Genome-Wide Identification of the Potato GGPS Gene Family and Analysis of Its Response to Abiotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA Extraction and qRT-PCR Analysis
2.2. Identification and Sequence Analysis of Potato GGPS Genes
2.3. Phylogenetic Analysis
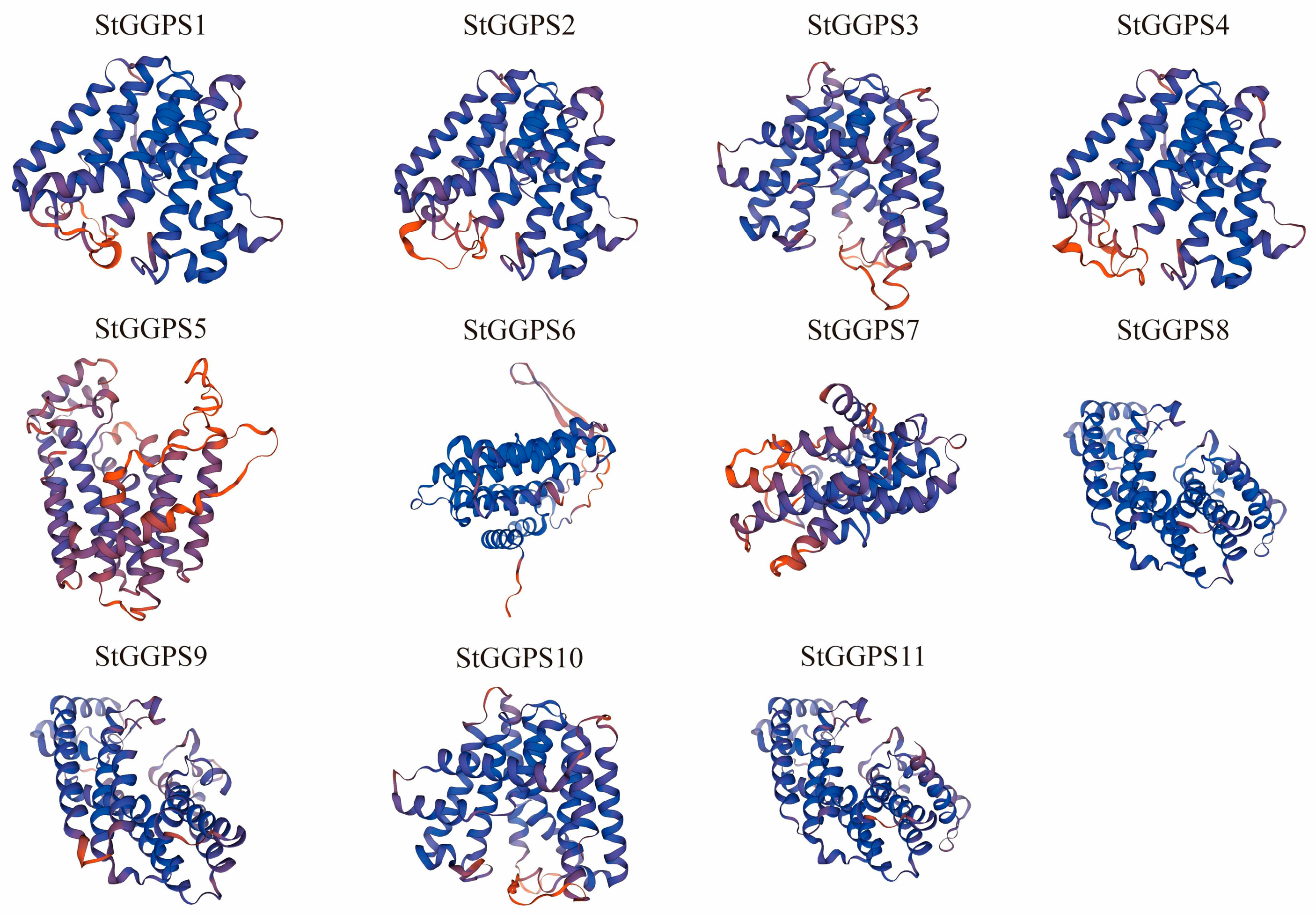
2.4. Gene Structure, Conserved Motifs, and 3D Structural Analysis
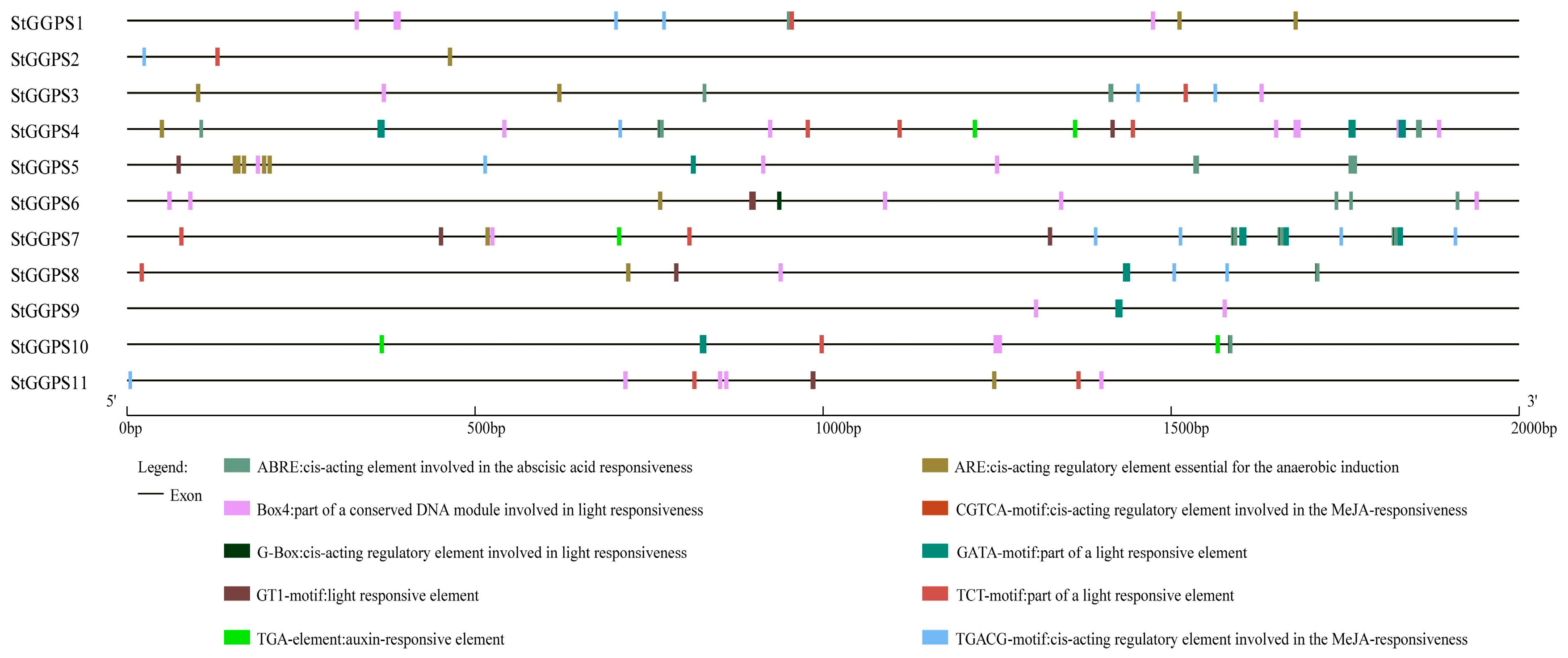
2.5. Analysis of Cis-Acting Elements in Promoter Regions
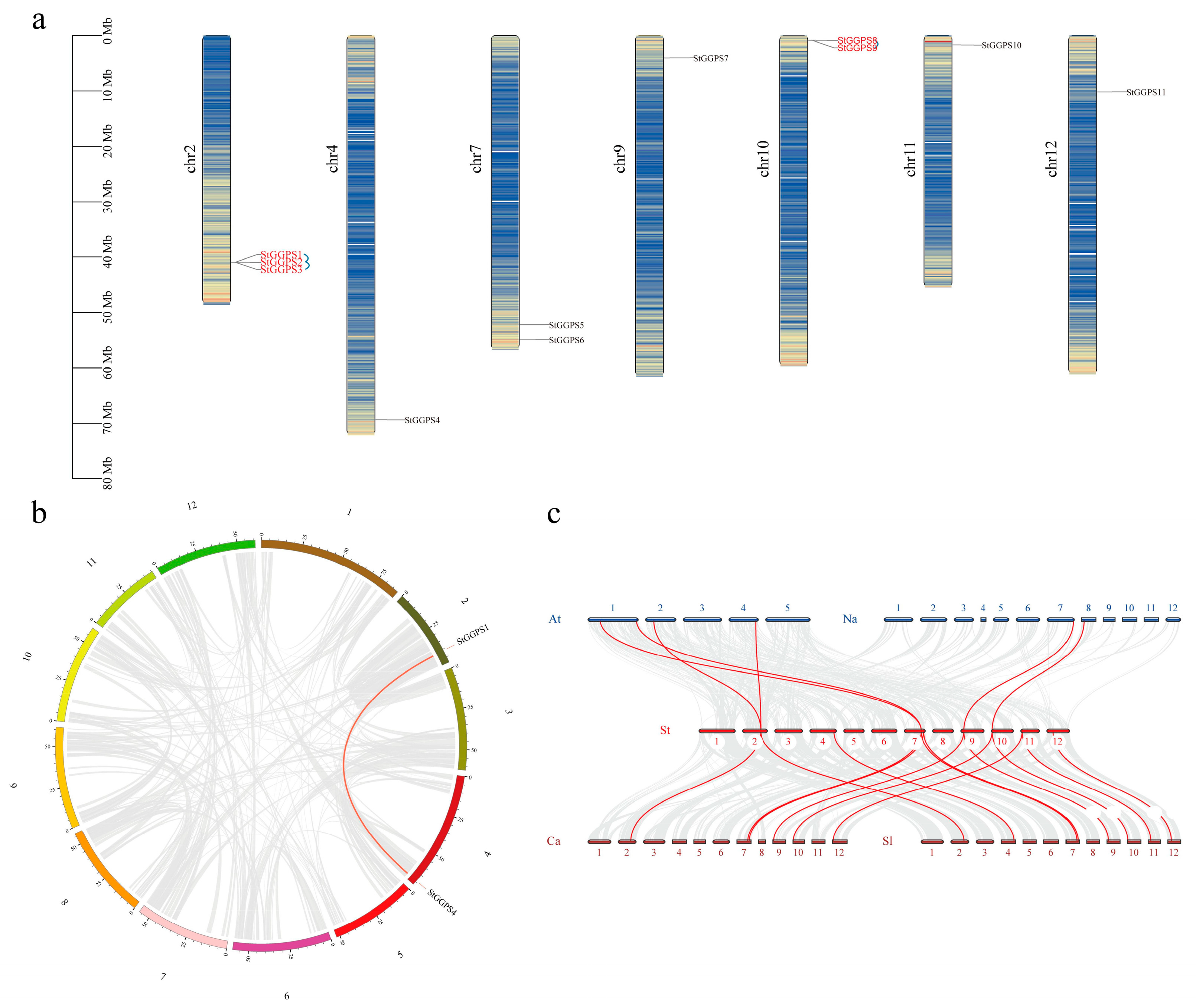
2.6. Chromosomal Location, Gene Distribution, and Synteny Analysis
2.7. Expression Analysis of RNA Data
3. Results
3.1. Identification of GGPS Gene Family Members and Analysis of Protein Physicochemical Properties in Potatoes
3.2. Potato GGPS Family Evolution Analysis, Gene Structure, and Conserved Motifs
3.3. Chromosome Localization of Genes and Analysis of Gene Duplication Events
3.4. Analysis of Cis-Acting Elements in StGGPS Gene Promoters
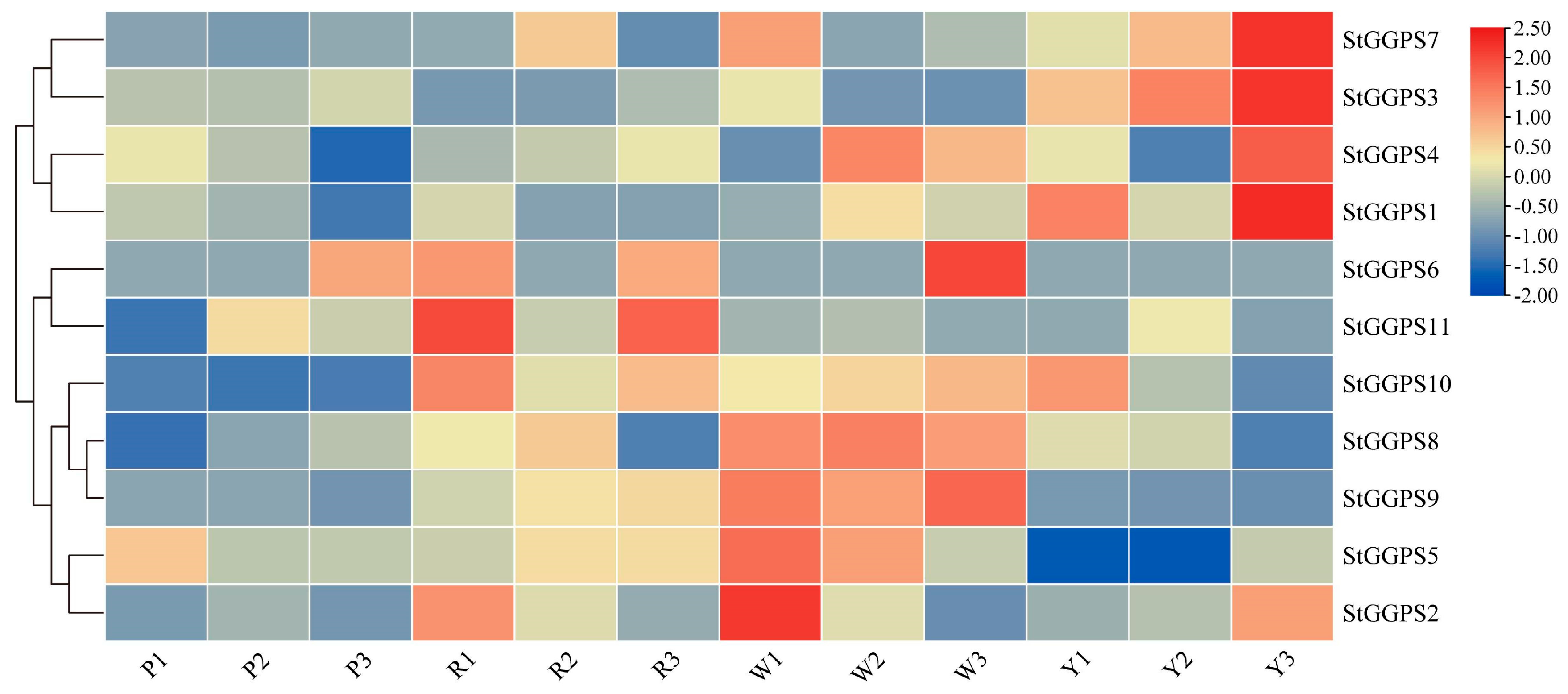
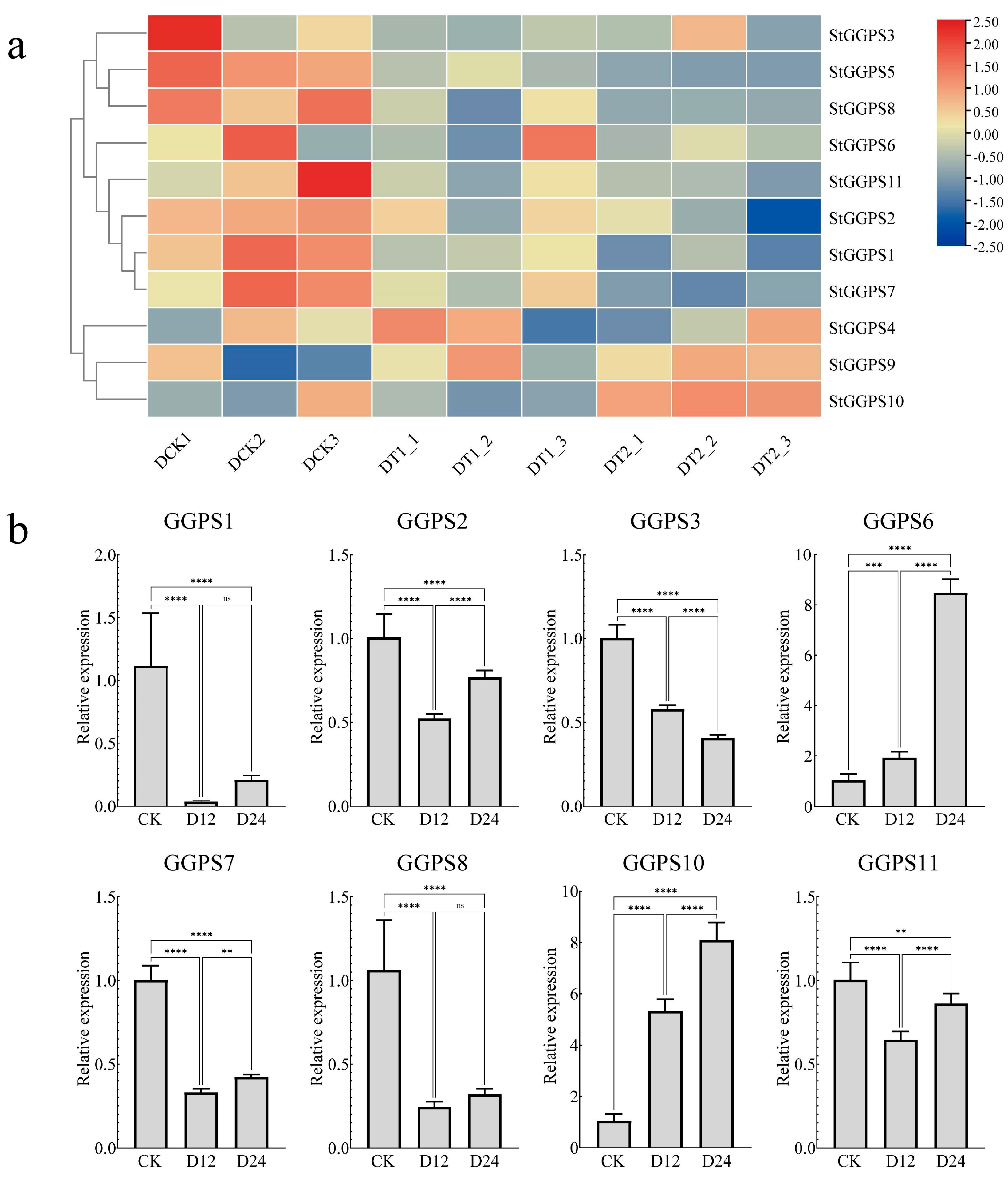
3.5. Expression Patterns of Genes in Tubers of Different Colors
3.6. Gene Expression Patterns in Response to Abiotic Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avalos, J.; Carmen Limón, M. Biological Roles of Fungal Carotenoids. Curr. Genet. 2015, 61, 309–324. [Google Scholar] [CrossRef] [PubMed]
- López, G.-D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoids and Their Biosynthesis in Fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and Function of Apocarotenoid Signals in Plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef]
- Xu, P.; Chukhutsina, V.U.; Nawrocki, W.J.; Schansker, G.; Bielczynski, L.W.; Lu, Y.; Karcher, D.; Bock, R.; Croce, R. Photosynthesis without β-Carotene. eLife 2020, 9, e58984. [Google Scholar] [CrossRef]
- Sierra, J.; McQuinn, R.P.; Leon, P. The Role of Carotenoids as a Source of Retrograde Signals: Impact on Plant Development and Stress Responses. J. Exp. Bot. 2022, 73, 7139–7154. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Jia, K.-P.; Baz, L.; Al-Babili, S. From Carotenoids to Strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef]
- Tata, S.K.; Jung, J.; Kim, Y.; Choi, J.Y.; Jung, J.; Lee, I.; Shin, J.S.; Ryu, S.B. Heterologous Expression of Chloroplast-localized Geranylgeranyl Pyrophosphate Synthase Confers Fast Plant Growth, Early Flowering and Increased Seed Yield. Plant Biotechnol. J. 2016, 14, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hivert, G.; Davidovich-Rikanati, R.; Bar, E.; Sitrit, Y.; Schaffer, A.; Dudareva, N.; Lewinsohn, E. Prenyltransferases Catalyzing Geranyldiphosphate Formation in Tomato Fruit. Plant Sci. 2020, 296, 110504. [Google Scholar] [CrossRef]
- Chen, W.; He, S.; Liu, D.; Patil, G.B.; Zhai, H.; Wang, F.; Stephenson, T.J.; Wang, Y.; Wang, B.; Valliyodan, B.; et al. A Sweetpotato Geranylgeranyl Pyrophosphate Synthase Gene, IbGGPS, Increases Carotenoid Content and Enhances Osmotic Stress Tolerance in Arabidopsis Thaliana. PLoS ONE 2015, 10, e0137623. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Mehari, T.G.; Fang, H.; Ji, M.; Qu, Z.; Jia, M.; Wang, D.; Ditta, A.; Khan, M.K.R.; Cao, Y.; et al. Genome-Wide Identification of the Geranylgeranyl Pyrophosphate Synthase (GGPS) Gene Family Involved in Chlorophyll Synthesis in Cotton. BMC Genom. 2023, 24, 176. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Zong, Y.; Tu, Z.; Li, H. Isolation, Expression, and Functional Analysis of the Geranylgeranyl Pyrophosphate Synthase (GGPPS) Gene from Liriodendron tulipifera. Plant Physiol. Biochem. PPB 2021, 166, 700–711. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Coman, D.; Beck, G.; Barja, M.V.; Colinas, M.; Graf, A.; Welsch, R.; Rütimann, P.; Bühlmann, P.; Bigler, L.; et al. Arabidopsis GERANYLGERANYL DIPHOSPHATE SYNTHASE 11 Is a Hub Isozyme Required for the Production of Most Photosynthesis-Related Isoprenoids. New Phytol. 2016, 209, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Gervais, T.; Creelman, A.; Li, X.-Q.; Bizimungu, B.; Koeyer, D.D.; Dahal, K. Potato Response to Drought Stress: Physiological and Growth Basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S.; et al. Mechanistic Concept of Physiological, Biochemical, and Molecular Responses of the Potato Crop to Heat and Drought Stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efimova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. 24-Epibrassinolide Alleviates the Toxic Effects of NaCl on Photosynthetic Processes in Potato Plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, N.; Si, H.; Calderón-Urrea, A. Selection and Validation of Reference Genes for RT-qPCR Analysis in Potato under Abiotic Stress. Plant Methods 2017, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Maren, N.A.; Duduit, J.R.; Huang, D.; Zhao, F.; Ranney, T.G.; Liu, W. Stepwise Optimization of Real-Time RT-PCR Analysis. Methods Mol. Biol. 2023, 2653, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Stajich, J.E. An Introduction to BioPerl. Methods Mol. Biol. 2007, 406, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Duarte, J.M.; Dutta, S.; Fayazi, M.; Feng, Z.; et al. RCSB Protein Data Bank: Celebrating 50 Years of the PDB with New Tools for Understanding and Visualizing Biological Macromolecules in 3D. Protein Sci. A Publ. Protein Soc. 2022, 31, 187–208. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating γ-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants1 [OPEN]. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, M.; Song, S.; Wei, F.; Qin, L.; Fan, P.; Shi, Y.; Wang, X.; Wang, R. A Small Subunit of Geranylgeranyl Diphosphate Synthase Functions as an Active Regulator of Carotenoid Synthesis in Nicotiana Tabacum. Int. J. Mol. Sci. 2023, 24, 992. [Google Scholar] [CrossRef]
- Wang, J.; Lin, H.-X.; Su, P.; Chen, T.; Guo, J.; Gao, W.; Huang, L.-Q. Molecular Cloning and Functional Characterization of Multiple Geranylgeranyl Pyrophosphate Synthases (ApGGPPS) from Andrographis paniculata. Plant Cell Rep. 2019, 38, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Block, A.; Fristedt, R.; Rogers, S.; Kumar, J.; Barnes, B.; Barnes, J.; Elowsky, C.G.; Wamboldt, Y.; Mackenzie, S.A.; Redding, K.; et al. Functional Modeling Identifies Paralogous Solanesyl-Diphosphate Synthases That Assemble the Side Chain of Plastoquinone-9 in Plastids. J. Biol. Chem. 2013, 288, 27594–27606. [Google Scholar] [CrossRef]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant Tolerance to Excess Light Energy and Photooxidative Damage Relies on Plastoquinone Biosynthesis. Sci. Rep. 2015, 5, 10919. [Google Scholar] [CrossRef]


| Gene ID | Gene Name | Chromosome Localization | Amino Acid Length (aa) | Molecular Weight (Da) | Theoretical Isoelectric Point | Subcellular Localization | |||
|---|---|---|---|---|---|---|---|---|---|
| PGSC0003DMG400047044 | StGGPS1 | chr02 | 40939136 | 40940224 | + | 362 | 39,321 | 6.35 | Chloroplast |
| PGSC0003DMG400041508 | StGGPS2 | chr02 | 40941168 | 40942286 | + | 372 | 41,120.1 | 8.38 | Mitochondrion |
| PGSC0003DMG400043267 | StGGPS3 | chr02 | 40945073 | 40946191 | + | 372 | 40,731.9 | 7.92 | Chloroplast |
| PGSC0003DMG400027856 | StGGPS4 | chr04 | 69344146 | 69345467 | − | 375 | 40,657.5 | 4.99 | Chloroplast |
| PGSC0003DMG400007081 | StGGPS5 | chr07 | 52190753 | 52195395 | − | 398 | 43,490.4 | 6.01 | Chloroplast |
| PGSC0003DMG400022214 | StGGPS6 | chr07 | 54896132 | 54897257 | − | 294 | 32,784.6 | 7.01 | Cytoskeleton |
| PGSC0003DMG400002687 | StGGPS7 | chr09 | 4028005 | 4032971 | + | 334 | 36,484.6 | 5.26 | Chloroplast |
| PGSC0003DMG400008690 | StGGPS8 | chr10 | 854194 | 861002 | + | 342 | 39,650.3 | 6.12 | Cytoplasm |
| PGSC0003DMG400014369 | StGGPS9 | chr10 | 871271 | 879652 | + | 306 | 35,198.2 | 6.77 | Nucleus |
| PGSC0003DMG400015673 | StGGPS10 | chr11 | 1682159 | 1683721 | − | 365 | 39,976.7 | 6.85 | Chloroplast |
| PGSC0003DMG400029788 | StGGPS11 | chr12 | 10169940 | 10174778 | − | 342 | 39,296.9 | 4.87 | Cytoplasm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Li, W.; Chen, X.; Gao, S.; Jia, M.; Zhang, S.; Cui, J. Genome-Wide Identification of the Potato GGPS Gene Family and Analysis of Its Response to Abiotic Stress. Genes 2025, 16, 646. https://doi.org/10.3390/genes16060646
Fu C, Li W, Chen X, Gao S, Jia M, Zhang S, Cui J. Genome-Wide Identification of the Potato GGPS Gene Family and Analysis of Its Response to Abiotic Stress. Genes. 2025; 16(6):646. https://doi.org/10.3390/genes16060646
Chicago/Turabian StyleFu, Changqing, Wei Li, Xiaotian Chen, Shunjuan Gao, Mingfei Jia, Shuqing Zhang, and Jianghui Cui. 2025. "Genome-Wide Identification of the Potato GGPS Gene Family and Analysis of Its Response to Abiotic Stress" Genes 16, no. 6: 646. https://doi.org/10.3390/genes16060646
APA StyleFu, C., Li, W., Chen, X., Gao, S., Jia, M., Zhang, S., & Cui, J. (2025). Genome-Wide Identification of the Potato GGPS Gene Family and Analysis of Its Response to Abiotic Stress. Genes, 16(6), 646. https://doi.org/10.3390/genes16060646






