A Fast TaqMan® Real-Time PCR Assay for the Detection of Mitochondrial DNA Haplotypes in a Wolf Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction, Primer and Probe Design
2.3. Real-Time PCR Conditions
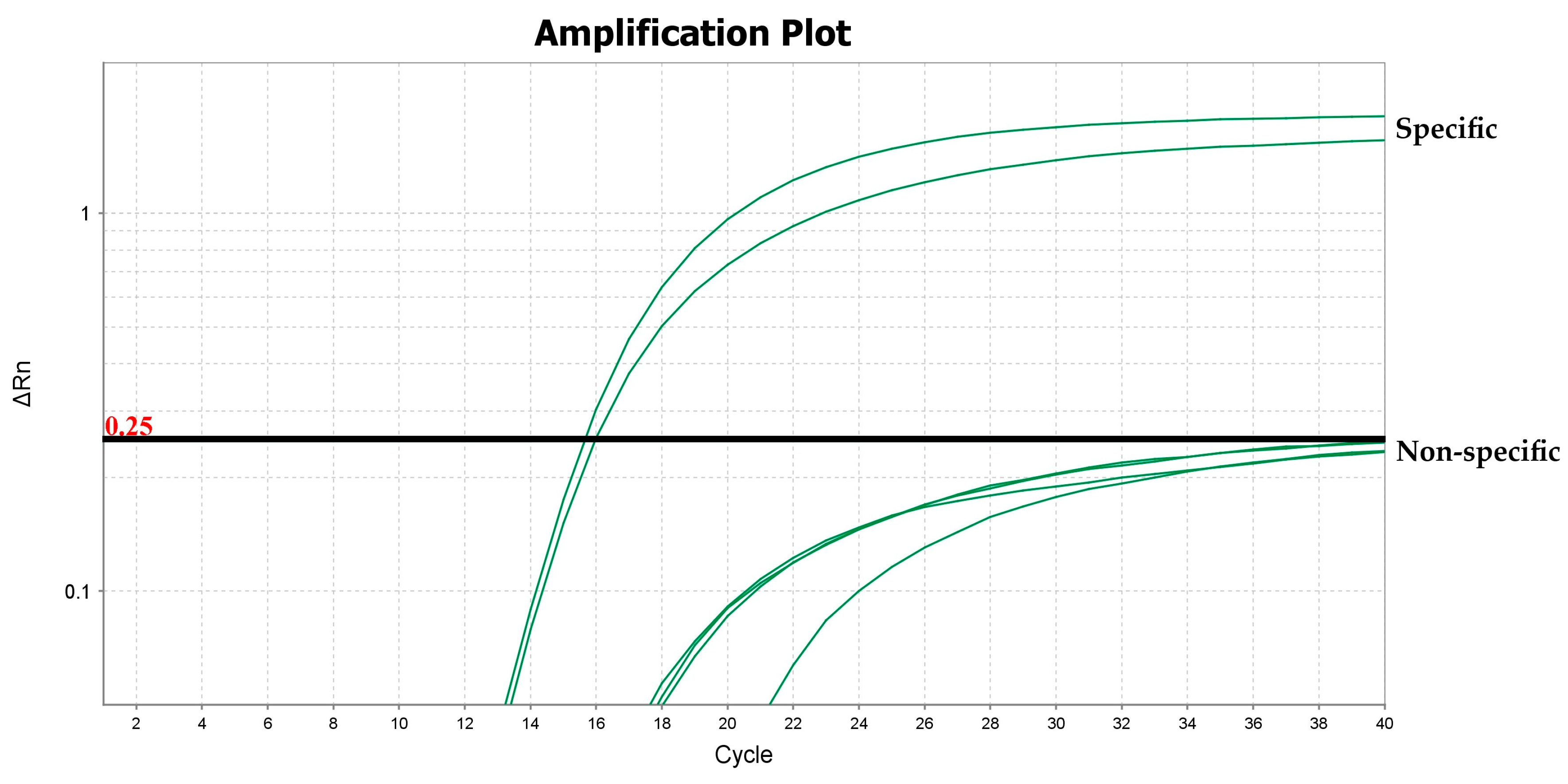
2.4. Validation Studies
3. Results
3.1. Evaluating Primers/Probe Sets
3.2. Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | Control region |
| CV | Coefficient of variation |
| MGB | Minor Groove Binder |
| mtDNA | Mitochondrial DNA |
| NDH-4 | NADH dehydrogenase 4 |
| PCR | Polymerase Chain Reaction |
| SD | Standard deviation |
| SNP | Single-nucleotide polymorphism |
| STR | Short tandem repeat |
References
- Silva, P.; Galaverni, M.; Ortega-Del Vecchyo, D.; Fan, Z.; Caniglia, R.; Fabbri, E.; Randi, E.; Wayne, R.; Godinho, R. Genomic Evidence for the Old Divergence of Southern European Wolf Populations. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201206. [Google Scholar] [CrossRef]
- Marucco, F.; Pilgrim, K.L.; Avanzinelli, E.; Schwartz, M.K.; Rossi, L. Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading. Animals 2022, 12, 1260. [Google Scholar] [CrossRef]
- Proposal for a Directive of the European Parliament and of the Council Amending Council Directive 92/43/EEC as Regards the Protection Status of the Wolf (Canis lupus). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=COM:2025:106:FIN (accessed on 7 March 2025).
- Musto, C.; Cerri, J.; Galaverni, M.; Caniglia, R.; Fabbri, E.; Apollonio, M.; Mucci, N.; Bonilauri, P.; Maioli, G.; Fontana, M.C. Men and Wolves: Anthropogenic Causes Are an Important Driver of Wolf Mortality in Human-Dominated Landscapes in Italy. Glob. Ecol. Conserv. 2021, 32, e01892. [Google Scholar] [CrossRef]
- Salvatori, V.; Godinho, R.; Braschi, C.; Boitani, L.; Ciucci, P. High Levels of Recent Wolf × Dog Introgressive Hybridization in Agricultural Landscapes of Central Italy. Eur. J. Wildl. Res. 2019, 65, 73. [Google Scholar] [CrossRef]
- Donfrancesco, V.; Ciucci, P.; Salvatori, V.; Benson, D.; Andersen, L.W.; Bassi, E.; Blanco, J.C.; Boitani, L.; Caniglia, R.; Canu, A. Unravelling the Scientific Debate on How to Address Wolf-Dog Hybridization in Europe. Front. Ecol. Evol. 2019, 7, 175. [Google Scholar] [CrossRef]
- Santostasi, N.L.; Bauduin, S.; Grente, O.; Gimenez, O.; Ciucci, P. Simulating the Efficacy of Wolf–Dog Hybridization Management with Individual-based Modeling. Conserv. Biol. 2025, 39, e14312. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, R.; Galaverni, M.; Velli, E.; Mattucci, F.; Canu, A.; Apollonio, M.; Mucci, N.; Scandura, M.; Fabbri, E. A Standardized Approach to Empirically Define Reliable Assignment Thresholds and Appropriate Management Categories in Deeply Introgressed Populations. Sci. Rep. 2020, 10, 2862. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, R.; Attili, L.; Tancredi, C.; Fanelli, R.; Garofalo, L. A Validated Molecular Protocol to Differentiate Pure Wolves, Dogs and Wolf x Dog Hybrids through a Panel of Multiplexed Canine STR Markers. Diversity 2022, 14, 511. [Google Scholar] [CrossRef]
- Randi, E.; Lucchini, V.; Christensen, M.F.; Mucci, N.; Funk, S.M.; Dolf, G.; Loeschcke, V. Mitochondrial DNA Variability in Italian and East European Wolves: Detecting the Consequences of Small Population Size and Hybridization. Conserv. Biol. 2000, 14, 464–473. [Google Scholar] [CrossRef]
- Caniglia, R.; Fabbri, E.; Mastrogiuseppe, L.; Randi, E. Who Is Who? Identification of Livestock Predators Using Forensic Genetic Approaches. Forensic Sci. Int. Genet. 2013, 7, 397–404. [Google Scholar] [CrossRef]
- Montana, L.; Caniglia, R.; Galaverni, M.; Fabbri, E.; Randi, E. A New Mitochondrial Haplotype Confirms the Distinctiveness of the Italian Wolf (Canis lupus) Population. Mamm. Biol. 2017, 84, 30–34. [Google Scholar] [CrossRef]
- Montana, L.; Caniglia, R.; Galaverni, M.; Fabbri, E.; Ahmed, A.; Bolfíková, B.Č.; Czarnomska, S.D.; Galov, A.; Godinho, R.; Hindrikson, M.; et al. Combining Phylogenetic and Demographic Inferences to Assess the Origin of the Genetic Diversity in an Isolated Wolf Population. PLoS ONE 2017, 12, e0176560. [Google Scholar] [CrossRef] [PubMed]
- Hindrikson, M.; Männil, P.; Ozolins, J.; Krzywinski, A.; Saarma, U. Bucking the Trend in Wolf-Dog Hybridization: First Evidence from Europe of Hybridization between Female Dogs and Male Wolves. PLoS ONE 2012, 7, e46465. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, U.; Mazzara, M.; Broll, H.; Giacomo, M.; Grohmann, L.; Herau, V.; Holst-Jensen, A.; Hougs, L.; Hübert, P.; Laurensse, E. European Network of GMO Laboratories (ENGL) Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Björnerfeldt, S.; Webster, M.T.; Vilà, C. Relaxation of Selective Constraint on Dog Mitochondrial DNA Following Domestication. Genome Res. 2006, 16, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Dawnay, N.; Ogden, R.; McEwing, R.; Carvalho, G.R.; Thorpe, R.S. Validation of the Barcoding Gene COI for Use in Forensic Genetic Species Identification. Forensic Sci. Int. 2007, 173, 1–6. [Google Scholar] [CrossRef]
- SWFS Technical Working Group. Standards and Guidelines for Wildlife Forensic Analysis, Version 3; Webster, L.M.I., Ed.; Society for Wildlife Forensic Science, Fort Collins, CO, USA, 2018; p. 21. Available online: https://www.wildlifeforensicscience.org/documents/ (accessed on 15 February 2025).
- Best Practice Manuals and Forensic Guidelines|ENFSI. Available online: https://enfsi.eu/about-enfsi/structure/working-groups/documents-page/documents/best-practice-manuals/ (accessed on 10 February 2025).
- Kusak, J.; Fabbri, E.; Galov, A.; Gomerčić, T.; Arbanasić, H.; Caniglia, R.; Galaverni, M.; Reljić, S.; Huber, D.; Randi, E. Wolf-Dog Hybridization in Croatia. Vet. Arh. 2018, 88, 375–395. [Google Scholar] [CrossRef]
- Fabbri, E.; Caniglia, R.; Kusak, J.; Galov, A.; Gomerčić, T.; Arbanasić, H.; Huber, D.; Randi, E. Genetic Structure of Expanding Wolf (Canis lupus) Populations in Italy and Croatia, and the Early Steps of the Recolonization of the Eastern Alps. Mamm. Biol. 2014, 79, 138–148. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, I.; Lee, S.-Y.; Hong, Y.; Kim, H.-Y. Quantitative Detection of Pork in Commercial Meat Products by TaqMan® Real-Time PCR Assay Targeting the Mitochondrial D-Loop Region. Food Chem. 2016, 210, 102–106. [Google Scholar] [CrossRef]
- Ma, X.; Xia, H.; Pan, Y.; Huang, Y.; Xu, T.; Guan, F. Double-Tube Multiplex TaqMan Real-Time PCR for the Detection of Eight Animal-Derived Dairy Ingredients. J. Agric. Food Chem. 2024, 72, 11640–11651. [Google Scholar] [CrossRef]
- Sales, K.G.D.S.; Miranda, D.E.D.O.; Paiva, M.H.S.; Figueredo, L.A.; Otranto, D.; Dantas-Torres, F. Fast Multiplex Real-Time PCR Assay for Simultaneous Detection of Dog and Human Blood and Leishmania Parasites in Sand Flies. Parasit. Vectors 2020, 13, 131. [Google Scholar] [CrossRef]
- Caniglia, R.; Galaverni, M.; Delogu, M.; Fabbri, E.; Musto, C.; Randi, E. Big Bad Wolf or Man’s Best Friend? Unmasking a False Wolf Aggression on Humans. Forensic Sci. Int. Genet. 2016, 24, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Della Valle, A.; Serino, A.; Corrado, F.; Di Nunzio, C. How the Forensic Multidisciplinary Approach Can Solve a Fatal Dog Pack Attack. Forensic Sci. Med. Pathol. 2023, 20, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Godinho, R.; Llaneza, L.; Blanco, J.C.; Lopes, S.; Álvares, F.; García, E.J.; Palacios, V.; Cortés, Y.; Talegón, J.; Ferrand, N. Genetic Evidence for Multiple Events of Hybridization between Wolves and Domestic Dogs in the Iberian Peninsula. Mol. Ecol. 2011, 20, 5154–5166. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, S.E.; Jeong, H.W.; Ha, J.H. The complete nucleotide sequence of the domestic dog (Canis familiaris) mitochondrial genome. Mol. Phylogenetics Evol. 1998, 10, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V.J.; Sawyer, S.K.; Greenfield, D.L.; Germonpré, M.B.; Sablin, M.V.; López-Giráldez, F.; Domingo-Roura, X.; et al. Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 2013, 342, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Hultin Jaderlund, K.; Nennesmo, I.; Holmqvist, E.; Heidrich, N.; Larsson, N.-G.; Andersson, G.; Wagner, G.H.; Hedhammar, A.; Wibom, R.; et al. Sensory ataxic neuropathy in golden retriever dogs is caused by a deletion in the mitochondrial tRNATyr gene. PLoS Genet. 2009, 5, E1000499. [Google Scholar] [CrossRef]
- Koblmüller, S.; Vilà, C.; Lorente-Galdos, B.; Dabad, M.; Ramirez, O.; Marques-Bonet, T.; Wayne, R.K.; Leonard, J.A. Whole mitochondrial genomes illuminate ancient intercontinental dispersals of grey wolves (Canis lupus). J. Biogeogr. 2016, 43, 1728–1738. [Google Scholar] [CrossRef]
- Webb, K.M.; Allard, M.W. Mitochondrial genome DNA analysis of the domestic dog: Identifying informative SNPs outside of the control region. J. Forensic Sci. 2009, 54, 275–288. [Google Scholar] [CrossRef]

| Target | SNP Position | Primer Sequence (5′ → 3′) | Tm (°C) | GC (%) | TaqMan Probe | Tm (°C) | GC (%) | Amplicon Size (bp) |
|---|---|---|---|---|---|---|---|---|
| ND4 | 11515 | F-TCAAACCATCATTCACACGAGAA R-GGATAGGAGGAGTAGGGGCAGTAG | 59.1 59.5 | 39 58 | VIC-CCTAATGgCCTTGCA-MGB NFQ | 65 | 53 | 68 |
| CR | 15654 | F-CTCAATCTCACAATTCACTGACCTATC R-GGCATGGTGATTAAGCCCTTAT | 58.3 58.0 | 41 45 | 6FAM-ACAGTAAtCGAATGCAT-MGB NFQ | 66 | 35 | 80 |
| Haplotype | Apennines (Italy) | Greece | Bulgaria | Slovenia | ||
|---|---|---|---|---|---|---|
| ND4 | CR | |||||
| WH14 | N5 (G) | W14 (T) | + [14] | |||
| WH19 | N5 (G) | W16 (T) | + [1] | |||
| WH17 | N5 (G) | W15 (C) | + | |||
| WH18 | N4 (A) | W16 (T) | + | + | + [1] | |
| WH15 | N4 (A) | W14 (T) | + | |||
| WH3 | N1 (A) | W3 (T) | + [19] | |||
| WH20 | N4 (A) | W17 (T) | + [1] |
| N | No Curve | One Curve | Two Curves | |
|---|---|---|---|---|
| Apennine wolf | 38 | 0 | 0 | 38 |
| Apennine wolf × dog hybrid | 33 | 0 | 0 | 33 |
| Slovenian wolf | 21 | 19 | 2 (6FAM) | 0 |
| Non-Apennine wolf × dog hybrid | 5 | 5 | 0 | 0 |
| Dog | 52 | 47 | 5 (6FAM) | 0 |
| Wolf | Dog | ||||
|---|---|---|---|---|---|
| DNA Content (ng) | Mean | SD | CV (%) | Mean | SD |
| 10 | 16.23 | 0.78 | 4.2 | 39.80 | 0.58 |
| 1 | 20.11 | 0.83 | 4.0 | ND | ND |
| 10−1 | 24.31 | 1.40 | 5.6 | ND | ND |
| 10−2 | 28.85 | 2.01 | 6.6 | ND | ND |
| 10−3 | 33.23 | 2.67 | 7.5 | ND | ND |
| 10−4 | 37.40 | 2.63 | 6.3 | ND | ND |
| 10−5 | 39.11 | 1.10 | 2.3 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzini, R.; Attili, L.; De Crescenzo, M.; Pizzarelli, A. A Fast TaqMan® Real-Time PCR Assay for the Detection of Mitochondrial DNA Haplotypes in a Wolf Population. Genes 2025, 16, 897. https://doi.org/10.3390/genes16080897
Lorenzini R, Attili L, De Crescenzo M, Pizzarelli A. A Fast TaqMan® Real-Time PCR Assay for the Detection of Mitochondrial DNA Haplotypes in a Wolf Population. Genes. 2025; 16(8):897. https://doi.org/10.3390/genes16080897
Chicago/Turabian StyleLorenzini, Rita, Lorenzo Attili, Martina De Crescenzo, and Antonella Pizzarelli. 2025. "A Fast TaqMan® Real-Time PCR Assay for the Detection of Mitochondrial DNA Haplotypes in a Wolf Population" Genes 16, no. 8: 897. https://doi.org/10.3390/genes16080897
APA StyleLorenzini, R., Attili, L., De Crescenzo, M., & Pizzarelli, A. (2025). A Fast TaqMan® Real-Time PCR Assay for the Detection of Mitochondrial DNA Haplotypes in a Wolf Population. Genes, 16(8), 897. https://doi.org/10.3390/genes16080897





