. Obesity, non-alcoholic fatty liver disease and hepatocellular carcinoma: Current status and therapeutic targets. Front. Endocrinol. 2023, 14, 1148934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinyol, R.; Torrecilla, S.; Wang, H.; Montironi, C.; Piqué-Gili, M.; Torres-Martin, M.; Wei-Qiang, L.; Willoughby, C.E.; Ramadori, P.; Andreu-Oller, C.; et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 865–878, Erratum in J. Hepatol. 2021, 75, 1515. https://doi.org/10.1016/j.jhep.2021.09.014. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Banini, B.A.; Sanyal, A.J. NAFLD-related HCC. Adv. Cancer Res. 2021, 149, 143–169. [Google Scholar] [CrossRef] [PubMed]
- MOnorato, A.; Fiore, E.; Bayo, J.; Casali, C.; Fernandez-Tomé, M.; Rodríguez, M.; Domínguez, L.; Argemi, J.; Hidalgo, F.; Favre, C.; et al. SPARC inhibition accelerates NAFLD-associated hepatocellular carcinoma development by dysregulating hepatic lipid metabolism. Liver Int. 2021, 41, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Huang, X.Y.; Yao, J.G.; Wang, C.; Xia, Q.; Long, X.D. Genetic polymorphisms in ataxin-3 and liver cirrhosis risk related to aflatoxin B1. Oncotarget 2018, 9, 27321–27332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Jin, J.; Kouznetsova, V.L.; Kesari, S.; Tsigelny, I.F. Synergism in actions of HBV with aflatoxin in cancer development. Toxicology 2023, 499, 153652. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lezana, T.; Lopez-Canovas, J.L.; Villanueva, A. Signaling pathways in hepatocellular carcinoma. Adv. Cancer Res. 2021, 149, 63–101. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.Y.; Dazert, E.; Boldanova, T.; Coto-Llerena, M.; Nuciforo, S.; Ercan, C.; Suslov, A.; Meier, M.A.; Bock, T.; Schmidt, A.; et al. Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat. Commun. 2022, 13, 2436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 561–577.e22, Erratum in Cell 2019, 179, 1240. https://doi.org/10.1016/j.cell.2019.10.038. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Meza, G.; von Felden, J.; Gonzalez-Kozlova, E.E.; Garcia-Lezana, T.; Peix, J.; Portela, A.; Craig, A.J.; Sayols, S.; Schwartz, M.; Losic, B.; et al. DNA Methylation Profiling of Human Hepatocarcinogenesis. Hepatology 2021, 74, 183–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD–NASH-–HCC Transition—Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hann, A.; Oo, Y.H.; Perera, M.T.P.R. Regulatory T-Cell Therapy in Liver Transplantation and Chronic Liver Disease. Front. Immunol. 2021, 12, 719954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, G.; Zhang, G.; Zhao, Y.; Wan, Y.; Jiang, B.; Wang, H. Unveiling the nexus of p53 and PD-L1: Insights into immunotherapy resistance mechanisms in hepatocellular carcinoma. Am. J. Cancer Res. 2025, 15, 1410–1435. [Google Scholar] [CrossRef]
- Law, W.; Zheng, J.; Kim, T.H.; Horvat, N.; Harding, J.J.; Sigel, C.; Arslan, M.E.; Wei, A.; Do, R.K.; Chernyak, V. CT and MRI features of Catenin Beta 1-mutated hepatocellular carcinoma in a Western cohort. In Abdominal Radiology; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Bonelli, P.; Tornesello, A.L.; Tuccillo, F.M.; Starita, N.; Cerasuolo, A.; Cimmino, T.P.; Amiranda, S.; Izzo, F.; Ferrara, G.; Buonaguro, L.; et al. HCV-related hepatocellular carcinoma: Gene signatures associated with TERT promoter mutations and sex. J. Transl. Med. 2025, 23, 639. [Google Scholar] [CrossRef]
- Gu, D.L.; Chen, Y.H.; Shih, J.H.; Lin, C.H.; Jou, Y.S.; Chen, C.F. Target genes discovery through copy number alteration analysis in human hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 8873–8879. [Google Scholar] [CrossRef]
- Zhu, C.; Soto-Feliciano, Y.M.; Morris, J.P.; Huang, C.H.; Koche, R.P.; Ho, Y.J.; Banito, A.; Chen, C.W.; Shroff, A.; Tian, S.; et al. MLL3 regulates the CDKN2A tumor suppressor locus in liver cancer. eLife 2023, 12, e80854. [Google Scholar] [CrossRef]
- Nomeir, H.; Elsheredy, H.; Nomeir, A.; Mostafa, N.R.; El-Hamshary, S. Diagnostic performance of RASSF1A and CDKN2A gene methylation versus α-fetoprotein in hepatocellular carcinoma. Clin. Exp. Hepatol. 2022, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.I.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fung, B.M.; Tabibian, J.H. Cholangiocarcinoma in patients with primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2020, 36, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Miyazu, T.; Ishida, N.; Asai, Y.; Tamura, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Osawa, S.; Baba, S.; et al. Intrahepatic cholangiocarcinoma in patients with primary sclerosing cholangitis and ulcerative colitis: Two case reports. World J. Gastrointest. Surg. 2023, 15, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamid, A.M.; Alshoabi, S.A.; Binnuhaid, A.A.; Alsultan, K.; Alzain, A.F.; Aman, A.M. Primary Sclerosing Cholangitis Associated With Ulcerative Colitis Coexisting With Cholangiocarcinoma: A Case Report. Cureus 2024, 16, e62531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, J.H.; Liau, J.Y.; Lee, C.H.; Jeng, Y.M. Lymphoepithelioma-like Intrahepatic Cholangiocarcinoma Is a Distinct Entity With Frequent pTERT/TP53 Mutations and Comprises 2 Subgroups Based on Epstein-Barr Virus Infection. Am. J. Surg. Pathol. 2021, 45, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; Tsokos, C.G.; Umetsu, S.E.; Shain, A.H.; Kelley, R.K.; Onodera, C.; Bowman, S.; Talevich, E.; Ferrell, L.D.; Kakar, S.; et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J. Pathol. 2019, 248, 164–178. [Google Scholar] [CrossRef]
- Luk, I.S.; Bridgwater, C.M.; Yu, A.; Boila, L.D.; Yáñez-Bartolomé, M.; Lampano, A.E.; Hulahan, T.S.; Boukhali, M.; Kathiresan, M.; Macarulla, T.; et al. SRC inhibition enables formation of a growth suppressive MAGI1-PP2A complex in isocitrate dehydrogenase-mutant cholangiocarcinoma. Sci. Transl. Med. 2024, 16, eadj7685. [Google Scholar] [CrossRef]
- Rasool, W.; Alami Idrissi, Y.; Ahmad, O.; Rath, S.; Saeed, F.; Sayed, M.S.; Sharma, B.; Ibrahim, A.A.; Kumari, A.; Ullah, I.; et al. Safety and efficacy of pemigatinib in patients with cholangiocarcinoma: A systematic review. J. Gastrointest. Oncol. 2025, 16, 699–710. [Google Scholar] [CrossRef]
- Marcus, R.; Ferri-Borgogno, S.; Hosein, A.; Foo, W.C.; Ghosh, B.; Zhao, J.; Rajapakshe, K.; Brugarolas, J.; Maitra, A.; Gupta, S. Oncogenic KRAS Requires Complete Loss of BAP1 Function for Development of Murine Intrahepatic Cholangiocarcinoma. Cancers 2021, 13, 5709. [Google Scholar] [CrossRef]
- Wagner, B.J.; Plum, P.S.; Apel, K.; Scherer, M.; Buchner, D.; Brinkmann, S.; Buettner, R.; Stippel, D.; Quaas, A.; Drebber, U. Protein-loss of SWI/SNF-complex core subunits influences prognosis dependent on histological subtypes of intra- and extrahepatic cholangiocarcinoma. Oncol. Lett. 2021, 21, 349. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Stecko, H.; Moris, D.; Pawlik, T.M. Genomic Profiling of Biliary Tract Cancers: Comprehensive Assessment of Anatomic and Geographic Heterogeneity, Co-Alterations and Outcomes. J. Surg. Oncol. 2025, 131, 1352–1361. [Google Scholar] [CrossRef]
- Alexander, W.B.; Wang, W.; Hill, M.A.; O’Dell, M.R.; Ruffolo, L.I.; Guo, B.; Jackson, K.M.; Ullman, N.; Friedland, S.C.; McCall, M.N.; et al. Smad4 restricts injury-provoked biliary proliferation and carcinogenesis. Dis. Models Mech. 2024, 17, dmm050358. [Google Scholar] [CrossRef]
- Saijuntha, W.; Sithithaworn, P.; Wangboon, C.; Andrews, R.H.; Petney, T.N. Liver Flukes: Clonorchis and Opisthorchis. Adv. Exp. Med. Biol. 2024, 1454, 239–284. [Google Scholar] [CrossRef] [PubMed]
- Spanu, D.; Pretta, A.; Lai, E.; Persano, M.; Donisi, C.; Mariani, S.; Dubois, M.; Migliari, M.; Saba, G.; Ziranu, P.; et al. Hepatocellular carcinoma and microbiota: Implications for clinical management and treatment. World J. Hepatol. 2022, 14, 1319–1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acharya, C.; Sahingur, S.E.; Bajaj, J.S. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight 2017, 2, e94416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Che, Y.; Chen, G.; Guo, Q.; Duan, Y.; Feng, H.; Xia, Q. Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology 2023, 78, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feng, J.; Li, J.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. The gut microbiome-bile acid axis in hepatocarcinogenesis. Biomed. Pharmacother. 2021, 133, 111036. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, J.; O’Neil, M.; Vittal, A.; Weinman, S.A.; Tikhanovich, I. PRMT1-Dependent Macrophage IL-6 Production Is Required for Alcohol-Induced HCC Progression. Gene Expr. 2019, 19, 137–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.H.; Yan, H.Q.; Wang, F.; Wang, Y.Y.; Jiang, Y.N.; Wang, Y.N.; Gao, F.G. TIPE2 inhibits TNF-α-induced hepatocellular carcinoma cell metastasis via Erk1/2 downregulation and NF-κB activation. Int. J. Oncol. 2015, 46, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Yoshida, O.; Watanabe, T.; Yukimoto, A.; Koizumi, Y.; Ikeda, Y.; Tokumoto, Y.; Hirooka, M.; Abe, M.; Hiasa, Y. Stimulated hepatic stellate cell promotes progression of hepatocellular carcinoma due to protein kinase R activation. PLoS ONE 2019, 14, e0212589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, R.; Luo, S.; Zhang, M.; Wang, W.; Zhuo, S.; Wu, Y.; Qiu, Q.; Yuan, Y.; Jiang, X. Ginsenoside Rh4 inhibits inflammation-related hepatocellular carcinoma progression by targeting HDAC4/IL-6/STAT3 signaling. Mol. Genet. Genom. 2023, 298, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Lai, P.; Zhang, S.; Wang, Y.; Qu, N.; Lu, D.; Gao, L.; Xu, L.; Yang, Y.; Zhang, T.; et al. The Relationship between Hepatic Myeloid-Derived Suppressor Cells and Clinicopathological Parameters in Patients with Chronic Liver Disease. BioMed Res. Int. 2021, 2021, 6612477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saab, M.; Mestivier, D.; Sohrabi, M.; Rodriguez, C.; Khonsari, M.R.; Faraji, A.; Sobhani, I. Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma. PLoS ONE 2021, 16, e0247798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rao, B.; Ren, T.; Wang, X.; Wang, H.; Zou, Y.; Sun, Y.; Liu, S.; Ren, Z.; Yu, Z. Dysbiosis in the Human Microbiome of Cholangiocarcinoma. Front. Physiol. 2021, 12, 715536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvaro, D.; Cannizzaro, R.; Labianca, R.; Valvo, F.; Farinati, F.; Italian Society of Gastroenterology (SIGE); Italian Association of Hospital Gastroenterology (AIGO); Italian Association of Medical Oncology (AIOM); Italian Association of Oncological Radiotherapy (AIRO). Cholangiocarcinoma: A position paper by the Italian Society of Gastroenterology (SIGE), the Italian Association of Hospital Gastroenterology (AIGO), the Italian Association of Medical Oncology (AIOM) and the Italian Association of Oncological Radiotherapy (AIRO). Dig. Liver Dis. 2010, 42, 831–838. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Brusnic, O.; Onișor, D.M. Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines 2023, 11, 2930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; Liu, Y.; Ma, T.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Nie, H.; Huang, J.; Mu, G. Fermented Dairy Products as Precision Modulators of Gut Microbiota and Host Health: Mechanistic Insights, Clinical Evidence, and Future Directions. Foods 2025, 14, 1946. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Wang, X.; Dong, J.; Li, J. Gamma -aminobutyric acid ameliorates neurological impairments in type 1 diabetes mellitus mice by regulating the “gut flora-LPS-TLR4-NF-ΚB” signalling Axis. Diabetol. Metab. Syndr. 2025, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.M.C.; Feng, S.; Ng, E.W.L.; Qiao, Y. Biochemical characterization of Bifidobacterium bifidum peptidoglycan d,l-endopeptidase BbMep that generates NOD2 ligands. RSC Chem. Biol. 2025, 6, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, K.; Zhao, Y.; Ni, Y.; Zhang, Z.; Yuan, X.; Ning, J.; Song, Q.; Zhao, F.; He, L.; et al. Arbutin ameliorated depression by inhibiting neuroinflammation and modulating intestinal flora. Phytomedicine Int. J. Phytother. Phytopharm. 2025, 145, 156944. [Google Scholar] [CrossRef]
- He, L.; Yang, G.; Li, T.; Li, W.; Yang, R. Metabolic profile of procyanidin A2 by human intestinal microbiota and their antioxidant and hypolipidemic potential in HepG2 cells. Eur. J. Nutr. 2025, 64, 113. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Tang, W.; Li, D.; Ma, L.; Liu, H.; Zhang, S.; Zhang, X.; Dong, L.; Shen, X.; et al. Hepatic NOD2 promotes hepatocarcinogenesis via a RIP2-mediated proinflammatory response and a novel nuclear autophagy-mediated DNA damage mechanism. J. Hematol. Oncol. 2021, 14, 9. [Google Scholar] [CrossRef]
- Tzeng, H.T.; Lee, W.C. Impact of Transgenerational Nutrition on Nonalcoholic Fatty Liver Disease Development: Interplay between Gut Microbiota, Epigenetics and Immunity. Nutrients 2024, 16, 1388. [Google Scholar] [CrossRef]
- Marcelino, B.D.R.; Vieira, M.C.D.S.; Silva, M.J.A.; da Silva, L.C.S.S.; Gurrão, E.P.D.C.; Dos Santos, E.C.; Cabral, J.G.; Souza, A.B.; Sardinha, D.M.; Marinho, R.L.; et al. Study of TNF-α, IFN-γ, IL-10, TGF-β and IL-6 Gene Polymorphisms in a Cohort of Professionals Who Worked in the First Pandemic Wave in the Brazilian Amazon. Crit. Rev. Immunol. 2025, 45, 39–61. [Google Scholar] [CrossRef]
- Khdair, S.I.; Al-Naimat, O.S.; Jarrar, W.; Al-Qerem, W.; Khudeir, F.A. The Influence of TNF-α, IL-6, TGF-β1, IFN-γ, IL-10 Polymorphisms on Predisposition to Diabetes Mellitus among Jordanian Patients. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 681–691. [Google Scholar] [CrossRef]
- Tun, K.M.; Hong, A.S.; Batra, K.; Naga, Y.; Ohning, G. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Hepatic Encephalopathy and Clostridioides difficile Infection in Patients With Cirrhosis. Cureus 2022, 14, e25537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinlaor, P.; Kaewpitoon, N.; Laha, T.; Sripa, B.; Kaewkes, S.; Morales, M.E.; Mann, V.H.; Parriott, S.K.; Suttiprapa, S.; Robinson, M.W.; et al. Cathepsin F cysteine protease of the human liver fluke, Opisthorchis viverrini. PLoS Negl. Trop. Dis. 2009, 3, e398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buza, T.M.; Tonui, T.; Stomeo, F.; Tiambo, C.; Katani, R.; Schilling, M.; Lyimo, B.; Gwakisa, P.; Cattadori, I.M.; Buza, J.; et al. iMAP: An integrated bioinformatics and visualization pipeline for microbiome data analysis. BMC Bioinform. 2019, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Franzén, O.; Hu, J.; Bao, X.; Itzkowitz, S.H.; Peter, I.; Bashir, A. Improved OTU-picking using long-read 16S rRNA gene amplicon sequencing and generic hierarchical clustering. Microbiome 2015, 3, 43. [Google Scholar] [CrossRef]
- Navas-Molina, J.A.; Peralta-Sánchez, J.M.; González, A.; McMurdie, P.J.; Vázquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [CrossRef] [PubMed]
- Pallozzi, M.; De Gaetano, V.; Di Tommaso, N.; Cerrito, L.; Santopaolo, F.; Stella, L.; Gasbarrini, A.; Ponziani, F.R. Role of Gut Microbial Metabolites in the Pathogenesis of Primary Liver Cancers. Nutrients 2024, 16, 2372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasan, R.; Bose, S.; Roy, R.; Paul, D.; Rawat, S.; Nilwe, P.; Chauhan, N.K.; Choudhury, S. Tumor tissue-specific bacterial biomarker panel for colorectal cancer: Bacteroides massiliensis, Alistipes species, Alistipes onderdonkii, Bifidobacterium pseudocatenulatum, Corynebacterium appendicis. Arch. Microbiol. 2022, 204, 348. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria Provide Genetic and Molecular Reporter Systems to Identify Specific Diseases. Int. J. Mol. Sci. 2024, 25, 4431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albhaisi, S.A.M.; Bajaj, J.S.; Sanyal, A.J. Role of gut microbiota in liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G84–G98. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, C.E.; Yoon, L.S.; Michels, K.B.; Tranfield, W.; Jacobs, J.P.; May, F.P. The Impact of Prebiotic, Probiotic, and Synbiotic Supplements and Yogurt Consumption on the Risk of Colorectal Neoplasia among Adults: A Systematic Review. Nutrients 2022, 14, 4937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribera, C.; Sánchez-Ortí, J.V.; Clarke, G.; Marx, W.; Mörkl, S.; Balanzá-Martínez, V. Probiotic, prebiotic, synbiotic and fermented food supplementation in psychiatric disorders: A systematic review of clinical trials. Neurosci. Biobehav. Rev. 2024, 158, 105561. [Google Scholar] [CrossRef] [PubMed]
- Beyoğlu, D.; Idle, J.R. The gut microbiota—A vehicle for the prevention and treatment of hepatocellular carcinoma. Biochem. Pharmacol. 2022, 204, 115225. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kuang, X.; Yan, H.; Ren, P.; Yang, X.; Liu, H.; Liu, Q.; Yang, H.; Kang, X.; Shen, X.; et al. A Novel Synbiotic Alleviates Autoimmune Hepatitis by Modulating the Gut Microbiota-Liver Axis and Inhibiting the Hepatic TLR4/NF-κB/NLRP3 Signaling Pathway. mSystems 2023, 8, e0112722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Suk, K.T. The Role of the Gut Microbiome in Liver Cirrhosis Treatment. Int. J. Mol. Sci. 2020, 22, 199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Margáin, A.; Román-Calleja, B.M.; Moreno-Guillén, P.; González-Regueiro, J.A.; Kúsulas-Delint, D.; Campos-Murguía, A.; Flores-García, N.C.; Macías-Rodríguez, R.U. Nutritional therapy for hepatocellular carcinoma. World J. Gastrointest. Oncol. 2021, 13, 1440–1452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahdavi, R.; Faramarzi, E.; Nikniaz, Z.; FarshiRadvar, F. Role of Probiotics and Synbiotics in Preventing Chemoradiotherapy-Associated Toxicity in Colorectal Cancer Patients: A Systematic Review. Iran. J. Med. Sci. 2023, 48, 110–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, J.; Ke, S.; Chen, Y.; Xiao, J.; Tang, Z.; Wang, L.; Ren, Y.; Alnaggar, M.; Qiu, H.; et al. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: An open-label, single-arm, phase II trial (RENMIN-215). EClinicalMedicine 2023, 66, 102315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal microbiota transplantation: Current challenges and future landscapes. Clin. Microbiol. Rev. 2024, 37, e0006022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Gong, C.; Xu, J.; Chen, D.; Yang, B.; Chen, Z.; Wei, L. Research Progress of Fecal Microbiota Transplantation in Liver Diseases. J. Clin. Med. 2023, 12, 1683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abenavoli, L.; Maurizi, V.; Rinninella, E.; Tack, J.; Di Berardino, A.; Santori, P.; Rasetti, C.; Procopio, A.C.; Boccuto, L.; Scarpellini, E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina 2022, 58, 1559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, B.; Zhu, J.; Wang, Y.; Chen, W.; Fang, S.; Mao, W.; Xu, Z.; Yang, Y.; Weng, Q.; Zhao, Z.; et al. Targeted xCT-mediated Ferroptosis and Protumoral Polarization of Macrophages Is Effective against HCC and Enhances the Efficacy of the Anti-PD-1/L1 Response. Adv. Sci. 2023, 10, e2203973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, H.; Wang, H.; Li, C.; Fang, J.Y.; Xu, J. Cancer Cell-Intrinsic PD-1 and Implications in Combinatorial Immunotherapy. Front. Immunol. 2018, 9, 1774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P.; Krawczyk, P.; Milanowski, J. Diet, Microbiome, and Cancer Immunotherapy—A Comprehensive Review. Nutrients 2021, 13, 2217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, Y.; Sichler, A.; Ecker, J.; Laschinger, M.; Liebisch, G.; Höring, M.; Basic, M.; Bleich, A.; Zhang, X.J.; Kübelsbeck, L.; et al. Gut microbiota promote liver regeneration through hepatic membrane phospholipid biosynthesis. J. Hepatol. 2023, 78, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, X.; Liu, W.; Wei, H.; Liang, W.; Zhou, Y.; Ding, Y.; Ji, F.; Ho-Kwan Cheung, A.; Wong, N.; et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J. Hepatol. 2023, 79, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.D.; Haidacher, S.J.; Engevik, M.A.; Luck, B.; Ruan, W.; Ihekweazu, F.; Bajaj, M.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; et al. Interrogation of the mammalian gut–brain axis using LC–MS/MS-based targeted metabolomics with in vitro bacterial and organoid cultures and in vivo gnotobiotic mouse models. Nat. Protoc. 2023, 18, 490–529. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teng, H.; Wang, Y.; Sui, X.; Fan, J.; Li, S.; Lei, X.; Shi, C.; Sun, W.; Song, M.; Wang, H.; et al. Gut microbiota-mediated nucleotide synthesis attenuates the response to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Cell 2023, 41, 124–138.e6. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; He, X.; Liu, Y.; Gao, F.; Lu, W.; Fan, Y.; Gao, Y.; Wang, W.; Zhu, F.; Wang, Y.; et al. Longitudinal Gut Microbiota Dysbiosis Underlies Olanzapine-Induced Weight Gain. Microbiol. Spectr. 2023, 11, e0005823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Gene | Function/Role | Cancer Type | Mutation Frequency/Relevance | References |
|---|---|---|---|---|
| TP53 | Tumor suppressor, DNA repair, apoptosis | HCC, CCA | ~30–50% in HCC; also common in eCCA | [45,63] |
| CTNNB1 | Wnt/β-catenin pathway activation | HCC | Activating mutations that drive proliferation | [40,46] |
| TERT | Telomerase reverse transcriptase | HCC, CCA | Promoter mutations in early stages of HCC | [33,42,47] |
| IDH1/2 | Metabolism, epigenetic modulation | iCCA | ~10–20% in iCCA, rare in HCC | [57,59] |
| FGFR2 | Tyrosine kinase receptor, cell growth | iCCA | Fusions/rearrangements targetable by inhibitors | [60] |
| ARID1A | Chromatin remodeling | HCC, CCA | Frequently mutated; epigenetic dysregulation | [48,62] |
| BAP1 | Tumor suppressor, chromatin regulation | CCA | Loss-of-function linked to poor prognosis | [61] |
| KRAS | RAS signaling pathway | eCCA | Commonly mutated in eCCA, less frequent in iCCA or HCC | [63] |
| SMAD4 | TGF-β signaling, tumor suppression | eCCA | Frequently altered in eCCA | [63] |
| AXIN1 | Wnt pathway inhibitor | HCC | Inactivation promotes β-catenin signaling | [48] |
| KEAP1/NFE2L2 | Oxidative stress response | HCC | Mutations impair ROS detox pathways | [48] |
| PBRM1 | SWI/SNF complex, chromatin regulation | CCA | Frequently mutated; epigenetic dysregulation | [62] |
| MLL3/KMT2C | Histone methyltransferase | HCC | Mutated in HCC; linked to tumor suppressor CDKN2A | [50] |
| CDKN2A | Cell cycle regulation | HCC, CCA | Methylated or deleted in both cancers | [49,50] |
| RASSF1A | Tumor suppressor | HCC | Silenced via promoter methylation | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Bonifacio-Mundaca, J.; Desterke, C.; Casafont, Í.; Mata-Garrido, J. Genomic Alterations and Microbiota Crosstalk in Hepatic Cancers: The Gut–Liver Axis in Tumorigenesis and Therapy. Genes 2025, 16, 920. https://doi.org/10.3390/genes16080920
Fu Y, Bonifacio-Mundaca J, Desterke C, Casafont Í, Mata-Garrido J. Genomic Alterations and Microbiota Crosstalk in Hepatic Cancers: The Gut–Liver Axis in Tumorigenesis and Therapy. Genes. 2025; 16(8):920. https://doi.org/10.3390/genes16080920
Chicago/Turabian StyleFu, Yuanji, Jenny Bonifacio-Mundaca, Christophe Desterke, Íñigo Casafont, and Jorge Mata-Garrido. 2025. "Genomic Alterations and Microbiota Crosstalk in Hepatic Cancers: The Gut–Liver Axis in Tumorigenesis and Therapy" Genes 16, no. 8: 920. https://doi.org/10.3390/genes16080920
APA StyleFu, Y., Bonifacio-Mundaca, J., Desterke, C., Casafont, Í., & Mata-Garrido, J. (2025). Genomic Alterations and Microbiota Crosstalk in Hepatic Cancers: The Gut–Liver Axis in Tumorigenesis and Therapy. Genes, 16(8), 920. https://doi.org/10.3390/genes16080920






