The Complex Role of the miR-17-92 Cluster in Stroke: Mechanistic Insights and Biomarker Potential
Abstract
:1. Introduction
2. miR-17-92 Cluster and Its Paralogs
3. Genetic Variations in the miR-17-92 Cluster and Its Paralogs: Implications for Stroke
4. miR-17-92 Cluster: Implications of Altered Expression Levels as a Biomarker of Stroke
| Type of Stroke | Expression Level | Biological Specimens and Approach Used for Evaluation | Relevant Findings of the Study | Reference |
|---|---|---|---|---|
| IS and HS | ↓ miR-17-5p, ↓ miR-18a-5p, ↓ miR-19a-3p, ↓ miR-19b-3p ↓ miR-20a-5p, ↓ miR-92a-3p | Serum from IS patients (n = 58) and healthy controls (n = 50); GSE117064 cohort (173 IS cases and 1612 HC cases); days 1 and 7. | Diagnostic value for IS; moderate discrimination ability for distinguishing IS from HC. | [17] |
| IS | ↓ miR-17-5p, ↓ miR-17-3p, ↓ miR-18a-3p, ↓ miR-18a-5p, ↓ miR-19b-3p, ↓ miR-19a-3p, ↓ miR-92a-3p | GSE110993, from IS (n = 20) and control group (n = 20). | circ_0011474-hsa-miR-20a-5p/hsa-miR-17-5p-CDKN1A ceRNA regulatory axis in IS. | [2] |
| IS | ↑ miR-17-5p, ↓ miR-19a-3p | PBMC; IS (n = 398) and control group (n = 397). | Diagnostic role. | [7] |
| IS | ↑ miR-17-5p | Serum; IS (n = 106) and healthy control group (n = 102). | MiR-17-5p an independent predictor; a higher diagnostic value of miR-17-5p in combination with miR-15a and miR-16. | [31] |
| IS | ↑ miR-17-5p | Plasma from IS patients (n = 83) and healthy controls (n = 37). | Diagnostic and recurrence role. | [32] |
| IS | ↓ miR-19a | Leukocytes; IS (n = 24) and control group (n = 24). | miR-19a is part of an altered signature, along with downregulated miR-122, miR-148a, let-7i, miR-19a, miR-320d, and miR-4429 and overexpressed miR-363 and miR-487b; involved in thrombus formation. | [33] |
| IS and TIA | ↓ miR-18a-5p, ↓ miR-20a-5p | Serum; IS (191) and TIA (61). | Part of an 11-miRNA signature to discriminate IS versus TIA. | [35] |
| IS | ↓ miR-93 | Plasma and neutrophils; IS (n = 33) and control group (n = 20). | Diagnostic and prognostic role; correlated with neurological function score; correlated with the expression of TNFα and IL10. | [34] |
| IS | ↓ miR-19a, ↑ miR-363 | Leukocytes; IS (n = 24) and vascular risk factor controls (n = 24). | miR-363 part of altered signature along with overexpression of miR-122, miR-148a, let-7i, miR-19a, miR-320d, and miR-4429 and downregulation of miR-487b. | [33] |
| IS | ↓ miR-92a | Serum; IS (70) and control group (n = 25). | miR-92a part of altered signature along with downregulation of miR-375 and overexpression of miR-134. | [37] |
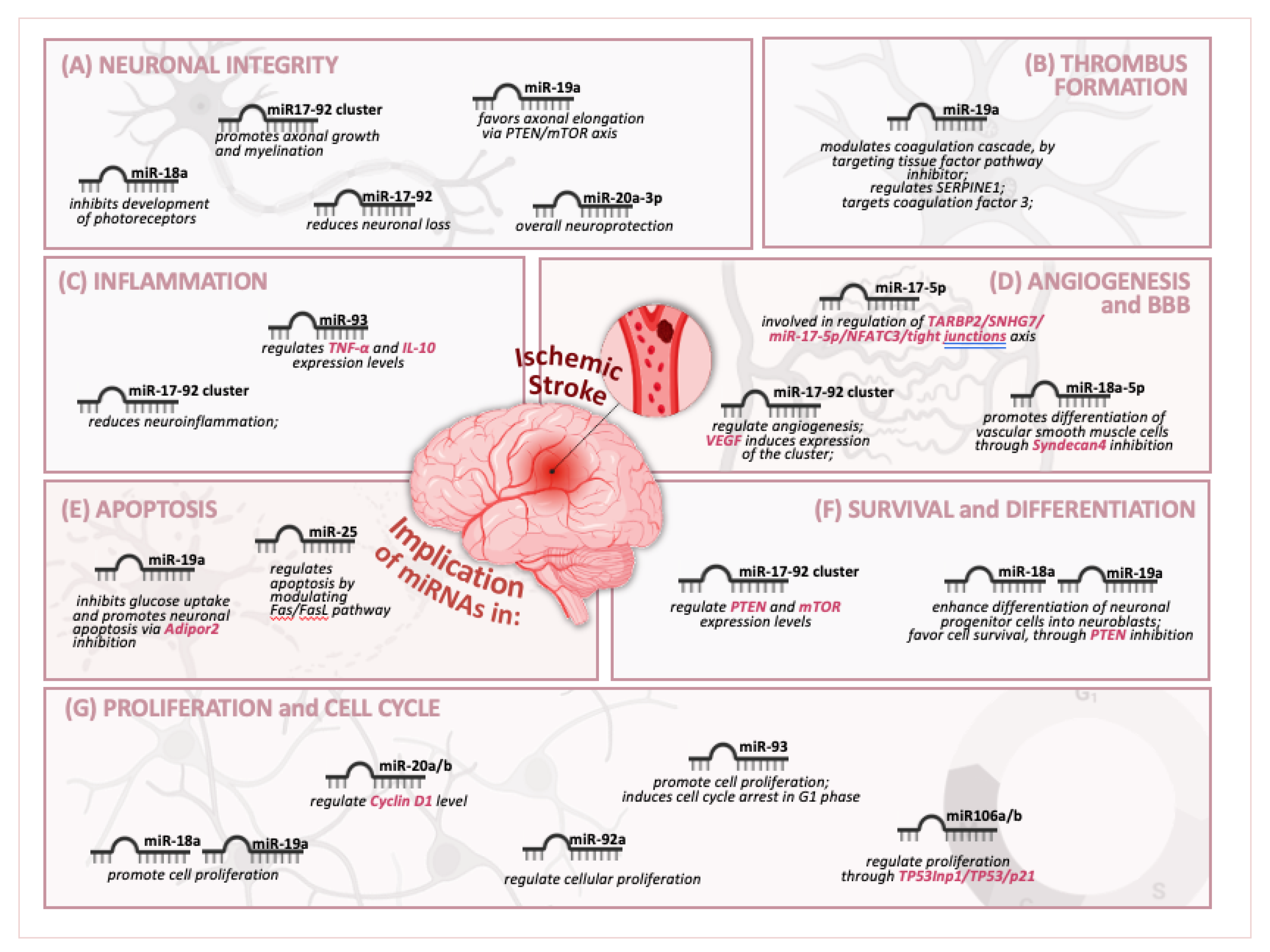
5. miR-17-92 Cluster and Its Paralogs Affect Key Physiological and Pathological Processes
5.1. miR-17-92 Cluster and Its Paralogs Affect Cell Death/Survival and Differentiation
5.2. miR-17-92 Cluster and Its Paralogs’ Regulation of Angiogenesis
5.3. miR-17-92 Cluster and Its Paralogs Are Involved in the Blood–Brain Barrier (BBB)
5.4. miR-17-92 Cluster and Its Paralogs’ Regulation of Thrombus Formation
5.5. miR-17-92 Cluster and Its Paralogs Affect Neuroinflammation, Neurogenesis, and Neural Repair
| MiRNA Species | Experimental Model | Observation | Reference |
|---|---|---|---|
| miR-17-5p | arterial endothelial cells | Promotes endothelialization and facilitates the vascular repair of aneurysms via PTEN-mediated PI3K/AKT/VEGFA pathway. | [44] |
| miR-19a | primary murine cortical neurons | Axonal outgrows via PTEN and mTOR axis. | [29] |
| miR-20a/b | spinal cord injury (SCI); astrocytes and microglia culture | Cell proliferation, apoptosis, and neuronal differentiation; miR-20a/b regulates the developmental stage of cortical neurons by targeting cyclin D1 and HspB1; enhances neurite outgrowth in cortical neurons and axonal growth and neuronal branching in hippocampal neurons. Provides neuroprotection and ameliorates IS. | [41] |
| miR-25 | human SH-SY5Y and IMR-32 cells; OGDR model | Downregulated in an OGDR model related to the released Fas/FasL; regulated cell death pathways. | [53] |
| miR-93 | OGD of BV2 microglial cells; miR-93 mimic | Promotes cell proliferation in OGD and induces G1 phase cell-cycle arrest. | [34] |
| miR-106b | neural stem/progenitor cells | Regulates proliferation and differentiation via the Tp53inp1-Tp53-Cdkn1a axis. | [49] |
6. miR-17-92 as a Therapeutic Strategy for Stroke
| Pathology | Cluster Member | In Vitro System | Observation | Reference |
|---|---|---|---|---|
| IS | ↑ miR17-92 cluster | Animal model | ↓ PTEN and mTOR regulate axonal growth. | [28] |
| IS | ↑ miR-17-92 cluster | Enriched exosomes; MACO models | Increase functional recovery after stroke via PTEN-mediated PI3K/Akt/mTOR; regulate axon remodeling. | [43] |
| IS | ↑ miR-17-92 | TBI model/miR-19-92 exosomal delivery | miR-17-92-enriched exosomes reduce hippocampal neuronal cell loss in rats after TBI and enhance sensorimotor function. | [56] |
| IS | miR-17-92 | MACAO | miR-17-92-enriched exosomes increase neural plasticity in the IBZ and promote neurite outgrowth and myelination vis the PTEN/PI3K/Akt/mTOR pathway. | [43] |
| TBI | ↑ miR17-92 cluster | Traumatic brain injury (TBI) model; engineered exosomes carrying the elevated miR-17-92 cluster | Improve sensorimotor functional recovery; improve spatial learning and memory; reduce hippocampal neuronal cell loss; reduce brain inflammation; ↓ CD68+ microglia/macrophages and GFAP+ astrocytes in the LBZ and DG (anti-neuroinflammation). | [56] |
| IS | ↑ miR-19a ↑ miR-18a | SVZ neural progenitor cells; MACAO model | Overexpression of miR-17-92 cluster in MACAO versus non-MACAO model; mediates neural progenitor cell function and Shh signaling. | [54] |
| IS | ↓ miR-19a-3p | I/R and OGD model; primary neurons and astrocytes | ↓ miR-19a-3p in rat neurons; ↑ miR-19a-3p in R/OGD models; MiR-19a inhibits glucose uptake and promotes neuronal apoptosis by targeting Adipor2. | [57] |
| IS | ↓ miR-20a | MCAO; TBI injury | Downregulated in the first 24 h of cerebral ischemia in the blood and brain; intravenous injection of miR-20a-3p after stroke within the first 4h improved patient outcomes after MCAO compared to the control group. | [41] |
| IS | ↓ miR-20a | OGD/R and MACAO | miR-20b regulates neuron apoptosis and ischemic brain injury by targeting TXNIP. | [58] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBI | traumatic brain injury |
| IS | ischemic stroke |
| HS | hemorrhagic stroke |
| I/R | in vivo ischemic/reperfusion neuronal injury |
| OGD | oxygen–glucose deprivation (OGD) |
| OGDR | oxygen–glucose deprivation/reperfusion |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 |
| PI3K | phosphoinositide 3-kinase |
| mTOR | mammalian target of rapamycin |
| miRNAs | microRNAs |
| MCAO | model of middle cerebral artery occlusion |
| SVZ | subventricular zone |
| ADIPOR2 | adiponectin receptor 2 |
| TXNIP | thioredoxin-interacting protein |
| GFAP | glial fibrillary acidic protein |
| LBZ | lesion boundary zone |
| IBZ | ischemic boundary zone |
| DG | dentate gyrus |
| MSCs | mesenchymal stem cells |
References
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Hu, D.; Wang, M.; Zheng, H.; Zhou, Y.; Zhang, L. Integrated Analysis of circRNA-Associated ceRNA Network in Ischemic Stroke. Front. Genet. 2023, 14, 1153518. [Google Scholar] [CrossRef] [PubMed]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Tamura, S.; Miyata, K.; Kobayashi, S.; Takeda, R.; Iwamoto, H. Grading of Balance Function in Subacute Stroke Patients by Using the Berg Balance Scale Together with Latent Rank Theory. Phys. Ther. Res. 2024, 27, 76–83. [Google Scholar] [CrossRef]
- Callegari, K.; Dash, S.; Uchida, H.; Shingai, Y.; Liu, C.; Khodarkovskaya, A.; Lee, Y.; Ito, A.; Lopez, A.; Zhang, T.; et al. Molecular Profiling of the Stroke-Induced Alterations in the Cerebral Microvasculature Reveals Promising Therapeutic Candidates. Proc. Natl. Acad. Sci. USA 2023, 120, e2205786120. [Google Scholar] [CrossRef]
- Huang, H.; Wei, G.; Wang, C.; Lu, Y.; Liu, C.; Wang, R.; Shi, X.; Yang, J.; Wei, Y. A Functional Polymorphism in the Promoter of miR-17-92 Cluster Is Associated with Decreased Risk of Ischemic Stroke. BMC Med. Genom. 2019, 12, 159. [Google Scholar] [CrossRef]
- Stevanato, L.; Sinden, J.D. The Effects of microRNAs on Human Neural Stem Cell Differentiation in Two- and Three-Dimensional Cultures. Stem Cell Res. Ther. 2014, 5, 49. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Sonea, L.; Zimta, A.A.; Cojocneanu-Petric, R.; Tonchev, K.; Mehterov, N.; Diudea, D.; Buduru, S.; Berindan-Neagoe, I. A Looking-Glass of Non-Coding RNAs in Oral Cancer. Int. J. Mol. Sci. 2017, 18, 2620. [Google Scholar] [CrossRef]
- Irimie, A.I.; Zimta, A.-A.; Ciocan, C.; Mehterov, N.; Dudea, D.; Braicu, C.; Berindan-Neagoe, I. The Unforeseen Non-Coding RNAs in Head and Neck Cancer. Genes 2018, 9, 134. [Google Scholar] [CrossRef]
- Boxhammer, E.; Dienhart, C.; Rezar, R.; Hoppe, U.C.; Lichtenauer, M. Deciphering the Role of microRNAs: Unveiling Clinical Biomarkers and Therapeutic Avenues in Atrial Fibrillation and Associated Stroke—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 5568. [Google Scholar] [CrossRef] [PubMed]
- Mens, M.M.J.; Heshmatollah, A.; Fani, L.; Ikram, M.A.; Ikram, M.K.; Ghanbari, M. Circulatory MicroRNAs as Potential Biomarkers for Stroke Risk: The Rotterdam Study. Stroke 2021, 52, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zheng, M.; Sewani, M.A.; Wang, J. The miR-17-92 Cluster in Cardiac Health and Disease. Birth Defects Res. 2024, 116, e2273. [Google Scholar] [CrossRef]
- Khuu, C.; Utheim, T.P.; Sehic, A. The Three Paralogous MicroRNA Clusters in Development and Disease, miR-17-92, miR-106a-363, and miR-106b-25. Scientifica 2016, 2016, 1379643. [Google Scholar] [CrossRef] [PubMed]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 Cluster: A Comprehensive Update on Its Genomics, Genetics, Functions and Increasingly Important and Numerous Roles in Health and Disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef]
- Gruszka, R.; Zakrzewska, M. The Oncogenic Relevance of miR-17-92 Cluster and Its Paralogous miR-106b-25 and miR-106a-363 Clusters in Brain Tumors. Int. J. Mol. Sci. 2018, 19, 879. [Google Scholar] [CrossRef]
- Dong, L.; Ye, Y.; Huang, G.; Tao, H. The Diagnostic Value of Serum miR-17-92 Cluster in Ischemic Stroke. Folia Neuropathol. 2024, 62, 206–214. [Google Scholar] [CrossRef]
- Zhao, W.; Gupta, A.; Krawczyk, J.; Gupta, S. The miR-17-92 Cluster: Yin and Yang in Human Cancers. Cancer Treat. Res. Commun. 2022, 33, 100647. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.-Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA Cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Vasunilashorn, S.M.; Ngo, L.H.; Dillon, S.T.; Fong, T.G.; Carlyle, B.C.; Kivisäkk, P.; Trombetta, B.A.; Vlassakov, K.V.; Kunze, L.J.; Arnold, S.E.; et al. Plasma and Cerebrospinal Fluid Inflammation and the Blood-Brain Barrier in Older Surgical Patients: The Role of Inflammation after Surgery for Elders (RISE) Study. J. Neuroinflammation 2021, 18, 103. [Google Scholar] [CrossRef]
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. MicroRNA: An Emerging Predictive, Diagnostic, Prognostic and Therapeutic Strategy in Ischaemic Stroke. Cell Mol. Neurobiol. 2022, 42, 1301–1319. [Google Scholar] [CrossRef] [PubMed]
- Bejleri, J.; Jirström, E.; Donovan, P.; Williams, D.J.; Pfeiffer, S. Diagnostic and Prognostic Circulating MicroRNA in Acute Stroke: A Systematic and Bioinformatic Analysis of Current Evidence. J. Stroke 2021, 23, 162–182. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.L.; Chopp, M.; Fan, B.; Zhang, R.; Wang, X.; Hu, J.; Zhang, X.M.; Zhang, Z.G.; Liu, X.S. Ablation of the microRNA-17-92 Cluster in Neural Stem Cells Diminishes Adult Hippocampal Neurogenesis and Cognitive Function. FASEB J. 2019, 33, 5257–5267. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shao, Y.; Ruan, X.; Zhu, L.; Zang, Z.; Wei, T.; Nakyeyune, R.; Wei, W.; Liu, F. Genetic Variant in miR-17-92 Cluster Binding Sites Is Associated with Esophageal Squamous Cell Carcinoma Risk in Chinese Population. BMC Cancer 2022, 22, 1253. [Google Scholar] [CrossRef]
- Faluyi, O.O.; Eng, L.; Qiu, X.; Che, J.; Zhang, Q.; Cheng, D.; Ying, N.; Tse, A.; Kuang, Q.; Dodbiba, L.; et al. Validation of microRNA Pathway Polymorphisms in Esophageal Adenocarcinoma Survival. Cancer Med. 2017, 6, 361–373. [Google Scholar] [CrossRef]
- Li, C.-X.; Weng, H.; Zheng, J.; Feng, Z.-H.; Ou, J.-L.; Liao, W.-J. Association Between MicroRNAs Polymorphisms and Risk of Ischemic Stroke: A Meta-Analysis in Chinese Individuals. Front. Aging Neurosci. 2018, 10, 82. [Google Scholar] [CrossRef]
- Luo, H.-C.; Luo, Q.-S.; Wang, C.-F.; Lei, M.; Li, B.-L.; Wei, Y.-S. Association of miR-146a, miR-149, miR-196a2, miR-499 Gene Polymorphisms with Ischemic Stroke in a Chinese People. Oncotarget 2017, 8, 81295–81304. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Chopp, M. Promoting Brain Remodeling to Aid in Stroke Recovery. Trends Mol. Med. 2015, 21, 543–548. [Google Scholar] [CrossRef]
- Zhang, Y.; Ueno, Y.; Liu, X.S.; Buller, B.; Wang, X.; Chopp, M.; Zhang, Z.G. The MicroRNA-17–92 Cluster Enhances Axonal Outgrowth in Embryonic Cortical Neurons. J. Neurosci. 2013, 33, 6885–6894. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Czlonkowska, A.; Postula, M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke-A Comprehensive Review and Bioinformatic Analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef]
- Wu, J.; Du, K.; Lu, X. Elevated Expressions of Serum miR-15a, miR-16, and miR-17-5p Are Associated with Acute Ischemic Stroke. Int. J. Clin. Exp. Med. 2015, 8, 21071–21079. [Google Scholar] [PubMed]
- Kim, J.-M.; Jung, K.-H.; Chu, K.; Lee, S.-T.; Ban, J.; Moon, J.; Kim, M.; Lee, S.K.; Roh, J.-K. Atherosclerosis-Related Circulating MicroRNAs as a Predictor of Stroke Recurrence. Transl. Stroke Res. 2015, 6, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Ander, B.P.; Zhan, X.; Noblett, D.; Stamova, B.; Liu, D. microRNA Expression in Peripheral Blood Cells Following Acute Ischemic Stroke and Their Predicted Gene Targets. PLoS ONE 2014, 9, e99283. [Google Scholar] [CrossRef]
- Ma, Q.; Li, G.; Tao, Z.; Wang, J.; Wang, R.; Liu, P.; Luo, Y.; Zhao, H. Blood microRNA-93 as an Indicator for Diagnosis and Prediction of Functional Recovery of Acute Stroke Patients. J. Clin. Neurosci. 2019, 62, 121–127. [Google Scholar] [CrossRef]
- Toor, S.M.; Aldous, E.K.; Parray, A.; Akhtar, N.; Al-Sarraj, Y.; Abdelalim, E.M.; Arredouani, A.; El-Agnaf, O.; Thornalley, P.J.; Pananchikkal, S.V.; et al. Circulating MicroRNA Profiling Identifies Distinct MicroRNA Signatures in Acute Ischemic Stroke and Transient Ischemic Attack Patients. Int. J. Mol. Sci. 2023, 24, 108. [Google Scholar] [CrossRef]
- He, J.-R.; Zhang, Y.; Lu, W.-J.; Liang, H.-B.; Tu, X.-Q.; Ma, F.-Y.; Yang, G.-Y.; Zeng, L.-L. Age-Related Frontal Periventricular White Matter Hyperintensities and miR-92a-3p Are Associated with Early-Onset Post-Stroke Depression. Front. Aging Neurosci. 2017, 9, 328. [Google Scholar] [CrossRef]
- Salman, A.T.; Shaker, O.; Elshaer, S.S.; Elshafei, A. The Expression Profiling of Serum miR-92a, miR-134 and miR-375 in Acute Ischemic Stroke. Future Sci. OA 2022, 8, FSO829. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.-H.; Zhang, H.; Pang, X.-W.; Chen, L.; Zhou, L.-Q.; Chen, M.; Tian, D.-S.; Wang, W. Signaling Pathways Involved in Ischemic Stroke: Molecular Mechanisms and Therapeutic Interventions. Sig. Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.-C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, Treatment, and Animal and Cellular Models of Human Ischemic Stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Yang, P.; Cai, L.; Zhang, G.; Bian, Z.; Han, G. The Role of the miR-17-92 Cluster in Neurogenesis and Angiogenesis in the Central Nervous System of Adults. J. Neurosci. Res. 2017, 95, 1574–1581. [Google Scholar] [CrossRef]
- Arzhanov, I.; Sintakova, K.; Romanyuk, N. The Role of miR-20 in Health and Disease of the Central Nervous System. Cells 2022, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Olive, V.; Bennett, M.J.; Walker, J.C.; Ma, C.; Jiang, I.; Cordon-Cardo, C.; Li, Q.-J.; Lowe, S.W.; Hannon, G.J.; He, L. miR-19 Is a Key Oncogenic Component of Mir-17-92. Genes. Dev. 2009, 23, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, Z.; Buller, B.; Li, Y.; Golembieski, W.; Gan, X.; Wang, F.; Lu, M.; Ali, M.M.; Zhang, Z.G.; et al. MiR-17-92 Enriched Exosomes Derived from Multipotent Mesenchymal Stromal Cells Enhance Axon-Myelin Remodeling and Motor Electrophysiological Recovery after Stroke. J. Cereb. Blood Flow. Metab. 2021, 41, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Emami Nejad, A.; Mostafavi Zadeh, S.M.; Nickho, H.; Sadoogh Abbasian, A.; Forouzan, A.; Ahmadlou, M.; Nedaeinia, R.; Shaverdi, S.; Manian, M. The Role of microRNAs Involved in the Disorder of Blood–Brain Barrier in the Pathogenesis of Multiple Sclerosis. Front. Immunol. 2023, 14, 1281567. [Google Scholar] [CrossRef]
- Tian, Y.; Li, X.; Bai, C.; Yang, Z.; Zhang, L.; Luo, J. MiR-17-5p Promotes the Endothelialization of Endothelial Progenitor Cells to Facilitate the Vascular Repair of Aneurysm by Regulating PTEN-Mediated PI3K/AKT/VEGFA Pathway. Cell Cycle 2020, 19, 3608–3621. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Liu, G.; Zhang, B.; Jin, X.; Wang, Y. MicroRNA-17-5p, a Novel Endothelial Cell Modulator, Controls Vascular Re-Endothelialization and Neointimal Lesion Formation. Vascular 2023, 31, 392–401. [Google Scholar] [CrossRef]
- Yang, S.; Fan, T.; Hu, Q.; Xu, W.; Yang, J.; Xu, C.; Zhang, B.; Chen, J.; Jiang, H. Downregulation of microRNA-17-5p Improves Cardiac Function after Myocardial Infarction via Attenuation of Apoptosis in Endothelial Cells. Mol. Genet. Genom. 2018, 293, 883–894. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, L.; Zhu, B.; Zhou, X.; Zhang, T.; Ma, T. TARBP2-Stablized SNHG7 Regulates Blood-Brain Barrier Permeability by Acting as a Competing Endogenous RNA to miR-17-5p/NFATC3 in Aβ-Microenvironment. Cell Death Dis. 2022, 13, 457. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zheng, J.C. The microRNA-17 ~ 92 Family as a Key Regulator of Neurogenesis and Potential Regenerative Therapeutics of Neurological Disorders. Stem Cell Rev. Rep. 2022, 18, 401–411. [Google Scholar] [CrossRef]
- Xia, X.; Lu, H.; Li, C.; Huang, Y.; Wang, Y.; Yang, X.; Zheng, J.C. miR-106b Regulates the Proliferation and Differentiation of Neural Stem/Progenitor Cells through Tp53inp1-Tp53-Cdkn1a Axis. Stem Cell Res. Ther. 2019, 10, 282. [Google Scholar] [CrossRef]
- Kee, H.J.; Kim, G.R.; Cho, S.-N.; Kwon, J.-S.; Ahn, Y.; Kook, H.; Jeong, M.H. miR-18a-5p MicroRNA Increases Vascular Smooth Muscle Cell Differentiation by Downregulating Syndecan4. Korean Circ. J. 2014, 44, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Zhao, L.; Song, W.; Wang, L.; Liu, J.; Zhang, H.; Huang, Y.; Lau, C.W.; Yao, X.; Tian, X.Y.; et al. Inhibition of miR-92a Suppresses Oxidative Stress and Improves Endothelial Function by Upregulating Heme Oxygenase-1 in Db/Db Mice. Antioxid. Redox Signal 2018, 28, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Shi, L.-L.; Zhang, L.; Zhao, Z.-H.; Liang, F.; Xu, X.; Zhao, L.-Y.; Yang, P.-B.; Zhang, J.-S.; Tian, Y.-F. MicroRNA-25 Negatively Regulates Cerebral Ischemia/Reperfusion Injury-Induced Cell Apoptosis Through Fas/FasL Pathway. J. Mol. Neurosci. 2016, 58, 507–516. [Google Scholar] [CrossRef]
- Liu, X.S.; Chopp, M.; Wang, X.L.; Zhang, L.; Hozeska-Solgot, A.; Tang, T.; Kassis, H.; Zhang, R.L.; Chen, C.; Xu, J.; et al. MicroRNA-17-92 Cluster Mediates the Proliferation and Survival of Neural Progenitor Cells after Stroke. J. Biol. Chem. 2013, 288, 12478–12488. [Google Scholar] [CrossRef]
- Taylor, S.M.; Giuffre, E.; Moseley, P.; Hitchcock, P.F. The MicroRNA, miR-18a, Regulates NeuroD and Photoreceptor Differentiation in the Retina of Zebrafish. Dev. Neurobiol. 2019, 79, 202–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chopp, M.; Pang, H.; Zhang, Z.G.; Mahmood, A.; Xiong, Y. MiR-17-92 Cluster-Enriched Exosomes Derived from Human Bone Marrow Mesenchymal Stromal Cells Improve Tissue and Functional Recovery in Rats after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1535–1550. [Google Scholar] [CrossRef]
- Ge, X.-L.; Wang, J.-L.; Liu, X.; Zhang, J.; Liu, C.; Guo, L. Inhibition of miR-19a Protects Neurons against Ischemic Stroke through Modulating Glucose Metabolism and Neuronal Apoptosis. Cell. Mol. Biol. Lett. 2019, 24, 37. [Google Scholar] [CrossRef]
- Yang, D.; Tan, Y.; Li, H.; Zhang, X.; Li, X.; Zhou, F. Upregulation of miR-20b Protects Against Cerebral Ischemic Stroke by Targeting Thioredoxin Interacting Protein (TXNIP). Exp. Neurobiol. 2021, 30, 170–182. [Google Scholar] [CrossRef]
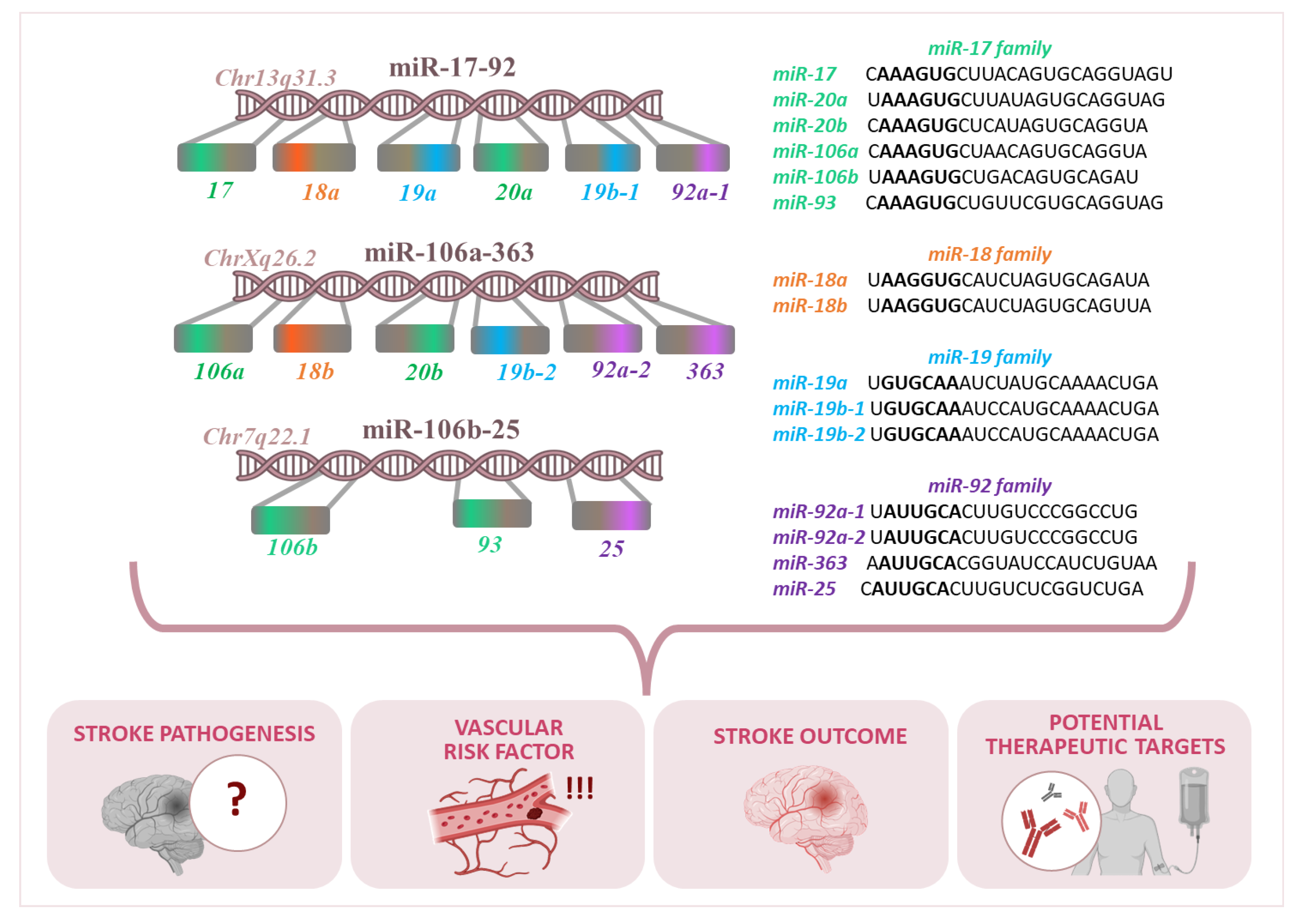
| Genome Context | Name | Accession | Chromosome | Start | End | Strand | Confidence |
|---|---|---|---|---|---|---|---|
| chr13: 91350605–91350688 | hsa-mir-17 | MI0000071 | chr13 | 91350605 | 91350688 | + | High |
| hsa-mir-18a | MI0000072 | chr13 | 91350751 | 91350821 | + | High | |
| hsa-mir-19a | MI0000073 | chr13 | 91350891 | 91350972 | + | High | |
| hsa-mir-19b-1 | MI0000074 | chr13 | 91351192 | 91351278 | + | High | |
| hsa-mir-20a | MI0000076 | chr13 | 91351065 | 91351135 | + | High | |
| hsa-mir-92a-1 | MI0000093 | chr13 | 91351314 | 91351391 | + | High | |
| chrX: 134170198–134170278 | hsa-mir-18b | MI0001518 | chrX | 134170041 | 134170111 | − | High |
| hsa-mir-19b-2 | MI0000075 | chrX | 134169671 | 134169766 | − | High | |
| hsa-mir-20b | MI0001519 | chrX | 134169809 | 134169877 | − | High | |
| hsa-mir-92a-2 | MI0000094 | chrX | 134169538 | 134169612 | − | High | |
| hsa-mir-106a | MI0000113 | chrX | 134170198 | 134170278 | − | High | |
| hsa-mir-363 | MI0000764 | chrX | 134169378 | 134169452 | − | High | |
| chr7: 100093560–100093643 | hsa-mir-25 | MI0000082 | chr7 | 100093560 | 100093643 | − | High |
| hsa-mir-93 | MI0000095 | chr7 | 100093768 | 100093847 | − | High | |
| hsa-mir-106b | MI0000734 | chr7 | 100093993 | 100094074 | − | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braicu, C.; Molnar, M.; Isachesku, E.; Pană, A.; Mureșanu, D.; Strilciuc, S. The Complex Role of the miR-17-92 Cluster in Stroke: Mechanistic Insights and Biomarker Potential. Genes 2025, 16, 665. https://doi.org/10.3390/genes16060665
Braicu C, Molnar M, Isachesku E, Pană A, Mureșanu D, Strilciuc S. The Complex Role of the miR-17-92 Cluster in Stroke: Mechanistic Insights and Biomarker Potential. Genes. 2025; 16(6):665. https://doi.org/10.3390/genes16060665
Chicago/Turabian StyleBraicu, Cornelia, Mihaela Molnar, Ekaterina Isachesku, Adrian Pană, Dafin Mureșanu, and Stefan Strilciuc. 2025. "The Complex Role of the miR-17-92 Cluster in Stroke: Mechanistic Insights and Biomarker Potential" Genes 16, no. 6: 665. https://doi.org/10.3390/genes16060665
APA StyleBraicu, C., Molnar, M., Isachesku, E., Pană, A., Mureșanu, D., & Strilciuc, S. (2025). The Complex Role of the miR-17-92 Cluster in Stroke: Mechanistic Insights and Biomarker Potential. Genes, 16(6), 665. https://doi.org/10.3390/genes16060665







