Abstract
Background/Objectives: Bradycardia, an uncharacteristically low heart rate below 60 bpm, is a commonly reported adverse drug event (ADE) in individuals administered dexmedetomidine for sedation. Dexmedetomidine is frequently used as a sedative and analgesic for both intubated and non-intubated patients due to its low risk of respiratory depression. The purpose of this study was to further characterize the safety profile of dexmedetomidine using safety reports collected from the FDA Adverse Event Reporting System (FAERS) and transcriptomic data. Methods: Association rule mining was used to both identify additional ADEs that presented concurrently with bradycardia in patients sedated with dexmedetomidine, as well as to characterize potential drug–drug interactions (DDIs). Furthermore, public transcriptomic data were analyzed to identify differentially expressed genes that may elucidate the genetic drivers of elevated bradycardia risk in those administered dexmedetomidine. Results: Bradycardia was the most frequently reported ADE for individuals administered dexmedetomidine. Other cardiovascular-related ADEs commonly associated with bradycardia included syncope (lift = 4.711), loss of consciousness (lift = 3.997), cardiac arrest (lift = 2.850), and hypotension (lift = 2.770). Several possible DDIs were identified, including Lactated Ringer’s solution (lift = 5.441), bupivacaine (lift = 2.984), and risperidone (lift = 2.434), which may elevate bradycardia risk. Finally, eight genes related to cardiac muscle contraction were identified as possible regulators of dexmedetomidine-induced bradycardia, including COX5B, COX6A2, COX8B, MYH7, MYH6, MYL2, UQCRQ, and UQCR11 in mouse cardiac cells. Conclusions: Key clinical takeaways include the co-presentation of multiple cardiovascular ADEs, including cardiac arrest, hypotension, and syncope, alongside dexmedetomidine-associated bradycardia. Furthermore, several possible DDIs with dexmedetomidine were also identified.
Keywords:
FAERS; pharmacovigilance; drug interactions; bradycardia; RNA-seq; dexmedetomidine; association rules; DDI 1. Introduction
Bradycardia is a commonly occurring cardiac arrhythmia characterized by a prolonged and uncharacteristically low heart rate below 60 beats per minute (bpm) that is typically diagnosed through visualization of an abnormal electrocardiogram [1,2,3]. Bradycardia frequently arises due to complications in pulse conduction by the sinoatrial node, the primary pacemaker and regulator of heart rate, although other factors including myocardial infarction, congenital heart defects, certain medications, myocarditis, and blockages of atrioventricular conduction may also contribute [1,2,4]. Although many cases of bradycardia may present asymptomatically, particularly in those with high fitness levels or those during periods of rest, unstable bradycardia may result in significant fatigue, syncope, dizziness, confusion, chest pain, and cardiac arrest [1,2,5]. For patients presenting with sinus bradycardia and hemodynamic instability, intravenous treatment of 0.5 mg atropine for up to 30 min is the standard treatment modality; however, for patients that do not respond to atropine therapy and continue to present with heart rate abnormalities, a temporary pacemaker may be considered [1,2,6].
Dexmedetomidine, first approved for clinical use by the Food and Drug Administration (FDA) in 1999, is a highly selective α2-adrenergic receptor agonist that is commonly used as a sedative in clinical settings [7,8]. It suppresses sympathetic nervous system activity and reduces the release of norepinephrine, resulting in decreased blood pressure and the onset of sleep [8,9]. Dexmedetomidine is indicated for use in the sedation of intubated and mechanically ventilated patients, as well as for peri-operative use in non-intubated patients [8,10,11]. For intubated patients, the typical dosage ranges from 0.2 to 0.7 mcg/kg per hour; however, dosages upwards of 1.5 mcg/kg per hour are considered safe and are associated with minimal increases in side effects [8]. For anesthetic purposes in non-intubated patients, a loading dose of 0.5–1.0 mcg/kg is administered first followed by an hourly administration of 0.2–0.7 mcg/kg [8,12,13]. Regardless of patient status, manufacturers advise that dexmedetomidine administration does not exceed a 24 h period [8].
Because of its 1600:1 selectivity for α2-adrenergic receptors compared to the α1 isoform, dexmedetomidine is associated with reduced deleterious effects on respiratory function [8,13]. In addition, pretreatment with dexmedetomidine has demonstrated cardioprotective effects against ischemia–reperfusion injury (IRI) by stabilizing cardiac electrophysiology and modulating inflammatory signaling pathways [8,14,15]. Despite this, multiple studies have demonstrated a significant correlation between dexmedetomidine treatment and the risk of bradycardia [16,17,18,19,20]. A meta-analysis and systematic review of 15 studies indicating performance of laparoscopic surgical procedures found that individuals treated with dexmedetomidine during surgery had an approximately three-fold increase in relative risk (RR = 2.81, 95% CI 1.34–5.91) of developing intraoperative bradycardia compared to those not sedated with dexmedetomidine [20]. Another meta-analysis of 18 studies collected between 2003 and 2016 found a nearly 26-fold increase in relative risk (RR = 26.3, 95% CI 16.3–42.4) of developing bradycardia in those sedated with dexmedetomidine compared to untreated controls [15]. Finally, a disproportionality analysis found that dexmedetomidine was associated with a near 57-fold increase (ROR = 56.66, 95% CI 49.90–64.35) in the likelihood of bradycardia [21]. Thus, despite its efficacy and reduced association with impaired respiratory function, it is important to explore the safety profile of dexmedetomidine and characterize the associated bradycardia risk, particularly for those with pre-existing cardiovascular complications and arrhythmias.
To address these gaps in dexmedetomidine characterization, this study employed a multidisciplinary approach combining the disproportionality analysis of safety reports derived from the FDA Adverse Event Reporting System (FAERS) with transcriptomic analysis of a public RNA-seq dataset to further elucidate the correlation between dexmedetomidine treatment and bradycardia risk. FAERS is one of the largest post-marketing drug surveillance databases available globally with over 30 million adverse event reports currently collected and an additional 1 million reports added annually [3,22,23]. FAERS provides an example of real-world data that more effectively captures the heterogeneity of patient experience while RNA-seq data provide experimental data that supplement the findings derived from FAERS analysis. When coupled together, a more robust conclusion can be drawn as to the association between dexmedetomidine and bradycardia if their directionality is comparable. Furthermore, association rule mining was used to identify concurrent adverse events that most frequently manifested alongside bradycardia in those treated with dexmedetomidine as well as to identify possible drug–drug interactions (DDIs). Finally, the potential underlying genetic mechanisms driving this elevated bradycardia risk were also explored.
2. Materials and Methods
2.1. FAERS Data
Adverse event reports submitted through FAERS were accessed using the publicly available FAERS dashboard tool. Each record derived from FAERS contains the following seven distinct data entries:
- (1)
- Drug information;
- (2)
- Drug-related adverse events (ADEs);
- (3)
- Patient outcomes for each indicated ADE;
- (4)
- Demographic information, including patients’ sex and age;
- (5)
- Origin of the reported ADE;
- (6)
- Date of submission;
- (7)
- Indications for use of each indicated drug.
Reports were extracted from the FAERS dashboard using both the generic drug term (dexmedetomidine and dexmedetomidine hydrochloride) and the most common brand name (Precedex) as input search terms. A report in which either the generic or brand name of the drug of interest was indicated in either the ‘suspect product names’ or ‘suspect product active ingredients’ data columns was eligible for inclusion in this study.
2.2. Removal of Duplicate FAERS Reports
The majority of the over 30 million records accessible through the FAERS dashboard are direct reports, indicating that the FDA was notified of the occurrence of an ADE through a submission made by a patient, healthcare provider, pharmaceutical company, or drug manufacturer. However, a relatively small subset of these reports may instead be indirect in nature. That is, rather than being directly submitted through the FAERS portal by an individual, some reports may be extracted from publications in the literature. As a result, multiple reports indicating the same ADE for the same patient albeit with different case IDs may arise in the database as multiple sources may identify and report the event after a review of the literature. To avoid the adverse effects of including spurious reports in the final analysis, any indirect reports extracted from publications were ultimately excluded from analysis for two reasons. First, some reports may be duplicated more than 10 times for the same patient-adverse event pair. By excluding these indirect reports, the strength of the potential safety signal is stabilized, and the elevated risk of false positive generation induced by inflated case counts is attenuated. Next, inconsistencies may be present among reports, either as a result of typographical errors, differences in interpretations, differing study goals, or direct exclusion of potentially relevant information. Data analysis was performed using R statistical software version 4.3.2.
2.3. Association Rule Mining
Next, association rule mining was used to identify additional co-presenting ADEs that were commonly reported alongside dexmedetomidine-associated bradycardia. Each association rule was analyzed using the arules package in R statistical software. The following formulas were used to calculate the support, confidence, and lift values for each association rule:
Despite its popularity in identifying significant patterns among data, use of the lift metric in association rule mining presents several limitations. First, the use of an arbitrary cutoff value to determine the significance of a given rule may lead to erroneous interpretations, particularly in datasets with skewed distributions or for those with low confidence (rare itemsets). Furthermore, the standard lift value does not provide confidence intervals or p-values, thus making it difficult to assess the statistical validity of observed associations deemed significant based on the cutoff criteria. Consequently, rules with high lift values may arise simply as a result of the sampling variability and not due to a true significant association among items. Thus, a complementary validation method such as disproportionality analysis is needed to differentiate significant rules from spurious rules arising by chance.
Disproportionality analyses evaluate the statistical significance of associations by comparing their observed frequencies against the expected frequency under the assumption of statistical independence. By applying this analysis, the robustness and statistical relevance of each association rule can be ascertained and the likelihood of false positive signals can be reduced.
The disproportionality analysis calculates the odds ratio and corresponding confidence intervals (CIs) using a standard 2 × 2 contingency table. For the identification of bradycardia-associated ADEs, referred to as ADE 2, the following table is used (Table 1):

Table 1.
A 2 × 2 contingency table for the validation of significant bradycardia-associated ADEs identified through association rule mining.
For the identification of significant DDIs with dexmedetomidine-associated bradycardia, the following table is used (Table 2):

Table 2.
A 2 × 2 contingency table for the validation of significant DDIs identified through association rule mining.
The odds ratio and corresponding CI for each rule were calculated using the fisher.test function in R.
2.4. Transcriptomic Data Analysis
Finally, a public transcriptomic dataset from the NCBI GEO database (GEO id: GSE210873) was analyzed to explore the genes in mouse-derived apical cardiac cells regulated by the administration of dexmedetomidine [24]. In this dataset, mouse-derived cardiac tissue was treated intravenously with either a preconditioning Yohimbine vehicle and 10 μg/kg of dexmedetomidine (treatment group) or just the preconditioning vehicle control group. Briefly, mice cardiac tissue was subjected to myocardial ischemia for 30 min followed by reperfusion for 120 min. In the treatment group, the preconditioning vehicle was administered 30 min prior to dexmedetomidine administration, and all drugs were intravenously delivered through the caudal vein for 10 min. Three replicates were available for both the control and treatment groups. Raw sequencing files in FASTQ were extracted to the online Galaxy interface and then aligned to the mm10 mouse genome using the HISAT2 alignment tool. Count tables were subsequently generated using the featureCounts tool V2.2.1, and differentially expressed genes were identified using DESeq2. This list of differentially expressed genes was then used as an input to identify enriched disease pathways using the ShinyGo V0.81 web tool.
3. Results
3.1. Top 10 ADEs of Dexmedetomidine Treatment
First, the top 10 most frequently reported ADEs were identified for patients administered the sedative medication dexmedetomidine. After removal of indirect reports, a total of 1611 reports were included in the analysis. As shown in Table 3, the most frequently reported ADE among these 1611 cases was bradycardia (n = 199) followed by hypotension (n = 124), cardiac arrest (n = 118), off-label use (n = 105), agitation (n = 67), drug ineffective (n = 58), drug interactions (n = 56), product use issues (n = 53), oxygen saturation decreased (n = 52), and pyrexia (n = 48).

Table 3.
Top 10 adverse drug events (ADEs) for the sedative dexmedetomidine.
3.2. ADEs Associated with Bradycardia
Next, association rule mining was used to identify other dexmedetomidine-associated ADEs that frequently co-occurred alongside bradycardia in patients administered the sedative. A lift value greater than one indicates that bradycardia and a given ADE were indicated together more frequently than the expected number of instances. A significant bradycardia–ADE pair was defined as one with a lift cutoff ≥1.5 and a minimum count of 10. As shown in Table 4, the most significant ADEs that commonly co-presented alongside bradycardia were syncope (lift = 4.711), loss of consciousness (lift = 3.997), cardiac arrest (lift = 2.850), hypotension (lift = 2.770), overdose (lift = 2.336), drug interaction (lift = 2.148), product administered to patient of inappropriate age (lift = 1.804), cardio-respiratory arrest (lift = 1.793), and respiratory arrest (lift = 1.562).

Table 4.
Top ADEs associated with bradycardia with lift ≥ 1.5 and count ≥ 10.
To validate the potential associations identified through association rule mining, a disproportionality analysis was also conducted. As shown in Table 5, syncope (OR = 11.04, 95% CI: 3.86–33.97), loss of consciousness (OR = 7.65, 95% CI: 3.28–17.98), cardiac arrest (OR = 4.71, 95% CI: 3.06–7.19), hypotension (OR = 4.54, 95% CI: 2.98–6.85), overdose (OR = 3.01, 95% CI: 1.26–6.69), and drug interactions (OR = 2.73, 95% CI: 1.43–4.99) were identified as significant potential co-occurring ADEs. Conversely, product administered to a patient of inappropriate age (OR = 2.11, 95% CI: 0.95–4.32), cardio-respiratory arrest (OR = 2.08, 95% CI: 0.90–4.41), and respiratory arrest (OR = 1.76, 95% CI: 0.91–3.22) were not considered statistically significant association rules, as their odds ratio confidence intervals included one.

Table 5.
Disproportionality analysis of co-presenting ADEs identified by association rules.
3.3. Possible DDIs with Dexmedetomidine
In addition to identifying common ADEs that co-occurred with bradycardia, association rule mining was also used to identify potential DDIs. Drugs co-administered with dexmedetomidine that were associated with bradycardia more frequently than expected were selected as candidate interactions. A significant DDI was defined as an association rule including dexmedetomidine–drug pair and bradycardia with a lift cutoff ≥ 1.5 and a minimum count of 10. As shown in Table 6, the top DDIs associated with bradycardia were Lactated Ringer’s solution (lift = 5.441), bupivacaine (lift = 2.984), risperidone (lift = 2.434), albuterol (lift = 1.927), potassium chloride (lift = 1.587), haloperidol (lift = 1.579), and sevoflurane (lift = 1.513).

Table 6.
Top DDIs associated with bradycardia with lift ≥ 1.5 and count ≥ 10.
To validate these potential associations identified through association rule mining, a disproportionality analysis was also conducted. As shown in Table 7, Lactated Ringer’s solution (OR = 16.96, 95% CI: 5.49–62.19), bupivacaine (OR = 4.43, 95% CI: 1.93–9.78), risperidone (OR = 3.22, 95% CI: 1.46–6.74), and albuterol (OR = 2.32, 95% CI: 1.11–4.53) were identified as significant potential DDIs. Conversely, potassium chloride (OR = 1.79, 95% CI: 0.90–3.35), haloperidol (OR = 1.79, 95% CI: 0.96–3.17), and sevoflurane (OR = 1.71, 95% CI: 0.98–2.86) were determined to not be significant association rules.

Table 7.
Disproportionality analysis of DDIs identified by association rules.
3.4. Genes Regulated by Dexmedetomidine in Mouse Cardiac Cells
Transcriptomic analysis was conducted using the publicly available RNA-seq dataset (GEO ID: GSE210873) to identify key regulatory genes differentially expressed between treatment and control groups that may contribute to drug-induced bradycardia. In this dataset, primary mouse-derived cardiac tissue was intravenously treated with either a preconditioning Yohimbine vehicle alone (control group) or the same vehicle followed by 10 μg/kg of dexmedetomidine (treatment group). As shown in Figure 1, a total of 950 genes were differentially expressed between the two groups, including 702 upregulated and 248 downregulated genes, based on an adjusted p-value < 0.05 and a fold change > 1.5.
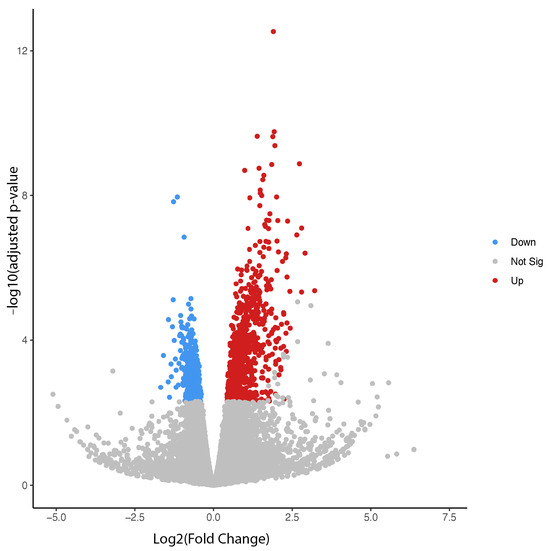
Figure 1.
Volcano plot for differentially expressed genes between dexmedetomidine treatment and control at p ≤ 0.05.
3.5. Disease Pathways Enriched in Dexmedetomidine-Treated Cardiac Cells
Finally, the list of differentially expressed genes was used as an input for the ShinyGO V0.81 enrichment tool in order to identify disease and molecular pathways that were enriched in dexmedetomidine-treated cardiac cells relative to the control. Figure 2 presents all of the enriched pathways for downregulated genes in dexmedetomidine-treated mouse cardiac cells with a false discovery rate (FDR) less than or equal to 0.05. As shown in Table 8, the top 10 enriched pathways for downregulated genes were ribosome (n = 20 genes; fold enrichment (FE) = 8.22), circadian rhythm (n = 4 genes; FE = 6.29), cardiac muscle contraction (n = 8 genes; FE = 4.92), oxidative phosphorylation (n = 12 genes; FE = 4.82), mitophagy-animal (n = 6 genes; FE = 4.72), acute myeloid leukemia (n = 6 genes; FE = 4.58), Parkinson’s disease (n = 21 genes; FE = 4.28), chemical carcinogenesis-reactive oxygen species (n = 17 genes; FE = 4.13), hypertrophic cardiomyopathy (n = 7 genes; FE = 4.11), and coronavirus disease COVID-19 (n = 18 genes; FE = 4.09). With regard to bradycardia, eight downregulated genes were mapped to the cardiac muscle contraction pathway including COX5B, COX6A2, COX8B, MYH7, MYH6, MYL2, UQCRQ, and UQCR11.
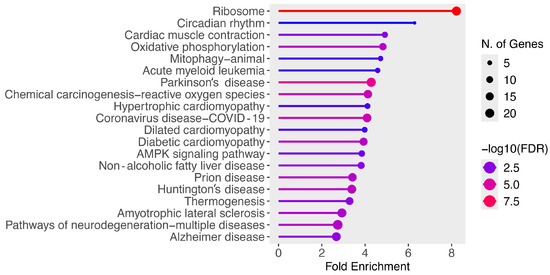
Figure 2.
Enriched pathways for downregulated genes at FDR ≤ 0.05.

Table 8.
Top 10 enriched pathways for downregulated genes in dexmedetomidine-treated mouse cardiac cells.
4. Discussion
A multidisciplinary approach was utilized in this study to assess the risk of bradycardia in patients treated with dexmedetomidine, as well as to further characterize the safety profile of dexmedetomidine by using association rule mining to identify potential co-presenting ADEs and DDIs. Furthermore, RNA-seq analysis was performed to identify possible genetic drivers of the observed elevated risk of bradycardia in those administered the sedative dexmedetomidine. Bradycardia was found to be the most commonly reported ADE for patients sedated with dexmedetomidine. Other cardiovascular-related ADEs that commonly co-presented with bradycardia in those sedated with dexmedetomidine included syncope (lift = 4.711), loss of consciousness (lift = 3.997), cardiac arrest (lift = 2.850), and hypotension (lift = 2.770). Ideally, anesthesia minimizes patient pain and discomfort by inhibiting brain function while maintaining cardiac activity [25,26]. Clinically, suppression of cardiac function in patients undergoing surgery may lead to complications, thus considerations for pre-existing conditions and patient cardiovascular health should be made prior to the use of dexmedetomidine.
Although dosing information is unavailable in FAERS reports, other studies in the literature indicate that elevation of dexmedetomidine-associated bradycardia risk may be linked to dexmedetomidine overdose, either due to high loading doses, more concentrated infusions, or prolonged periods of sedation [19,26,27,28]. For instance, a multicenter, double-blind randomized controlled trial comparing the efficacy of dexmedetomidine with another sedative midazolam found that the incidence of bradycardia was approximately three times higher in the dexmedetomidine group compared to those sedated with midazolam (14.2% vs. 5.2%, p < 0.001) at a maintenance dose of 0.45 μg/kg/h [26]. Another double-blind, randomized controlled trial found that both the incidence (p = 0.027) and severity (p = 0.017) of bradycardia were significantly higher in those sedated with a 1.0 μg/kg loading dose compared to those administered a 0.8 μg/kg loading dose during rhinoplasty surgery [29]. Finally, a retrospective study found that a lower baseline heart rate (odds ratio = 0.89, 95% CI 0.82–0.96) and longer tourniquet time during surgery (odds ratio = 1.06, 95% CI 1.02–1.10) were both associated with an elevated likelihood of bradycardia development during spinal anesthesia [16]. Thus, patient status should be closely monitored for signs of bradycardia presentation, particularly for those requiring high initial loading dosages, longer periods of sedation, or those presenting with risk factors of bradycardia including lower initial heart rate and pre-existing cardiovascular complications.
Lactated Ringer’s solution is a mixture of sodium lactate, sodium chloride, potassium chloride, and calcium chloride dissolved in water that is used to stabilize blood volume and electrolyte imbalances in patients undergoing surgery [30,31]. Although generally considered safe with minimal contraindications and commonly administered to patients sedated with dexmedetomidine, Lactated Ringer’s solution may exacerbate bradycardia risk by promoting hyponatremia and reducing electrical conduction of the heart or by reducing blood pressure significantly and disrupting proper blood flow [30,32,33]. D-lactate has been shown to increase the activity of inducible nitric oxide synthase (iNOS) by promoting the transformation of macrophages to the M2 subtypes and elevating the expression of the vasodilator nitric oxide [34,35]. Likewise, dexmedetomidine has also demonstrated the ability to elevate nitric oxide levels by promoting the activation of endothelial nitric oxide synthase (eNOS); however, it is important to note that dexmedetomidine may also modulate the production of iNOS-derived nitric oxide [36,37]. Thus, concurrent administration of dexmedetomidine and Lactated Ringer’s solution may synergistically elevate bradycardia risk through overproduction of nitric oxide and prolonged vasodilation, particularly in those in which a large volume of Lactated Ringer’s solution is intravenously delivered or in patients with pre-existing cardiovascular conditions [30,34,36,37].
Bupivacaine is a local anesthetic used to modulate patient pain both during and after surgery [38,39]. Dexmedetomidine is commonly used as an adjuvant therapy to prolong postoperative pain reduction and shorten the time for sedation to occur [40,41,42]. However, multiple studies have demonstrated an increase in bradycardia risk when a combination therapy of dexmedetomidine and bupivacaine is used [42,43,44]. A randomized study of 100 American Society of Anesthesiologists patients found that the incidence of bradycardia in the group administered 3 mL of 0.5% bupivacaine followed by intravenous delivery of a 1 μg/kg loading dose of dexmedetomidine was significantly higher than the incidence of bradycardia in the control group only administered bupivacaine (33% vs. 4%, p < 0.001) [43]. Furthermore, a systematic review of 25 studies found that adjuvant administration of dexmedetomidine in those previously administered bupivacaine was associated with a 59% increase in relative risk of bradycardia (RR = 1.59, 95% CI 1.07–2.37) compared to those administered bupivacaine exclusively [42]. The increased production of nitric oxide by dexmedetomidine may potentiate the sedative effects of bupivacaine by increasing the risk of methemoglobinemia, a condition characterized by decreased oxygen transport capabilities that may lead to bradycardia [45,46]. Furthermore, higher doses of bupivacaine have been shown to promote stimulatory phosphorylation events of eNOS and elevation of nitric oxide levels [46].
Risperidone is an antipsychotic medication with indications for use in the treatment of schizophrenia and bipolar disorder [47]. Both dexmedetomidine and risperidone function as suppressants of the central nervous system by decreasing dopaminergic signaling in the case of dexmedetomidine or both dopaminergic and serotonin-mediated signaling in the case of risperidone [48,49,50,51,52]. Although the mechanism in which these two drugs interact to elevate bradycardia risk is not well characterized, it may be related to the additive effects on eNOS activity and overproduction of nitric oxide by macrophages [53].
Eight genes that regulate cardiac muscle contraction, including COX5B, COX6A2, COX8B, MYH7, MYH6, MYL2, UQCRQ, and UQCR11, were found to be downregulated in cardiac cells treated with dexmedetomidine. COX5B, COX6A2, and COX8B encode subunits of the cytochrome c oxidase complex in the mitochondrial electron transport chain [54,55]. Deficiencies in COX6A2 have been linked to significant cardiac tissue remodeling and various cardiomyopathies including bradycardia [56,57]. MYL2 encodes myosin light chain 2, a critical structural protein involved in the contraction of cardiovascular tissue with both missense mutations in this gene and downregulation of protein expression being associated with electrophysiological abnormalities, poor mechanical conduction, and bradycardia [58,59]. Likewise, downregulation of the sarcomeric structural proteins encoded by MYH6 and MYH7 have also been linked to impaired heart tissue contractility and bradycardia [60,61,62,63]. In vivo mechanistic studies have demonstrated significant bradycardia in MYH6 knockout models while another study found that low expression levels of MYH6 are associated with ischemic cardiomyopathies and heart failure [62,64]. In humans, MYH7 may be the more dominant driver of the bradycardia phenotype, as it is highly expressed in the adult human heart, while MYH6 is the predominant myosin isoform in mice [63,65]. Knockout of URCRQ, which encodes a prominent component of the mitochondrial electron transport chain, has shown to lead to the development of contractile dysfunction and reduced cardiomyocyte electrical activity due to disruptions in ATP production [66,67,68].
Proper cardiac health is dependent on the ability of the heart to rhythmically contract and relax to pump blood throughout the body and provide oxygen, among other nutrients, to systems throughout the body [69,70]. This process can be divided into multiple stages in which the electrical signals generated from the sinoatrial node promote the release of calcium from the sarcoplasmic reticulum, binding of calcium to the structural myosin proteins, sliding of myosin and actin filaments to drive cardiac contractility, and repolarization [69,70]. Because of the significant energetic requirements for the continual cycling of cardiac tissue between resting and contracting states, ATP production is closely linked to the ability of the heart to contract, with approximately 70% of all ATP produced by cardiocytes being directed toward this process [69,71]. Decreased ATP production and overall energetic deficiency may prolong the repolarization phase and extend the length of a single cardiac muscle contraction, reducing the overall number of full contractile cycles in a given period of time [71,72,73,74]. Thus, the suppression of mitochondrial genes related to energy production, such as UQCRQ, UQCR11, COX5B, COX6A2, and COX8B, may extend the length of a contractile cycle by prolonging the initiation of cardiac muscle contraction, thus lowering the heart rate and driving bradycardia progression. Similarly, suppression of structural genes, such as MYH6, MYH7, and MYL2, impairs the ability of myosin and actin filaments to associate and reduces the amount of contractile force generated, resulting in slower heart beats and bradycardia.
Proteomic analyses have also demonstrated impaired energetics and altered cardiac muscle contractility in multiple cardiovascular pathophysiologies. For instance, a study comparing the proteomic profiles of human hypertrophic cardiomyopathy found 88 differentially expressed proteins related to both contraction mechanisms, which included the downregulation of sarcomeric proteins MYH6 and MYBPC3, as well as the downregulation of metabolic proteins, including creatine kinase and the sarcoplasmic reticulum calcium transport protein ATP2A2 [75,76]. A proteomic analysis of sinoatrial node cells coupled with FAERS analysis of targets for bradycardia-associated drugs found seven downregulated proteins linked to contractile complications, including the potassium channels KCNH2, HCN1, and HCN4, as well as structural proteins such as CDH2 [77]. Finally, the upregulation of the vitamin-D-binding protein (VDB) in myocardial infarction samples may indirectly promote bradycardia by reducing cardiac concentrations of vitamin D, a crucial driver of calcium regulation and cardiac contractility, a deficiency of which has been linked to various arrhythmias including bradycardia [78,79,80]. Thus, there is a strong precedence of bradycardia phenotypes due to disruptions in cardiac energy production and contractility.
Despite these promising findings, the present study is not without limitations. First, due to the observational nature of the data derived from FAERS, a causal relationship between dexmedetomidine and an elevated risk of bradycardia cannot be determined. Consequently, the incidence of new bradycardia cases in those administered dexmedetomidine cannot be estimated. As many indirect reports were removed prior to the analysis (>1000), the ability of the study to identify safety signals for rare ADEs may be hindered, and the study may possibly be underpowered. However, as bradycardia was still the most frequently reported ADE among dexmedetomidine reports, the effects of this exclusion should be minimal with regard to estimating the strength of the association between dexmedetomidine and bradycardia risk. Furthermore, reports derived from the FAERS dashboard lack potentially important information such as dosage, which may influence whether dexmedetomidine is associated with an increase in the risk of bradycardia development, and they are subject to various biases including underreporting and other reporting biases. To combat this, future studies will utilize electronic health records to explore the role of potential confounders and refine risk estimates. In addition, potential DDIs identified in this study are hypothetical in nature and require additional clinical studies to validate their potential effects on bradycardia risk. Finally, the RNA-seq data utilized in this study were derived from mouse cardiac tissue, which may not be entirely comparable to human models due to physiological or genetic differences among species. In addition, the differentially expressed genes identified in this study may not be extrapolated across cell types or across species. Although these genes are functionally conserved between mice and humans, differences in kinetic requirements for proper cardiac function, mitochondria concentration, and the lack of more robust compensatory mechanisms in mice to modulate irregular heart rates may influence the risk of bradycardia between species treated with dexmedetomidine [81,82]. Nonetheless, this study further characterizes the safety profile of dexmedetomidine and provides potential mechanisms by which dexmedetomidine may elevate bradycardia risk in some patients. Further studies will seek to validate these potential DDIs and to determine differences in gene expression in human cells treated with dexmedetomidine to see whether different regulatory mechanisms modulate bradycardia risk in humans.
5. Conclusions
Bradycardia is a commonly reported adverse event in patients administered the sedative dexmedetomidine. Additional adverse events that co-occurred alongside bradycardia included syncope, loss of consciousness, cardiac arrest, and hypotension. Several potential DDIs were identified including Lactated Ringer’s solution, bupivacaine, and risperidone, which may elevate bradycardia risk through impaired cardiac contraction mechanisms and decreased mitochondrial energy production. Finally, eight downregulated genes mapped to cardiac muscle contraction were identified as possible regulators of dexmedetomidine-induced bradycardia, including COX5B, COX6A2, COX8B, MYH7, MYH6, MYL2, UQCRQ, and UQCR11 in mouse cardiac cells.
Author Contributions
Conceptualization, R.M. and F.C.; Data curation, R.M., X.Z. and F.C.; Formal analysis, R.M., S.A.K., X.Z., K.B., W.H. and F.C.; Funding acquisition, F.C.; Investigation, R.M., S.A.K. and F.C.; Methodology, R.M. and F.C.; Project administration, F.C.; Resources, F.C.; Software, R.M. and F.C.; Supervision, F.C.; Validation, R.M. and F.C.; Visualization, R.M. and F.C.; Writing—original draft, R.M. and F.C.; Writing—review and editing, R.M. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NCBI | National Center for Biotechnology Information |
| GEO | Gene Expression Omnibus |
| DDI | Drug–drug interaction |
| OR | Odds ratio |
| CI | Confidence interval |
| FDR | False discovery rate |
| FDA | Food and Drug Administration |
| FAERS | FDA Adverse Event Reporting System |
| ADE | Adverse drug event |
| BPM | Beats per minute |
| ATP | Adenosine triphosphate |
| MYBPC3 | Cardiac myosin-binding protein C |
| ATP2A2 | Sarcoplasmic reticulum calcium ATPase 2 |
| KCNH2 | Potassium voltage-gated channel subfamily H member 2 |
| HCN1 | Hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 |
| HCN4 | Hyperpolarization-activated cyclic nucleotide-gated potassium channel 4 |
| CDH2 | N-cadherin |
| VDB | Vitamin-D-binding protein |
| iNOS | Inducible nitric oxide synthase |
| eNOS | Endothelial nitric oxide synthase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| COX5B | Cytochrome c oxidase subunit 5B |
| COX6A2 | Cytochrome c oxidase subunit 6A2 |
| COX8B | Cytochrome c oxidase subunit 8B |
| MYH6 | Myosin heavy chain, α isoform |
| MYH7 | Myosin heavy chain, β isoform |
| MYL2 | Myosin regulatory light chain 2 |
| UQCRQ | Ubiquinol-cytochrome c reductase, complex III subunit VII |
| UQCR11 | Ubiquinol-cytochrome c reductase, complex III subunit XI |
References
- Sidhu, S.; Marine, J.E. Evaluating and managing bradycardia. Trends Cardiovasc. Med. 2020, 30, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, Y.; Grossman, S.A. Sinus Bradycardia. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Morris, R.; Luboff, H.; Jose, R.P.; Eckhoff, K.; Bu, K.; Pham, M.; Rohlsen-Neal, D.; Cheng, F. Bradycardia Due to Donepezil in Adults: Systematic Analysis of FDA Adverse Event Reporting System. J. Alzheimer’s Dis. 2021, 81, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wung, S.F. Bradyarrhythmias: Clinical Presentation, Diagnosis, and Management. Crit. Care Nurs. Clin. N. Am. 2016, 28, 297–308. [Google Scholar] [CrossRef]
- Barstow, C.; McDivitt, J.D. Cardiovascular Disease Update: Bradyarrhythmias. FP Essent. 2017, 454, 18–23. [Google Scholar]
- Oken, K.; Schoenfeld, M.H.; Kusumoto, F. Evaluation and Treatment of Patients with Bradycardia and Cardiac Conduction Delay: Recommendations for Permanent Pacing. JAMA Cardiol. 2019, 4, 823–824. [Google Scholar] [CrossRef]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Reel, B.; Maani, C.V. Dexmedetomidine. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef]
- Carollo, D.S.; Nossaman, B.D.; Ramadhyani, U. Dexmedetomidine: A review of clinical applications. Curr. Opin. Anaesthesiol. 2008, 21, 457–461. [Google Scholar] [CrossRef]
- Weerink, M.A.S.; Struys, M.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef]
- Manning, A.N.; Bezzo, L.K.; Hobson, J.K.; Zoeller, J.E.; Brown, C.A.; Henderson, K.J. Dexmedetomidine Dosing to Prevent Pediatric Emergence Delirium. AANA J. 2020, 88, 359–364. [Google Scholar]
- Chen, H.Y.; Deng, F.; Tang, S.H.; Liu, W.; Yang, H.; Song, J.C. Effect of different doses of dexmedetomidine on the median effective concentration of propofol during gastrointestinal endoscopy: A randomized controlled trial. Br. J. Clin. Pharmacol. 2023, 89, 1799–1808. [Google Scholar] [CrossRef]
- Takahashi, K.; Yoshikawa, Y.; Kanda, M.; Hirata, N.; Yamakage, M. Dexmedetomidine as a cardioprotective drug: A narrative review. J. Anesth. 2023, 37, 961–970. [Google Scholar] [CrossRef]
- Gong, Z.; Ma, L.; Zhong, Y.L.; Li, J.; Lv, J.; Xie, Y.B. Myocardial protective effects of dexmedetomidine in patients undergoing cardiac surgery: A meta-analysis and systematic review. Exp. Ther. Med. 2017, 13, 2355–2361. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, E. Risk factors for dexmedetomidine-associated bradycardia during spinal anesthesia: A retrospective study. Medicine 2022, 101, e31306. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, A.T.; Murphy, C.V. Dexmedetomidine-associated bradycardia progressing to pulseless electrical activity: Case report and review of the literature. Pharmacotherapy 2009, 29, 1492. [Google Scholar] [CrossRef]
- Banc-Husu, A.M.; Badke, C.M.; Sanchez-Pinto, L.N.; Alonso, E.M. Dexmedetomidine leading to profound bradycardia in a pediatric liver transplant recipient. Pediatr Transpl. 2021, 25, e13895. [Google Scholar] [CrossRef] [PubMed]
- Ice, C.J.; Personett, H.A.; Frazee, E.N.; Dierkhising, R.A.; Kashyap, R.; Oeckler, R.A. Risk Factors for Dexmedetomidine-Associated Hemodynamic Instability in Noncardiac Intensive Care Unit Patients. Anesth. Analg. 2016, 122, 462–469. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Sella, N.; Geraldini, F.; Zarantonello, F.; Pettenuzzo, T.; Pasin, L.; Iuzzolino, M.; Rossini, N.; Pesenti, E.; Zecchino, G.; et al. Preoperative dexmedetomidine and intraoperative bradycardia in laparoscopic cholecystectomy: A meta-analysis with trial sequential analysis. Korean J. Anesthesiol. 2022, 75, 245–254. [Google Scholar] [CrossRef]
- Shuai, Y.; Chen, Z.; Wan, Q.; Wu, J.; Wang, X. Dexmedetomidine: A real-world safety analysis based on FDA adverse event reporting system database. Front. Pharmacol. 2024, 15, 1419196. [Google Scholar] [CrossRef]
- Morris, R.; Todd, M.; Aponte, N.Z.; Salcedo, M.; Bruckner, M.; Garcia, A.S.; Webb, R.; Bu, K.; Han, W.; Cheng, F. The association between warfarin usage and international normalized ratio increase: Systematic analysis of FDA Adverse Event Reporting System (FAERS). J. Cardiovasc. Aging 2023, 3, 39. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, Y.; Wei, S.; Liu, Y. Multidimensional assessment of adverse events of finasteride: A real-world pharmacovigilance analysis based on FDA Adverse Event Reporting System (FAERS) from 2004 to April 2024. PLoS ONE 2025, 20, e0309849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.R.; Hong, Y.; Wen, S.H.; Zhan, Y.Q.; Huang, W.Q. Dexmedetomidine Pretreatment Protects Against Myocardial Ischemia/Reperfusion Injury by Activating STAT3 Signaling. Anesth. Analg. 2023, 137, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; D’Cruz, J.R.; Rondeau, B.; Goldman, J. General Anesthesia for Surgeons. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jakob, S.M.; Ruokonen, E.; Grounds, R.M.; Sarapohja, T.; Garratt, C.; Pocock, S.J.; Bratty, J.R.; Takala, J.; Dexmedetomidine for Long-Term Sedation, I. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012, 307, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.J.; Hall, J.E.; Barney, J.A.; Uhrich, T.D.; Colinco, M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000, 93, 382–394. [Google Scholar] [CrossRef]
- Bahraini, A.; Banerjee, O.; Ra, J. Bradycardia resulting in cardiac arrest in a critically ill patient receiving dexmedetomidine. Trauma Case Rep. 2021, 36, 100548. [Google Scholar] [CrossRef] [PubMed]
- Djalali Motlagh, S.; Rokhtabnak, F.; Ghodraty, M.R.; Maleki Delarestaghi, M.; Saadat, S.; Araghi, Z. Effect of Different Loading Doses of Dexmedetomidine on Controlled Hypotension and the Incidence of Bradycardia During Rhinoplasty: A Clinical Trial. Anesth. Pain Med. 2021, 11, e118857. [Google Scholar] [CrossRef]
- Singh, S.; Kerndt, C.C.; Davis, D. Ringer’s Lactate. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ocskay, K.; Matrai, P.; Hegyi, P.; Parniczky, A. Lactated Ringer’s Solution Reduces Severity, Mortality, Systemic and Local Complications in Acute Pancreatitis: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 321. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Faeh, L.; Muller, M.; Eberle, B.; Jenni, H.; Zante, B.; Prazak, J.; Englberger, L.; Takala, J.; Jakob, S.M. Fluid management in patients undergoing cardiac surgery: Effects of an acetate- versus lactate-buffered balanced infusion solution on hemodynamic stability (HEMACETAT). Crit. Care 2019, 23, 159. [Google Scholar] [CrossRef]
- Chan, L.; Slater, J.; Hasbargen, J.; Herndon, D.N.; Veech, R.L.; Wolf, S. Neurocardiac toxicity of racemic D,L-lactate fluids. Integr Physiol. Behav. Sci. 1994, 29, 383–394. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, H.; Huang, J. The role of lactate in cardiovascular diseases. Cell Commun. Signal. 2023, 21, 317. [Google Scholar] [CrossRef]
- Burgos, R.A.; Manosalva, C.; Alarcon, P.; Navarro, M.; Quiroga, J.; Moran, G.; Gallastegui, J.; Brauchi, S.; Carretta, M.D. The D-lactate enigma: Exploring the inflammatory influence of D-lactate in cattle. Front. Vet. Sci. 2024, 11, 1509399. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Qian, W.; Zhao, J.; Sun, L.; Qian, Y.; Xiao, H. Dexmedetomidine inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated microglia by suppression of extracellular signal-regulated kinase. Neurol. Res. 2015, 37, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Nong, L.; Ma, J.; Zhang, G.; Deng, C.; Mao, S.; Li, H.; Cui, J. Dexmedetomidine inhibits vasoconstriction via activation of endothelial nitric oxide synthase. Korean J. Physiol. Pharmacol. 2016, 20, 441–447. [Google Scholar] [CrossRef]
- Shafiei, F.T.; McAllister, R.K.; Lopez, J. Bupivacaine. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Paganelli, M.A.; Popescu, G.K. Actions of bupivacaine, a widely used local anesthetic, on NMDA receptor responses. J. Neurosci. 2015, 35, 831–842. [Google Scholar] [CrossRef]
- Agarwal, S.; Aggarwal, R.; Gupta, P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 36–40. [Google Scholar] [CrossRef]
- Benito, J.; Evangelista, M.C.; Doodnaught, G.M.; Watanabe, R.; Beauchamp, G.; Monteiro, B.P.; Steagall, P. Analgesic Efficacy of Bupivacaine or Bupivacaine-Dexmedetomidine After Intraperitoneal Administration in Cats: A Randomized, Blinded, Clinical Trial. Front. Vet. Sci. 2019, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, P.; Cui, Y.; Lu, C.; Ji, M.; Liu, W.; Jiang, W.; Zhu, Z.; Sun, Q. Effect of 5-mug Dose of Dexmedetomidine in Combination with Intrathecal Bupivacaine on Spinal Anesthesia: A Systematic Review and Meta-analysis. Clin. Ther. 2020, 42, 676–690.e675. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, C.N.; Sai Tej, N.A.; Yatish, B.; Pujari, V.S.; Mohan Kumar, R.M.; Mohan, C.V. Effects of intravenous dexmedetomidine on hyperbaric bupivacaine spinal anesthesia: A randomized study. Saudi J. Anaesth. 2014, 8, 202–208. [Google Scholar]
- Kang, S.; Chae, Y.J.; Park, S.K.; Kim, T.G.; Joe, H.B. Prevention of Bradycardia during Spinal Anesthesia under Dexmedetomidine Sedation in Older Adults. J. Clin. Med. 2022, 11, 6349. [Google Scholar] [CrossRef]
- Bilgin, B.; Gursoy, H.; Basmak, H.; Ozkurt, M.; Tuncel, N.; Canaz, F.; Isiksoy, S.; Colak, E. The effects of bupivacaine injection and oral nitric oxide on extraocular muscle in the rabbit. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2227–2233. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, C.S.; Ok, S.H.; Kim, D.; Kim, K.N.; Hong, J.M.; Kim, J.Y.; Bae, S.I.; An, S.; Sohn, J.T. Bupivacaine-induced contraction is attenuated by endothelial nitric oxide release modulated by activation of both stimulatory and inhibitory phosphorylation (Ser1177 and Thr495) of endothelial nitric oxide synthase. Eur. J. Pharmacol. 2019, 853, 121–128. [Google Scholar] [CrossRef] [PubMed]
- McNeil, S.E.; Gibbons, J.R.; Cogburn, M. Risperidone. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kumar, R.; Sinha, R.; Kundu, R.; Ranjan, B. Role of dexmedetomidine for sedation in a patient with schizophrenia for strabismus surgery. Indian J. Anaesth. 2016, 60, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Mu, J.; Wang, Y.; He, H. Perioperative dexmedetomidine-induced delirium in a patient with schizophrenia: A case report. BMC Anesthesiol. 2024, 24, 278. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.A.; Virag, L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth. Analg. 2006, 102, 448–455. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Genetic differences associated with dopamine and serotonin release mediate fear-induced bradycardia in the human brain. Transl. Psychiatry 2024, 14, 24. [Google Scholar] [CrossRef]
- Alvarez-Herrera, S.; Rosel Vales, M.; Perez-Sanchez, G.; Becerril-Villanueva, E.; Flores-Medina, Y.; Maldonado-Garcia, J.L.; Saracco-Alvarez, R.; Escamilla, R.; Pavon, L. Risperidone Decreases Expression of Serotonin Receptor-2A (5-HT2A) and Serotonin Transporter (SERT) but Not Dopamine Receptors and Dopamine Transporter (DAT) in PBMCs from Patients with Schizophrenia. Pharmaceuticals 2024, 17, 167. [Google Scholar] [CrossRef]
- Alvarez-Herrera, S.; Escamilla, R.; Medina-Contreras, O.; Saracco, R.; Flores, Y.; Hurtado-Alvarado, G.; Maldonado-Garcia, J.L.; Becerril-Villanueva, E.; Perez-Sanchez, G.; Pavon, L. Immunoendocrine Peripheral Effects Induced by Atypical Antipsychotics. Front. Endocrinol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Kadenbach, B.; Huttemann, M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015, 24, 64–76. [Google Scholar] [CrossRef]
- Cunatova, K.; Reguera, D.P.; Houstek, J.; Mracek, T.; Pecina, P. Role of cytochrome c oxidase nuclear-encoded subunits in health and disease. Physiol. Res. 2020, 69, 947–965. [Google Scholar] [CrossRef]
- Jiang, M.; Song, Y.; Chen, X.; Lu, W.; Zhu, M.; Wei, M.; Lan, F.; Cui, M.; Bai, Y. COX6A2 deficiency leads to cardiac remodeling in human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2023, 14, 357. [Google Scholar] [CrossRef]
- Inoue, M.; Uchino, S.; Iida, A.; Noguchi, S.; Hayashi, S.; Takahashi, T.; Fujii, K.; Komaki, H.; Takeshita, E.; Nonaka, I.; et al. COX6A2 variants cause a muscle-specific cytochrome c oxidase deficiency. Ann. Neurol. 2019, 86, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, G.; Tang, Q.; Huang, C.; Jiang, H.; Shi, L.; Tu, X.; Huang, J.; Zhu, X.; Wang, H. Slow cardiac myosin regulatory light chain 2 (MYL2) was down-expressed in chronic heart failure patients. Clin. Cardiol. 2011, 34, 30–34. [Google Scholar] [CrossRef]
- De Bortoli, M.; Vio, R.; Basso, C.; Calore, M.; Minervini, G.; Angelini, A.; Melacini, P.; Vitiello, L.; Vazza, G.; Thiene, G.; et al. Novel Missense Variant in MYL2 Gene Associated with Hypertrophic Cardiomyopathy Showing High Incidence of Restrictive Physiology. Circ. Genom. Precis. Med. 2020, 13, e002824. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Gudbjartsson, D.F.; Sulem, P.; Masson, G.; Helgadottir, H.T.; Zanon, C.; Magnusson, O.T.; Helgason, A.; Saemundsdottir, J.; Gylfason, A.; et al. A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nat. Genet. 2011, 43, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Zimmermann, M.T.; Evans, J.M.; Eckloff, B.W.; Wieben, E.D.; Qureshi, M.Y.; O’Leary, P.W.; Olson, T.M. Recessive MYH6 Mutations in Hypoplastic Left Heart with Reduced Ejection Fraction. Circ. Cardiovasc. Genet. 2015, 8, 564–571. [Google Scholar] [CrossRef]
- Anfinson, M.; Fitts, R.H.; Lough, J.W.; James, J.M.; Simpson, P.M.; Handler, S.S.; Mitchell, M.E.; Tomita-Mitchell, A. Significance of alpha-Myosin Heavy Chain (MYH6) Variants in Hypoplastic Left Heart Syndrome and Related Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2022, 9, 144. [Google Scholar]
- Gacita, A.M.; Fullenkamp, D.E.; Ohiri, J.; Pottinger, T.; Puckelwartz, M.J.; Nobrega, M.A.; McNally, E.M. Genetic Variation in Enhancers Modifies Cardiomyopathy Gene Expression and Progression. Circulation 2021, 143, 1302–1316. [Google Scholar] [CrossRef]
- Chen, J.H.; Wang, L.L.; Tao, L.; Qi, B.; Wang, Y.; Guo, Y.J.; Miao, L. Identification of MYH6 as the potential gene for human ischaemic cardiomyopathy. J. Cell. Mol. Med. 2021, 25, 10736–10746. [Google Scholar] [CrossRef]
- Dainis, A.; Zaleta-Rivera, K.; Ribeiro, A.; Chang, A.C.H.; Shang, C.; Lan, F.; Burridge, P.W.; Liu, W.R.; Wu, J.C.; Chang, A.C.Y.; et al. Silencing of MYH7 ameliorates disease phenotypes in human iPSC-cardiomyocytes. Physiol Genom. 2020, 52, 293–303. [Google Scholar] [CrossRef]
- Spielmann, N.; Schenkl, C.; Komlodi, T.; da Silva-Buttkus, P.; Heyne, E.; Rohde, J.; Amarie, O.V.; Rathkolb, B.; Gnaiger, E.; Doenst, T.; et al. Knockout of the Complex III subunit Uqcrh causes bioenergetic impairment and cardiac contractile dysfunction. Mamm Genome 2023, 34, 229–243. [Google Scholar] [CrossRef]
- Barel, O.; Shorer, Z.; Flusser, H.; Ofir, R.; Narkis, G.; Finer, G.; Shalev, H.; Nasasra, A.; Saada, A.; Birk, O.S. Mitochondrial complex III deficiency associated with a homozygous mutation in UQCRQ. Am. J. Hum. Genet. 2008, 82, 1211–1216. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, Y.; Chen, Z.B.; Rhee, J.W.; Deng, Y.; Wang, Z.V. Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells 2023, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.D.; Makaryus, A.N. Physiology, Cardiac Cycle. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Fukuta, H.; Little, W.C. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail. Clin. 2008, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Garfinkel, A.; Karagueuzian, H.S.; Chen, P.S.; Qu, Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010, 7, 1891–1899. [Google Scholar] [CrossRef]
- Luptak, I.; Sverdlov, A.L.; Panagia, M.; Qin, F.; Pimentel, D.R.; Croteau, D.; Siwik, D.A.; Ingwall, J.S.; Bachschmid, M.M.; Balschi, J.A.; et al. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J. Mol. Cell. Cardiol. 2018, 116, 106–114. [Google Scholar] [CrossRef]
- Yang, H.M. Mitochondrial Dysfunction in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 1917. [Google Scholar] [CrossRef]
- Previs, M.J.; O’Leary, T.S.; Morley, M.P.; Palmer, B.M.; LeWinter, M.; Yob, J.M.; Pagani, F.D.; Petucci, C.; Kim, M.S.; Margulies, K.B.; et al. Defects in the Proteome and Metabolome in Human Hypertrophic Cardiomyopathy. Circ. Heart. Fail. 2022, 15, e009521. [Google Scholar] [CrossRef]
- Karpov, O.A.; Stotland, A.; Raedschelders, K.; Chazarin, B.; Ai, L.; Murray, C.I.; Van Eyk, J.E. Proteomics of the heart. Physiol. Rev. 2024, 104, 931–982. [Google Scholar] [CrossRef]
- Kahnert, K.; Soattin, L.; Mills, R.W.; Wilson, C.; Maurya, S.; Sorrentino, A.; Al-Othman, S.; Tikhomirov, R.; van de Vegte, Y.J.; Hansen, F.B.; et al. Proteomics couples electrical remodelling to inflammation in a murine model of heart failure with sinus node dysfunction. Cardiovasc. Res. 2024, 120, 927–942. [Google Scholar] [CrossRef]
- Fanari, Z.; Hammami, S.; Hammami, M.B.; Hammami, S.; Abdellatif, A. Vitamin D deficiency plays an important role in cardiac disease and affects patient outcome: Still a myth or a fact that needs exploration? J. Saudi Heart Assoc. 2015, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Lopez, R.; Fitzpatrick-Wong, S.; Ramjiawan, B. Understanding the Role of Vitamin D in Heart Failure. Rev. Cardiovasc. Med. 2023, 24, 111. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, C.; Curcio, A.; Torella, D.; Gaspari, M.; Celi, V.; Salituri, F.; Boncompagni, D.; Torella, M.; Gulletta, E.; Cuda, G.; et al. Proteomics reveals high levels of vitamin D binding protein in myocardial infarction. Front. Biosci. 2010, 2, 796–804. [Google Scholar]
- Janssen, P.M.; Biesiadecki, B.J.; Ziolo, M.T.; Davis, J.P. The Need for Speed: Mice, Men, and Myocardial Kinetic Reserve. Circ. Res. 2016, 119, 418–421. [Google Scholar] [CrossRef]
- Lindovsky, J.; Nichtova, Z.; Dragano, N.R.V.; Pajuelo Reguera, D.; Prochazka, J.; Fuchs, H.; Marschall, S.; Gailus-Durner, V.; Sedlacek, R.; Hrabe de Angelis, M.; et al. A review of standardized high-throughput cardiovascular phenotyping with a link to metabolism in mice. Mamm. Genome 2023, 34, 107–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).