Natural Bioproducts with Epigenetic Properties for Treating Cardiovascular Disorders
Abstract
1. Introduction
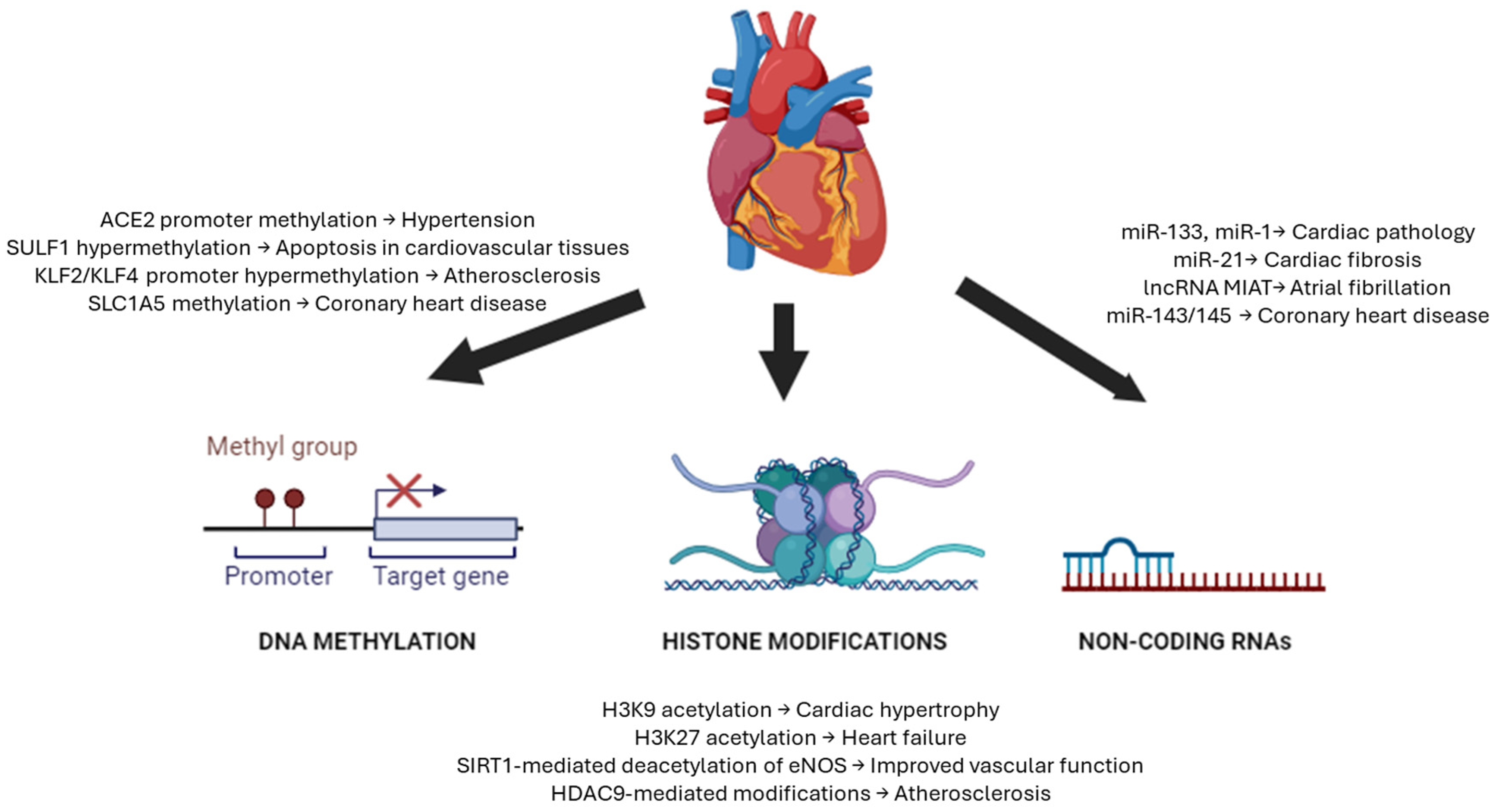
2. Epigenetics in Cardiovascular Disorders
2.1. DNA Methylation in Cardiovascular Disorders
| Diseases | Target | Mechanism | Reference |
|---|---|---|---|
| Coronary heart disease | cg22304262 | Alters SLC1A5 amino acid transporter expression | [44] |
| Global DNA | Hypermethylation correlates with increased disease risk | [44,45] | |
| cg04988978 | Modulates MPO expression affecting vascular inflammation | [54] | |
| Acute myocardial infarction (MI) | Global DNA | Differential methylation of genes involved in MI pathways | [44] |
| Heart failure | DNMT3a | Impairs cardiomyocyte metabolism and contractility | [55] |
| CTGF, MMP2, miRNA-155, HEY2, MSR1, MYOM3, COX17, miRNA-24-1 | Alters methylation patterns affecting cardiac remodeling | [56] | |
| KCNA4, KCNIP4, SMOC2 | Regulates cardiac ion channel function | [57] | |
| DNMT2, glutathione peroxidase 1 | Mediates oxidative stress response in cardiomyocytes | [58] | |
| Vascular calcification | DNMT3b, H19 | Promotes osteogenic transdifferentiation of VSMCs | [59] |
| G3BP1 | Mediates Wnt signaling in arterial calcification | [60] | |
| SM22a | Regulates VSMC phenotypic switching during calcification | [61] | |
| Hypertension | mitochondrial fusion 2 | Regulates mitochondrial dynamics affecting vascular function | [62] |
| Interferon | Modulates immune response in essential hypertension | [63] |
2.2. Histone Modifications in Cardiovascular Disorders
2.3. Non-Coding RNAs in Cardiovascular Disorders
3. Treatment of Cardiovascular Disorders with Epibioproducts
3.1. Natural DNA Methylation Modifiers
3.1.1. Polyphenols and Flavonoids
3.1.2. Folic Acid and Other B Vitamins as Methyl Donors
3.1.3. RCI-1502
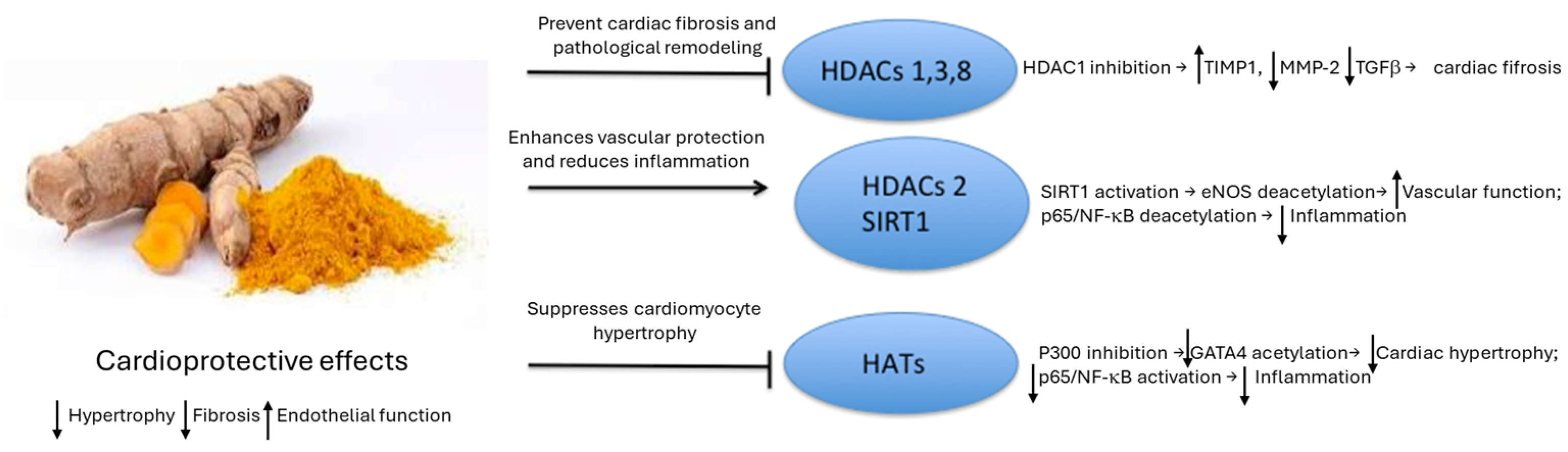
3.2. Natural Compounds Regulating Histone Modifications
Natural HDAC and HAT Modifiers
3.3. Natural Non-Coding RNA Modifiers
3.3.1. Natural miRNAs Modifiers
3.3.2. Natural LncRNAs Modifiers
3.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Kamato, D.; Little, P.J.; Nakagawa, S.; Pelisek, J.; Jin, Z.G. Targeting epigenetics and non-coding RNAs in atherosclerosis: From mechanisms to therapeutics. Pharmacol. Ther. 2019, 196, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Chen, L.; Qian, Z.; Xia, H.; Zhang, Z.; Zhang, J.; Wang, C.; Vaughn, M.G.; Tabet, M.; Lin, H. Ranking age-specific modifiable risk factors for cardiovascular disease and mortality: Evidence from a population-based longitudinal study. eClinicalMedicine 2023, 64, 102230. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Albert, M.A.; Elkind, M. The American Heart Association’s Focus on Primordial Prevention. Circulation 2021, 144, E233–E235. [Google Scholar] [CrossRef]
- Wołowiec, A.; Wołowiec, Ł.; Grześk, G.; Jaśniak, A.; Osiak, J.; Husejko, J.; Kozakiewicz, M. The Role of Selected Epigenetic Pathways in Cardiovascular Diseases as a Potential Therapeutic Target. Int. J. Mol. Sci. 2023, 24, 13723. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef]
- Gladwell, L.R.; Ahiarah, C.; Rasheed, S.; Rahman, S.M.; Choudhury, M. Traditional Therapeutics and Potential Epidrugs for CVD: Why Not Both? Life 2023, 14, 23. [Google Scholar] [CrossRef]
- Liu, X.; Pei, J.; Li, J.; Zhu, H.; Zheng, X.; Zhang, X.; Ruan, B.; Chen, L. Recent Advances in Resveratrol Derivatives: Structural Modifications and Biological Activities. Molecules 2025, 30, 958. [Google Scholar] [CrossRef]
- Bird, A. The essentials of DNA methylation. Cell 1992, 70, 5–8. [Google Scholar] [CrossRef]
- Duan, L.; Liu, C.; Hu, J.; Liu, Y.; Wang, J.; Chen, G.; Li, Z.; Chen, H. Epigenetic mechanisms in coronary artery disease: The current state and prospects. Trends Cardiovasc. Med. 2017, 28, 311–319. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun-Waterhouse, D.; Chen, Y.; Li, F.; Li, D. Epigenetic mechanisms underlying the benefits of flavonoids in cardiovascular health and diseases: Are long non-coding RNAs rising stars? Crit. Rev. Food Sci. Nutr. 2021, 62, 3855–3872. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Koromani, F.; Portilla, E.; O’Connor, A.; Bramer, W.M.; Troup, J.; Chowdhury, R.; Dehghan, A.; Franco, O.H. The role of epigenetic modifications in cardiovascular disease: A systematic review. Int. J. Cardiol. 2016, 212, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Pasipoularides, A. Implementing genome-driven personalized cardiology in clinical practice. J. Mol. Cell. Cardiol. 2018, 115, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Libby, P.; Kishore, R.; Tardif, J.-C.; El-Osta, A.; Paneni, F. Epigenetics and precision medicine in cardiovascular patients: From basic concepts to the clinical arena. Eur. Heart J. 2018, 39, 4150–4158. [Google Scholar] [CrossRef]
- van der Harst, P.; de Windt, L.J.; Chambers, J.C. Translational Perspective on Epigenetics in Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Williams-Ignarro, S.; de Nigris, F.; Lerman, L.O.; Rossi, L.; Guarino, C.; Mansueto, G.; Di Tuoro, F.; Pignalosa, O.; De Rosa, G.; et al. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 8797–8802, Erratum in: Proc. Natl. Acad Sci. USA 2004, 101, 18262. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Blazina, I.; Daeges, M.; Jeanne, T.L. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 316, 2008–2024, Erratum in: JAMA 2020, 323, 669. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Treatment of cardiovascular pathology with epigenetically active agents: Focus on natural and synthetic inhibitors of DNA methylation and histone deacetylation. Int. J. Cardiol. 2017, 227, 66–82. [Google Scholar] [CrossRef]
- Johnston, T.; Korolenko, T.; Pirro, M.; Sahebkar, A. Preventing cardiovascular heart disease: Promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol. Res. 2017, 120, 219–225. [Google Scholar] [CrossRef]
- Szczepańska, E.; Białek-Dratwa, A.; Janota, B.; Kowalski, O. Dietary Therapy in Prevention of Cardiovascular Disease (CVD)-Tradition or Modernity? A Review of the Latest Approaches to Nutrition in CVD. Nutrients 2022, 14, 2649. [Google Scholar] [CrossRef]
- Jiang, Y.-Z.; Jiménez, J.M.; Ou, K.; McCormick, M.E.; Zhang, L.-D.; Davies, P.F. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ. Res. 2014, 115, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Silva, G.D.B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Peng, C.; Huang, L.; Luo, X.; Mao, Q.; Wu, S.; Zhang, H. EGCG prevents pressure overload-induced myocardial remodeling by downregulating overexpression of HDAC5 in mice. Int. J. Mol. Med. 2021, 49, 11. [Google Scholar] [CrossRef]
- Wang, X.; Teng, X.; Luo, C.; Kong, L. Mechanisms and Advances of Epigenetic Regulation in Cardiovascular Disease. Front. Biosci. 2024, 29, 205. [Google Scholar] [CrossRef]
- Bontempo, P.; Capasso, L.; De Masi, L.; Nebbioso, A.; Rigano, D. Therapeutic Potential of Natural Compounds Acting through Epigenetic Mechanisms in Cardiovascular Diseases: Current Findings and Future Directions. Nutrients 2024, 16, 2399. [Google Scholar] [CrossRef]
- Soflaei, S.S.; Momtazi-Borojeni, A.A.; Majeed, M.; Derosa, G.; Maffioli, P.; Sahebkar, A. Curcumin: A Natural Pan-HDAC Inhibitor in Cancer. Curr. Pharm. Des. 2018, 24, 123–129. [Google Scholar] [CrossRef]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Lee, W.J.; Shim, J.-Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Ciesielski, O.; Biesiekierska, M.; Balcerczyk, A. Epigallocatechin-3-gallate (EGCG) Alters Histone Acetylation and Methylation and Impacts Chromatin Architecture Profile in Human Endothelial Cells. Molecules 2020, 25, 2326. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Hawsawi, Y.M.; Alzahrani, F.; Khan, M.I. Epigenetic regulation of inflammation: The metabolomics connection. Semin. Cell Dev. Biol. 2022, 154 Pt C, 355–363. [Google Scholar] [CrossRef]
- Dan, J.; Chen, T. Genetic Studies on Mammalian DNA Methyltransferases. Adv. Exp. Med. Biol. 2022, 1389, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Demetriadou, C.; Koufaris, C.; Kirmizis, A. Histone N-alpha terminal modifications: Genome regulation at the tip of the tail. Epigenetics Chromatin 2020, 13, 29. [Google Scholar] [CrossRef]
- He, M.; Han, Z.; Liu, L.; Zheng, Y.G. Chemical Biology Approaches for Investigating the Functions of Lysine Acetyltransferases. Angew. Chem. Int. Ed. Engl. 2017, 57, 1162–1184. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 29, 402. [Google Scholar]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA biogenesis, mechanisms of action and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as biomarkers in disease:latest findings regarding their role in diagnosis and prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Roy, B.; Lee, E.; Li, T.; Rampersaud, M. Role of miRNAs in Neurodegeneration: From disease cause to tools of biomarker discovery and therapeutics. Genes 2022, 13, 425. [Google Scholar] [CrossRef]
- Iacomino, G. miRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Naidoo, V.; Corzo, L.; Carrera, I.; Seoane, S.; Rodríguez, S.; Alcaraz, M.; Muñiz, A.; Cacabelos, N.; Cacabelos, R. Proteomic and Global DNA Methylation Modulation in Lipid Metabolism Disorders with a Marine-Derived Bioproduct. Biology 2023, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Domingo-Relloso, A.; Subedi, P.; Riffo-Campos, A.L.; Xia, R.; Gomez, L.; Haack, K.; Goldsmith, J.; Howard, B.V.; Best, L.G.; et al. Blood DNA Methylation and Incident Coronary Heart Disease: Evidence From the Strong Heart Study. JAMA Cardiol. 2021, 6, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Palou-Márquez, G.; Subirana, I.; Nonell, L.; Fernández-Sanlés, A.; Elosua, R. DNA methylation and gene expression integration in cardiovascular disease. Clin. Epigenetics 2021, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hu, Y.; Shen, J.; Liu, X.; Wang, T.; Li, L.; Li, J. Integrative analysis of DNA methylation and gene expression reveals key molecular signatures in acute myocardial infarction. Clin. Epigenetics 2022, 14, 46. [Google Scholar] [CrossRef]
- Halldorsson, B.V.; Eggertsson, H.P.; Moore, K.H.S.; Hauswedell, H.; Eiriksson, O.; Ulfarsson, M.O.; Palsson, G.; Hardarson, M.T.; Oddsson, A.; Jensson, B.O.; et al. The sequences of 150,119 genomes in the UK Biobank. Nature 2022, 607, 732–740. [Google Scholar] [CrossRef]
- GTEx Consortium; Laboratory, Data Analysis & Coordinating Center (LDACC)—Analysis Working Group; Statistical Methods groups—Analysis Working Group; Enhancing GTEx (eGTEx) groups; NIH Common Fund; NIH/NCI; NIH/NHGRI; NIH/NIMH; NIH/NIDA; Biospecimen Collection Source Site—NDRI; et al. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213, Erratum in: Nature 2018, 553, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, J.; Li, J.; Zhang, Y.; Zhou, Q.; Xu, F. DNA Methylation in Atherosclerosis: A New Perspective. Evid. Based Complement. Altern. Med. 2021, 2021, 6623657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Vikram, A.; Hoffman, T.A.; Naqvi, A.; Lewarchik, C.M.; Kim, Y.-R.; Irani, K. Histone and DNA methylation-mediated epigenetic downregulation of endothelial Kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1936–1942. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; Liu, S.; Liu, D.; Li, Y.; Liu, M.; Zhang, X.; Yan, C.; Han, Y. DNA hypermethylation: A novel mechanism of CREG gene suppression and atherosclerogenic endothelial dysfunction. Redox Biol. 2020, 32, 101444. [Google Scholar] [CrossRef]
- Luan, Y.; Liu, H.; Luan, Y.; Yang, Y.; Yang, J.; Ren, K.-D. New Insight in HDACs: Potential Therapeutic Targets for the Treatment of Atherosclerosis. Front. Pharmacol. 2022, 13, 863677. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, C.; Yang, Y.; Sun, F.; Zhang, Y.; Wang, H.; Liu, R.; Yuan, M. DNA methylation and histone post-translational modifications in atherosclerosis and a novel perspective for epigenetic therapy. Cell Commun. Signal. 2023, 21, 344. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-B.; Zhang, H.-P.; Cao, C.-J.; Wang, Y.-H.; Tian, J.; Yang, X.-L.; Yang, A.-N.; Wang, J.; Jiang, Y.-D.; Xu, H. Aberrant DNA methylation of the PDGF gene in homocysteine-mediated VSMC proliferation and its underlying mechanism. Mol. Med. Rep. 2014, 10, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, W.; Qu, K.; Lin, X.; Zeng, Z.; Chen, J.; Wei, D.; Wang, Z. TET2: A Novel Epigenetic Regulator and Potential Intervention Target for Atherosclerosis. DNA Cell Biol. 2018, 37, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.; Maghzal, G.J.; Talib, J.; Rashid, I.; Lau, A.K.; Stocker, R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2016, 22, 51–73. [Google Scholar] [CrossRef]
- Madsen, A.; Höppner, G.; Krause, J.; Hirt, M.N.; Laufer, S.D.; Schweizer, M.; Tan, W.L.W.; Mosqueira, D.; Anene-Nzelu, C.G.; Lim, I.; et al. An Important Role for DNMT3A-Mediated DNA Methylation in Cardiomyocyte Metabolism and Contractility. Circulation 2020, 142, 1562–1578, Erratum in: Circulation 2021, 143, e830. https://doi.org/10.1161/CIR.0000000000000978. [Google Scholar] [CrossRef]
- Glezeva, N.; Moran, B.; Collier, P.; Moravec, C.S.; Phelan, D.; Donnellan, E.; Russell-Hallinan, A.; O’connor, D.P.; Gallagher, W.M.; Gallagher, J.; et al. Targeted DNA Methylation Profiling of Human Cardiac Tissue Reveals Novel Epigenetic Traits and Gene Deregulation Across Different Heart Failure Patient Subtypes. Circ. Heart Fail. 2019, 12, e005765. [Google Scholar] [CrossRef]
- Laugier, L.; Frade, A.F.; Ferreira, F.M.; Baron, M.A.; Teixeira, P.C.; Cabantous, S.; Ferreira, L.R.P.; Louis, L.; Rigaud, V.O.C.; Gaiotto, F.A.; et al. Whole-Genome Cardiac DNA Methylation Fingerprint and Gene Expression Analysis Provide New Insights in the Pathogenesis of Chronic Chagas Disease Cardiomyopathy. Clin. Infect. Dis. 2017, 65, 1103–1111. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Meng, X.; Kong, Y.; Li, Y.; Yang, C.; Guo, Y.; Yang, H.; Liu, Z.; Wang, F. Selenium Supplementation Improved Cardiac Functions by Suppressing DNMT2-Mediated GPX1 Promoter DNA Methylation in AGE-Induced Heart Failure. Oxidative Med. Cell. Longev. 2022, 2022, 5402997. [Google Scholar] [CrossRef]
- Dai, X.; Liu, S.; Cheng, L.; Huang, T.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Epigenetic Upregulation of H19 and AMPK Inhibition Concurrently Contribute to S-Adenosylhomocysteine Hydrolase Deficiency-Promoted Atherosclerotic Calcification. Circ. Res. 2022, 130, 1565–1582. [Google Scholar] [CrossRef]
- Ramachandran, B.; Stabley, J.N.; Cheng, S.-L.; Behrmann, A.S.; Gay, A.; Li, L.; Mead, M.; Kozlitina, J.; Lemoff, A.; Mirzaei, H.; et al. A GTPase-activating protein-binding protein (G3BP1)/antiviral protein relay conveys arteriosclerotic Wnt signals in aortic smooth muscle cells. J. Biol. Chem. 2018, 293, 7942–7968. [Google Scholar] [CrossRef] [PubMed]
- de Oca, A.M.; A Madueño, J.; Martinez-Moreno, J.M.; Guerrero, F.; Muñoz-Castañeda, J.; E Rodriguez-Ortiz, M.; Mendoza, F.J.; Almaden, Y.; Lopez, I.; Rodriguez, M.; et al. High-phosphate-induced calcification is related to SM22α promoter methylation in vascular smooth muscle cells. J. Bone Miner. Res. 2010, 25, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, X.; Wang, Z.; Liu, Y.; Liu, J.; Sun, D.; Jin, Y.; Wang, S.; Wen, S.; Wei, Y. Association of mitofusin 2 methylation and essential hypertension: A case-control study in a Chinese population. Hypertens. Res. 2018, 41, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.-J.; Mao, S.-Q.; Gu, T.-L.; Zheng, S.-Y.; Zhao, J.-S.; Zhang, L.-N. Hypomethylation of the Interferon γ Gene as a Potential Risk Factor for Essential Hypertension: A Case-Control Study. Tohoku J. Exp. Med. 2018, 244, 283–290. [Google Scholar] [CrossRef]
- Yang, Y.; Luan, Y.; Yuan, R.-X.; Luan, Y. Histone Methylation Related Therapeutic Challenge in Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 710053. [Google Scholar] [CrossRef]
- Funamoto, M.; Imanishi, M.; Tsuchiya, K.; Ikeda, Y. Roles of histone acetylation sites in cardiac hypertrophy and heart failure. Front. Cardiovasc. Med. 2023, 10, 1133611. [Google Scholar] [CrossRef] [PubMed]
- Papait, R.; Serio, S.; Pagiatakis, C.; Rusconi, F.; Carullo, P.; Mazzola, M.; Salvarani, N.; Miragoli, M.; Condorelli, G. Histone Methyltransferase G9a Is Required for Cardiomyocyte Homeostasis and Hypertrophy. Circulation 2017, 136, 1233–1246. [Google Scholar] [CrossRef]
- Kurozumi, A.; Nakano, K.; Yamagata, K.; Okada, Y.; Nakayamada, S.; Tanaka, Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone 2019, 124, 53–61. [Google Scholar] [CrossRef]
- Maleszewska, M.; Gjaltema, R.A.; Krenning, G.; Harmsen, M.C. Enhancer of zeste homolog-2 (EZH2) methyltransferase regulates transgelin/smooth muscle-22α expression in endothelial cells in response to interleukin-1β and transforming growth factor-β2. Cell. Signal. 2015, 27, 1589–1596. [Google Scholar] [CrossRef]
- Li, W.; Feng, W.; Su, X.; Luo, D.; Li, Z.; Zhou, Y.; Zhu, Y.; Zhang, M.; Chen, J.; Liu, B.; et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease. J. Clin. Investig. 2021, 132, e150051. [Google Scholar] [CrossRef]
- Abend, A.; Shkedi, O.; Fertouk, M.; Caspi, L.H.; Kehat, I. Salt-inducible kinase induces cytoplasmic histone deacetylase 4 to promote vascular calcification. Embo Rep. 2017, 18, 1166–1185. [Google Scholar] [CrossRef]
- Malhotra, R.; Mauer, A.C.; Cardenas, C.L.L.; Guo, X.; Yao, J.; Zhang, X.; Wunderer, F.; Smith, A.V.; Wong, Q.; Pechlivanis, S.; et al. HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat. Genet. 2019, 51, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Inglott, F.S.; Alexander, M.Y.; Weston, R. Suppression of SIRT1 in Diabetic Conditions Induces Osteogenic Differentiation of Human Vascular Smooth Muscle Cells via RUNX2 Signalling. Sci. Rep. 2019, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci. Signal. 2014, 7, ra106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mu, Y.; Zhou, X.; Ji, H.; Gao, X.; Cai, W.W.; Guan, Q.; Xu, T. SIRT2-mediated FOXO3a deacetylation drives its nuclear translocation triggering FasL-induced cell apoptosis during renal ischemia reperfusion. Apoptosis 2017, 22, 519–530. [Google Scholar] [CrossRef]
- Bochaton, T.; Crola-Da-Silva, C.; Pillot, B.; Villedieu, C.; Ferreras, L.; Alam, M.R.; Thibault, H.; Strina, M.; Gharib, A.; Ovize, M.; et al. Inhibition of myocardial reperfusion injury by ischemic postconditioning requires sirtuin 3-mediated deacetylation of cyclophilin D. J. Mol. Cell. Cardiol. 2015, 84, 61–69. [Google Scholar] [CrossRef]
- Leng, Y.; Wu, Y.; Lei, S.; Zhou, B.; Qiu, Z.; Wang, K.; Xia, Z. Inhibition of HDAC6 Activity Alleviates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats: Potential Role of Peroxiredoxin 1 Acetylation and Redox Regulation. Oxidative Med. Cell. Longev. 2018, 2018, 9494052. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.-F.; Wang, N.-Y.; Wang, X.-M.; Liang, S.-T.; Zheng, W.; Lu, Y.-B.; Zhao, X.; Hao, D.-L.; Zhang, Z.-Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Bindu, S.; Pillai, V.B.; Samant, S.; Pan, Y.; Huang, J.-Y.; Gupta, M.; Nagalingam, R.S.; Wolfgeher, D.; Verdin, E.; et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell. Biol. 2016, 36, 678–692. [Google Scholar] [CrossRef]
- Luo, Y.-X.; Tang, X.; An, X.-Z.; Xie, X.-M.; Chen, X.-F.; Zhao, X.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 2017, 38, 1389–1398. [Google Scholar] [CrossRef]
- Shen, P.; Feng, X.; Zhang, X.; Huang, X.; Liu, S.; Lu, X.; Li, J.; You, J.; Lu, J.; Li, Z.; et al. SIRT6 suppresses phenylephrine-induced cardiomyocyte hypertrophy though inhibiting p300. J. Pharmacol. Sci. 2016, 132, 31–40. [Google Scholar] [CrossRef]
- Collesi, C.; Felician, G.; Secco, I.; Gutierrez, M.I.; Martelletti, E.; Ali, H.; Zentilin, L.; Myers, M.P.; Giacca, M. Reversible Notch1 acetylation tunes proliferative signalling in cardiomyocytes. Cardiovasc. Res. 2017, 114, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Byeon, H.-E.; Seo, E.; Nguyen, Q.-A.T.; Lee, W.; Jeong, Y.; Choi, J.; Pandey, D.; Berkowitz, D.E.; Kim, J.H.; et al. Histone deacetylase 6 inhibitor tubastatin A attenuates angiotensin II-induced hypertension by preventing cystathionine γ-lyase protein degradation. Pharmacol. Res. 2019, 146, 104281. [Google Scholar] [CrossRef] [PubMed]
- Arise, K.K.; Kumar, P.; Garg, R.; Samivel, R.; Zhao, H.; Pandya, K.; Nguyen, C.; Lindsey, S.; Pandey, K.N. Angiotensin II represses Npr1 expression and receptor function by recruitment of transcription factors CREB and HSF-4a and activation of HDACs. Sci. Rep. 2020, 10, 4337. [Google Scholar] [CrossRef]
- Lugenbiel, P.; Govorov, K.; Syren, P.; Rahm, A.-K.; Wieder, T.; Wunsch, M.; Weiberg, N.; Manolova, E.; Gramlich, D.; Rivinius, R.; et al. Epigenetic regulation of cardiac electrophysiology in atrial fibrillation: HDAC2 determines action potential duration and suppresses NRSF in cardiomyocytes. Basic Res. Cardiol. 2021, 116, 13. [Google Scholar] [CrossRef]
- Hall, I.F.; Climent, M.; Anselmi, C.V.; Papa, L.; Tragante, V.; Lambroia, L.; Farina, F.M.; E Kleber, M.; März, W.; Biguori, C.; et al. rs41291957 controls miR-143 and miR-145 expression and impacts coronary artery disease risk. EMBO Mol. Med. 2021, 13, e14060. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2020, 72, 156–166. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Li, Y.-S.; Wu, C.-C.; Wang, K.-C.; Huang, T.-C.; Chen, Z.; Chien, S. Extracellular MicroRNA-92a Mediates Endothelial Cell-Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Jones, M.; Thomas, B.J.; Wu, X.; Gilliland, T.; Qian, K.; Eskin, A.; Casero, D.; Zhang, Z.; Sandhu, J.; et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 2018, 24, 304–312. [Google Scholar] [CrossRef]
- Hu, Y.-W.; Guo, F.-X.; Xu, Y.-J.; Li, P.; Lu, Z.-F.; McVey, D.G.; Zheng, L.; Wang, Q.; Ye, J.H.; Kang, C.-M.; et al. Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J. Clin. Investig. 2019, 129, 1115–1128. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, J.-L.; Peng, Z.-Y.; Xu, W.-F.; Yu, G.-L. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020, 11, 317, Erratum in: Cell Death Dis. 2020, 11, 791. https://doi.org/10.1038/s41419-020-02996-8. Erratum in: Cell Death Dis. 2020, 11, 845. https://doi.org/10.1038/s41419-020-03025-4. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Mai, Z.; Zhu, X.; Wu, T.; Chen, Y.; Geng, D.; Wang, J. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Xu, B.; Liu, Y.; Liu, Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J. Med Sci. 2018, 34, 626–633. [Google Scholar] [CrossRef]
- Piccoli, M.-T.; Gupta, S.K.; Viereck, J.; Foinquinos, A.; Samolovac, S.; Kramer, F.L.; Garg, A.; Remke, J.; Zimmer, K.; Batkai, S.; et al. Inhibition of the Cardiac Fibroblast-Enriched lncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ. Res. 2017, 121, 575–583. [Google Scholar] [CrossRef]
- Micheletti, R.; Plaisance, I.; Abraham, B.J.; Sarre, A.; Ting, C.-C.; Alexanian, M.; Maric, D.; Maison, D.; Nemir, M.; Young, R.A.; et al. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci. Transl. Med. 2017, 9, eaai9118. [Google Scholar] [CrossRef]
- Ma, W.-Q.; Sun, X.-J.; Wang, Y.; Zhu, Y.; Han, X.-Q.; Liu, N.-F. Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis. Mol. Cell. Endocrinol. 2019, 479, 39–53. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Li, F.; Ji, Q. MiR-128-3p accelerates cardiovascular calcification and insulin resistance through ISL1-dependent Wnt pathway in type 2 diabetes mellitus rats. J. Cell. Physiol. 2018, 234, 4997–5010. [Google Scholar] [CrossRef]
- Chen, R.; Qiu, H.; Tong, Y.; Liao, F.; Hu, X.; Qiu, Y.; Liao, Y. MiRNA-19a-3p alleviates the progression of osteoporosis by targeting HDAC4 to promote the osteogenic differentiation of hMSCs. Biochem. Biophys. Res. Commun. 2019, 516, 666–672. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Xie, F.; Guo, S.; Liu, F.; Dong, N.; Wang, Y. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc. Res. 2018, 114, 168–179. [Google Scholar] [CrossRef]
- Jeong, G.; Kwon, D.-H.; Shin, S.; Choe, N.; Ryu, J.; Lim, Y.-H.; Kim, J.; Park, W.J.; Kook, H.; Kim, Y.-K. Long noncoding RNAs in vascular smooth muscle cells regulate vascular calcification. Sci. Rep. 2019, 9, 5848. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhan, J.-F.; Chen, Y.-X.; Xu, C.-Y. LncRNA-SNHG29 inhibits vascular smooth muscle cell calcification by downregulating miR-200b-3p to activate the α-Klotho/FGFR1/FGF23 axis. Cytokine 2020, 136, 155243. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cui, X.; Zhan, J.; Wang, Y.; Li, S.; Lin, X.; Xiang, Q.; Ni, Y.; Liu, L.; Liu, Y. LncRNA-ES3 inhibition by Bhlhe40 is involved in high glucose-induced calcification/senescence of vascular smooth muscle cells. Ann. N. Y. Acad. Sci. 2020, 1474, 61–72. [Google Scholar] [CrossRef]
- Wang, Y.; Lumbers, E.R.; Arthurs, A.L.; de Meaultsart, C.C.; Mathe, A.; A Avery-Kiejda, K.; Roberts, C.T.; Pipkin, F.B.; Marques, F.Z.; Morris, B.J.; et al. Regulation of the human placental (pro)renin receptor-prorenin-angiotensin system by microRNAs. Mol. Hum. Reprod. 2018, 24, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qi, F.; Wei, S.; Lin, L.; Liu, X. The Transcription Factor C/EBPβ Promotes HFL-1 Cell Migration, Proliferation, and Inflammation by Activating lncRNA HAS2-AS1 in Hypoxia. Front. Cell Dev. Biol. 2021, 9, 651913. [Google Scholar] [CrossRef]
- Fang, G.; Qi, J.; Huang, L.; Zhao, X. LncRNA MRAK048635_P1 is critical for vascular smooth muscle cell function and phenotypic switching in essential hypertension. Biosci. Rep. 2019, 39, BSR20182229. [Google Scholar] [CrossRef]
- Costantino, S.; Akhmedov, A.; Melina, G.; A Mohammed, S.; Othman, A.; Ambrosini, S.; Wijnen, W.J.; Sada, L.; Ciavarella, G.M.; Liberale, L.; et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur. Heart J. 2019, 40, 997–1008. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Wang, Z.; Wu, Y.; Chen, J.; Wang, G.; Lu, C.; Jia, W.; Xi, J.; Zhu, S.; et al. A Linc1405/Eomes Complex Promotes Cardiac Mesoderm Specification and Cardiogenesis. Cell Stem Cell 2018, 22, 893–908.e6. [Google Scholar] [CrossRef]
- Cai, B.; Ma, W.; Ding, F.; Zhang, L.; Huang, Q.; Wang, X.; Hua, B.; Xu, J.; Li, J.; Bi, C.; et al. The Long Noncoding RNA CAREL Controls Cardiac Regeneration. J. Am. Coll. Cardiol. 2018, 72, 534–550. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Shen, D.; Ge, D.; Chen, J.; Pei, J.; Li, Y.; Yue, Z.; Feng, J.; Chu, M.; et al. A long noncoding RNA NR_045363 controls cardiomyocyte proliferation and cardiac repair. J. Mol. Cell. Cardiol. 2019, 127, 105–114. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Li, X.; Jin, G.; Chen, X.; Chen, G.; Chen, Y.; Huang, S.; Liao, W.; Liao, Y.; et al. Sirt1 Antisense Long Noncoding RNA Promotes Cardiomyocyte Proliferation by Enhancing the Stability of Sirt1. J. Am. Heart Assoc. 2018, 7, e009700. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, B.; Liu, S.; Miao, C.; Li, Y. Inhibition of the LncRNA Gpr19 attenuates ischemia-reperfusion injury after acute myocardial infarction by inhibiting apoptosis and oxidative stress via the miR-324-5p/Mtfr1 axis. IUBMB Life 2019, 72, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-S.; Zhou, J.; Li, X. LncRNA UCA1 protects cardiomyocytes against hypoxia/reoxygenation induced apoptosis through inhibiting miR-143/MDM2/p53 axis. Genomics 2020, 112, 574–580. [Google Scholar] [CrossRef]
- Yao, L.; Zhou, B.; You, L.; Hu, H.; Xie, R. LncRNA MIAT/miR-133a-3p axis regulates atrial fibrillation and atrial fibrillation-induced myocardial fibrosis. Mol. Biol. Rep. 2020, 47, 2605–2617. [Google Scholar] [CrossRef]
- Honer, M.A.; Ferman, B.I.; Gray, Z.H.; Bondarenko, E.A.; Whetstine, J.R. Epigenetic modulators provide a path to understanding disease and therapeutic opportunity. Genes Dev. 2024, 38, 473–503. [Google Scholar] [CrossRef]
- Gorica, E.; Mohammed, S.A.; Ambrosini, S.; Calderone, V.; Costantino, S.; Paneni, F. Epi-Drugs in Heart Failure. Front. Cardiovasc. Med. 2022, 9, 923014. [Google Scholar] [CrossRef]
- Ivanov, M.; Barragan, I.; Ingelman-Sundberg, M. Epigenetic mechanisms of importance for drug treatment. Trends Pharmacol. Sci. 2014, 35, 384–396. [Google Scholar] [CrossRef]
- Babu, E.; Ramachandran, S.; CoothanKandaswamy, V.; Elangovan, S.; Prasad, P.D.; Ganapathy, V.; Thangaraju, M. Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene 2011, 30, 4026–4037. [Google Scholar] [CrossRef]
- Nagai, M.; Conney, A.H.; Zhu, B.T. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2004, 32, 497–504. [Google Scholar] [CrossRef]
- Sánchez-Del-Campo, L.; Otón, F.; Tárraga, A.; Cabezas-Herrera, J.; Chazarra, S.; Rodríguez-López, J.N. Synthesis and biological activity of a 3,4,5-trimethoxybenzoyl ester analogue of epicatechin-3-gallate. J. Med. Chem. 2008, 51, 2018–2026. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Thakur, V.S.; Gupta, K. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int. J. Oncol. 2012, 41, 353–361. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and cardiovascular disease. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Misra, B.R.; Mahaney, J.E.; Li, Y.; Misra, H.P. EPR studies on the superoxide-scavenging capacity of the nutraceutical resveratrol. Mol. Cell. Biochem. 2008, 313, 187–194. [Google Scholar] [CrossRef]
- Holthoff, J.H.; Woodling, K.A.; Doerge, D.R.; Burns, S.T.; Hinson, J.A.; Mayeux, P.R. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem. Pharmacol. 2010, 80, 1260–1265. [Google Scholar] [CrossRef]
- Wang, J.; He, D.; Zhang, Q.; Han, Y.; Jin, S.; Qi, F. Resveratrol protects against Cisplatin-induced cardiotoxicity by alleviating oxidative damage. Cancer Biother. Radiopharm. 2009, 24, 675–680. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Sea, H. Resveratrol affects histone 3 lysine 27 methylation of vessels and blood biomarkers in DOCA salt-induced hypertension. Mol. Biol. Rep. 2015, 42, 35–42. [Google Scholar]
- Ma, S.C.; Zhang, H.P.; Jiao, Y.; Wang, Y.H.; Zhang, H.; Yang, X.L.; Yang, A.N.; Jiang, Y.D. Homocysteine-induced proliferation of vascular smooth muscle cells occurs via PTEN hypermethylation and is mitigated by Resveratrol. Mol. Med. Rep. 2018, 17, 5312–5319. [Google Scholar] [CrossRef]
- Mea, B. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol. Life Sci. 2014, 69, 2245–2260. [Google Scholar]
- Aea, B. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press. 2010, 19, 196–205. [Google Scholar]
- Akhondzadeh, F. Resveratrol suppresses interleukin-6 expression through activation of sirtuin 1 in hypertrophied H9c2 cardiomyoblasts. J. Cell. Physiol. 2020, 235, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Ma, J.-Q.; Xie, W.-R.; Liu, S.-S.; Feng, Z.-J.; Zheng, G.-H.; Wang, A.-M. Quercetin promtects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NFkB pathway. Food Chem. Toxicol. 2015, 82, 19–26. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Effects of Puerarin on the Prevention and Treatment of Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 771793. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.-K.; Lin, J.; Fuchs, J.R.; Marcucci, G.; et al. Curcumin is a potent DNA hypomethylation agent. Bioorganic Med. Chem. Lett. 2008, 19, 706–709. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; López-Vallejo, F.; Kuck, D.; Lyko, F. Natural products as DNA methyltransferase inhibitors: A computer-aided discovery approach. Mol. Divers. 2010, 15, 293–304. [Google Scholar] [CrossRef]
- Bautista-Garcia, P.; González-López, L.; González-Esparza, B.; Del Castillo-Rosas, C. Effect of bioactive nutriments in health and disease: The role of epigenetic modifications. Funct. Foods 2017, 7, 12. [Google Scholar]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef]
- McNulty, H.; Pentieva, K.; Hoey, L.; Ward, M. Homocysteine, B-vitamins and CVD. Proc. Nutr. Soc. 2008, 67, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Naidoo, V.; Corzo, L.; Pego, R.; Seoane, S.; Rodríguez, S.; Alcaraz, M.; Muñiz, A.; Cacabelos, N.; Cacabelos, R. DNA Methylation as a Biomarker for Monitoring Disease Outcome in Patients with Hypovitaminosis and Neurological Disorders. Genes 2023, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Kuo, B.I.-T.; Kong, C.-W.; Chau, W.-K.; Hsu, H.-C.; Gau, J.-P.; Yu, Y.-B. Influence of methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism, B vitamins and other factors on plasma homocysteine and risk of thromboembolic disease in Chinese. J. Chin. Med. Assoc. 2005, 68, 560–565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Qin, X.; Demirtas, H.; Li, J.; Mao, G.; Huo, Y.; Sun, N.; Liu, L.; Xu, X. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar] [CrossRef]
- Lombardi, V.R.M.; Cacabelos, R. E-SAR-94010: A marine fish extract obtained by advanced biotechnological methods. Drugs Future 1999, 24, 167–176. [Google Scholar] [CrossRef]
- Lombardi, V.R.; Cagiao, A.; Fernández-Novoa, L.; Álvarez, X.A.; Corzo, M.D.; Zas, R.; Sampedro, C.; Cacabelos, R. Short-term food supplementation effects of a fish-derived extract on the immunological status of pregnant rats and their sucking pups. Nutr. Res. 2001, 21, 1425–1434. [Google Scholar] [CrossRef]
- Corzo, L.; Fernández-Novoa, L.; Carrera, I.; Martínez, O.; Rodríguez, S.; Alejo, R.; Cacabelos, R. Nutrition, Health, and Disease: Role of Selected Marine and Vegetal Nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar] [CrossRef]
- Rizos, E.C.; Elisaf, M.S. Does Supplementation with Omega-3 PUFAs Add to the Prevention of Cardiovascular Disease? Curr. Cardiol. Rep. 2017, 19, 47. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: Present, past and future. Expert Rev. Clin. Pharmacol. 2017, 10, 865–873. [Google Scholar] [CrossRef]
- Zivkovic, S.; Maric, G.; Cvetinovic, N.; Lepojevic-Stefanovic, D.; Cvijan, B.B. Anti-Inflammatory Effects of Lipid-Lowering Drugs and Supplements-A Narrative Review. Nutrients 2023, 15, 1517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelwahab, A.H.; Negm, A.M.; Mahmoud, E.S.; Salama, R.M.; Schaalan, M.F.; El-Sheikh, A.A.K.; Ramadan, B.K. The cardioprotective effects of secoisolariciresinol diglucoside (flaxseed lignan) against cafeteria diet-induced cardiac fibrosis and vascular injury in rats: An insight into apelin/AMPK/FOXO3a signaling pathways. Front. Pharmacol. 2023, 14, 1199294. [Google Scholar] [CrossRef] [PubMed]
- Den Ruijter, H.M.; Berecki, G.; Opthof, T.; Verkerk, A.O.; Zock, P.L.; Coronel, R. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc. Res. 2007, 73, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, J.T.; Soliman, E.Z.; Pester, J.; Danik, J.S.; Gomelskya, N.; Copeland, T.; Lee, I.-M.; Buring, J.E.; Manson, J.E.; Cook, N.R.; et al. A randomized clinical trial of omega-3 fatty acid and vitamin D supplementation on electrocardiographic risk profiles. Sci. Rep. 2023, 13, 11454. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fu, arate and succinate thata are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- Carrera, I.; Corzo, L.; Naidoo, V.; Martínez-Iglesias, O.; Cacabelos, R. Cardiovascular and lipid-lowering effects of a marine lipoprotein extract in a high-fat diet-induced obesity mouse model. Int. J. Med. Sci. 2023, 20, 292–306. [Google Scholar] [CrossRef]
- Karimi, M.; Vedin, I.; Levi, Y.F.; Basun, H.; Irving, G.F.; Eriksdotter, M.; Wahlund, L.-O.; Schultzberg, M.; Hjorth, E.; Cederholm, T.; et al. DNA-rich n-3 fatty acid supplementation decreases DNA methylation in blood leukocytes: The omegAD study. Am. J. Clin. Nutr. 2017, 106, 1157–1165. [Google Scholar] [CrossRef]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Jiang, S.; Han, J.; Li, T.; Xin, Z.; Ma, Z.; Di, W.; Hu, W.; Gong, B.; Di, S.; Wang, D.; et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol. Res. 2017, 119, 373–383. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Trepel, J.; Lee, S.; Chung, E.-J.; Lee, M.-J.; Jung, Y.-J.; Marcu, M.G. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.Q.; Shehadeh, L.A.; Mitrani, J.M.; Pessanha, M.; Slepak, T.I.; Webster, K.A.; Bishopric, N.H. Quantitative control of adaptive cardiac hypertrophy by acetyltransferase p300. Circulation 2008, 118, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Gusterson, R.J.; Jazrawi, E.; Adcock, I.M.; Latchman, D.S. The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J. Biol. Chem. 2003, 278, 6838–6847. [Google Scholar] [CrossRef]
- Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Takaya, T.; Wada, H.; Nagasawa, A.; Komeda, M.; Fujita, M.; Shimatsu, A.; Kita, T.; et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Investig. 2008, 118, 868–878. [Google Scholar] [CrossRef]
- Pittala, V.; Vanella, L.; Salerno, L.; Romeo, G.; Marrazzo, A.; Di Giacomo, C.; Sorrenti, V. Effects of Polyphenolic Derivatives on Heme Oxygenase-System in Metabolic Dysfunctions. Curr. Med. Chem. 2018, 25, 1577–1595. [Google Scholar] [CrossRef]
- Monfoulet, L.-E.; Mercier, S.; Bayle, D.; Tamaian, R.; Barber-Chamoux, N.; Morand, C.; Milenkovic, D. Curcumin modulates endothelial permeability and monocyte transendothelial migration by affecting endothelial cell dynamics. Free Radic. Biol. Med. 2017, 112, 109–120. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Funamoto, M.; Sono, S.; Shimizu, K.; Shimizu, S.; Genpei, M.; Miyazaki, Y.; Katanasaka, Y.; Morimoto, E.; Ueno, M.; et al. Curcumin and its demethoxy derivatives possess p300 HAT inhibitory activity and suppress hypertrophic responses in cardiomyocytes. J. Pharmacol. Sci. 2018, 136, 212–217. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, J.; Gao, L.; Zhang, Y.; Dai, M.; Bao, M. Dickkopf-3 upregulation mediates the cardioprotective effects of curcumin on chronic heart failure. Mol. Med. Rep. 2018, 17, 7249–7257. [Google Scholar] [CrossRef]
- Bora-Tatar, G.; Dayangaç-Erden, D.; Demir, A.S.; Dalkara, S.; Yelekçi, K.; Erdem-Yurter, H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Bioorg. Med. Chem. 2009, 17, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Meja, K.K.; Rajendrasozhan, S.; Adenuga, D.; Biswas, S.K.; Sundar, I.K.; Spooner, G.; Marwick, J.A.; Chakravarty, P.; Fletcher, D.E.; Whittaker, P.; et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 2008, 39, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shen, T.; Xie, J.; Wang, S.; He, Y.; Zhu, F. Curcumin modulates covalent histone modification and TIMP1 gene activation to protect against vascular injury in a hypertension rat model. Exp. Ther. Med. 2017, 14, 5896–5902. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2010, 22, 450–458. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, Z.-L.; Qin, Z.-H.; Liang, Z.-Q. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol. Sin. 2008, 29, 800–807. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef]
- Huang, Z.; Ye, B.; Dai, Z.; Wu, X.; Lu, Z.; Shan, P.; Huang, W. Curcumin inhibits autophagy and apoptosis in hypoxia/reoxygenation-induced myocytes. Mol. Med. Rep. 2015, 11, 4678–4684. [Google Scholar] [CrossRef]
- Ji, X.; Xiao, J.; Sheng, X.; Zhang, X.; Guo, M. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des. Dev. Ther. 2016, 10, 1267–1277, Erratum in: Drug Des. Dev. Ther. 2022, 16, 343–344. https://doi.org/10.2147/DDDT.S355505. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free. Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: Inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Magerko, K.A.; Lawson, B.R.; Durrant, J.R.; Lesniewski, L.A.; Seals, D.R. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011, 589 Pt 18, 4545–4554. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am. J. Physiol. Circ. Physiol. 2015, 308, H424–H434. [Google Scholar] [CrossRef]
- Zerr, P.; Palumbo-Zerr, K.; Huang, J.; Tomcik, M.; Sumova, B.; Distler, O.; Schett, G.; Distler, J.H.W. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 2016, 75, 226–233. [Google Scholar] [CrossRef]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Chira, S.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef]
- Liu, C.-W.; Sung, H.-C.; Lin, S.-R.; Wu, C.-W.; Lee, C.-W.; Lee, I.-T.; Yang, Y.-F.; Yu, I.-S.; Chiang, M.-H.; Liang, C.-J.; et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci. Rep. 2017, 7, 44689. [Google Scholar] [CrossRef]
- Sui, X.-Q.; Xu, Z.-M.; Xie, M.-B.; Pei, D.-A. Resveratrol inhibits hydrogen peroxide-induced apoptosis in endothelial cells via the activation of PI3K/Akt by miR-126. J. Atheroscler. Thromb. 2014, 21, 108–118. [Google Scholar] [CrossRef]
- Breen, D.M.; Dolinsky, V.W.; Zhang, H.; Ghanim, H.; Guo, J.; Mroziewicz, M.; Tsiani, E.L.; Bendeck, M.P.; Dandona, P.; Dyck, J.R.; et al. Resveratrol inhibits neointimal formation after arterial injury through an endothelial nitric oxide synthase-dependent mechanism. Atherosclerosis 2012, 222, 375–381. [Google Scholar] [CrossRef]
- Campagnolo, P.; Hong, X.; di Bernardini, E.; Smyrnias, I.; Hu, Y.; Xu, Q. Resveratrol-Induced Vascular Progenitor Differentiation towards Endothelial Lineage via MiR-21/Akt/β-Catenin Is Protective in Vessel Graft Models. PLoS ONE 2015, 10, e0125122. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Mukherjee, S.; Ahsan, K.; Bagchi, A.; Pacher, P.; Das, D.K. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS ONE 2010, 5, e15705. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, S.; Wang, Y.-B.; Xu, B.; Zhao, H.; He, Y.-Y.; Li, C.-W.; Zhang, J.; Cao, Y.-K.; Feng, Q.-Z. Resveratrol exerts protective effects on anoxia/reoxygenation injury in cardiomyocytes via miR-34a/Sirt1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2734–2741. [Google Scholar] [PubMed]
- Boshra, S.A. Resveratrol Modulates miR-34a in Cardiotoxicity Induced by Isoproterenol. J. Med. Food 2020, 23, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Ong’achwa, M.J.; Ge, L.; Qian, Y.; Chen, L.; Hu, X.; Li, F.; Wei, H.; Zhang, C.; et al. Resveratrol Inhibits the TGF-β1-Induced Proliferation of Cardiac Fibroblasts and Collagen Secretion by Downregulating miR-17 in Rat. BioMed Res. Int. 2018, 2018, 8730593. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, L.; Fang, K.; Huang, T.; Wan, L.; Liu, Y.; Zhang, S.; Yan, D.; Li, G.; Gao, Y.; et al. Resveratrol Ameliorates Cardiac Hypertrophy by Down-regulation of miR-155 Through Activation of Breast Cancer Type 1 Susceptibility Protein. J. Am. Heart Assoc. 2016, 5, e002648. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.-J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, Y.; Shen, A.; Cai, W. Resveratrol upregulates SOCS1 production by lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting miR-155. Int. J. Mol. Med. 2016, 39, 231–237, Erratum in: Int. J. Mol. Med. 2024, 54, 62. https://doi.org/10.3892/ijmm.2024.5386. [Google Scholar] [CrossRef]
- Ma, S.; Fan, L.; Li, J.; Zhang, B.; Yan, Z. Resveratrol promoted the M2 polarization of microglia and reduced neuroinflammation after cerebral ischemia by inhibiting miR-155. Int. J. Neurosci. 2020, 130, 817–825. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ros, J.A.R.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Li, D.; Hao, W.; Meng, F.; Wang, B.; Han, J.; Zheng, Q. Kaempferide Protects against Myocardial Ischemia/Reperfusion Injury through Activation of the PI3K/Akt/GSK-3β Pathway. Mediat. Inflamm. 2017, 2017, 5278218. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Moh, S.H.; Kang, H. Kaempferol inhibits vascular smooth muscle cell migration by modulating BMP-mediated miR-21 expression. Mol. Cell. Biochem. 2015, 407, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qi, Z. MiR-21 mediates the protection of kaempferol against hypoxia/reoxygenation-induced cardiomyocyte injury via promoting Notch1/PTEN/AKT signaling pathway. PLoS ONE 2020, 15, e0241007. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.X.; Fang, Y.; Chen, X.; Xue, J. Therapeutic Potential of Quercetin as an Antiatherosclerotic Agent in Atherosclerotic Cardiovascular Disease: A Review. Evid Based Complement Altern. Med. 2020, 2020, 5926381. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol. Lett. 2017, 277, 115–122. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Noroozadeh, A.; Mohammadi, M.T.; Johnston, T.P.; Sahebkar, A. Crocin Improves Oxidative Stress by Potentiating Intrinsic Anti-Oxidant Defense Systems in Pancreatic Cells During Uncontrolled Hyperglycemia. J. Pharmacopunct. 2019, 22, 83–89. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, B.; Li, J.; Shi, J.; Chu, L.; Han, X.; Chu, X.; Zhang, X.; Zhang, J. Crocin ameliorates arsenic trioxide-induced cardiotoxicity via Keap1-Nrf2/HO-1 pathway: Reducing oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2020, 131, 110713. [Google Scholar] [CrossRef]
- Fu, B.-C.; Lang, J.-L.; Zhang, D.-Y.; Sun, L.; Chen, W.; Liu, W.; Liu, K.-Y.; Ma, C.-Y.; Jiang, S.-L.; Li, R.-K.; et al. Suppression of miR-34a Expression in the Myocardium Protects Against Ischemia-Reperfusion Injury Through SIRT1 Protective Pathway. Stem Cells Dev. 2017, 26, 1270–1282. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, B.; Cheng, B.; Liu, Y.; Zhang, B.; Wang, X.; Lin, X.; Yang, B.; Gong, G. Crocin Alleviates Myocardial Ischemia/Reperfusion-Induced Endoplasmic Reticulum Stress via Regulation of miR-34a/Sirt1/Nrf2 Pathway. Shock 2019, 51, 123–130. [Google Scholar] [CrossRef]
- Ghorbanzadeh, V.; Mohammadi, M.; Dariushnejad, H.; Abhari, A.; Chodari, L.; Mohaddes, G. Cardioprotective Effect of Crocin Combined with Voluntary Exercise in Rat: Role of Mir-126 and Mir-210 in Heart Angiogenesis. Arq. Bras. Cardiol. 2017, 109, 54–62. [Google Scholar] [CrossRef]
- Kang, L.-L.; Zhang, D.-M.; Jiao, R.-Q.; Pan, S.-M.; Zhao, X.-J.; Zheng, Y.-J.; Chen, T.-Y.; Kong, L.-D. Pterostilbene Attenuates Fructose-Induced Myocardial Fibrosis by Inhibiting ROS-Driven Pitx2c/miR-15b Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1243215. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.-J.; Wu, Y.-L.; Yang, M.-Y.; Chan, K.-C.; Lee, H.-J.; Wang, C.-J. Nelumbo nucifera leaf polyphenol extract and gallic acid inhibit TNF-α-induced vascular smooth muscle cell proliferation and migration involving the regulation of miR-21, miR-143 and miR-145. Food Funct. 2020, 11, 8602–8611. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, R.; Alihemmati, A.; Naderi, R. The impacts of garlic and voluntary training alone or together on myocardial miR-126 and miR-210 gene expressions and angiogenesis in healthy rats. J. Cardiovasc. Thorac. Res. 2020, 12, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, J.; Lv, S.; Xie, T.; Wang, X. Apigenin Alleviates Myocardial Reperfusion Injury in Rats by Downregulating miR-15b. Med. Sci. Monit. 2019, 25, 2764–2776. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, K.; Zhao, Y.; Xie, M.-L. Apigenin attenuates TGF-β1-stimulated cardiac fibroblast differentiation and extracellular matrix production by targeting miR-155-5p/c-Ski/Smad pathway. J. Ethnopharmacol. 2021, 265, 113195. [Google Scholar] [CrossRef]
- Ning, B.-B.; Zhang, Y.; Wu, D.-D.; Cui, J.-G.; Liu, L.; Wang, P.-W.; Wang, W.-J.; Zhu, W.-L.; Chen, Y.; Zhang, T. Luteolin-7-diglucuronide attenuates isoproterenol-induced myocardial injury and fibrosis in mice. Acta Pharmacol. Sin. 2017, 38, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Pan, H.; Li, D.; Fang, F.; Chen, D.; Sun, H. Luteolin improves contractile function and attenuates apoptosis following ischemia-reperfusion in adult rat cardiomyocytes. Eur. J. Pharmacol. 2011, 668, 201–207. [Google Scholar] [CrossRef]
- Kou, X.; Liu, X.; Chen, X.; Li, J.; Yang, X.; Fan, J.; Yang, Y.; Chen, N. Ampelopsin attenuates brain aging of D-gal-induced rats through miR-34a-mediated SIRT1/mTOR signal pathway. Oncotarget 2016, 7, 74484–74495. [Google Scholar] [CrossRef]
- Xu, H.; Pan, W.; Qian, J.; Liu, F.; Dong, H.; Liu, Q. MicroRNA-21 contributes to the puerarin-induced cardioprotection via suppression of apoptosis and oxidative stress in a cell model of ischemia/reperfusion injury. Mol. Med. Rep. 2019, 20, 719–727. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Han, Q. Puerarin Attenuates Cardiac Hypertrophy Partly Through Increasing Mir-15b/195 Expression and Suppressing Non-Canonical Transforming Growth Factor beta (Tgfβ) Signal Pathway. Med Sci. Monit. 2016, 22, 1516–1523. [Google Scholar] [CrossRef]
- Cheng, S.; Zhou, F.; Xu, Y.; Liu, X.; Zhang, Y.; Gu, M.; Su, Z.; Zhao, D.; Zhang, L.; Jia, Y. Geniposide regulates the miR-101/MKP-1/p38 pathway and alleviates atherosclerosis inflammatory injury in ApoE-/- mice. Immunobiology 2019, 224, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, Y.; Zhao, K.; Gao, Y.; Cui, J.; Qi, L.; Huang, L. miR-21/PTEN pathway mediates the cardioprotection of geniposide against oxidized low-density lipoprotein-induced endothelial injury via suppressing oxidative stress and inflammatory response. Int. J. Mol. Med. 2020, 45, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, X.; Xu, Y.; Lu, J.; Chen, J.; Huang, X. Long non-coding RNAs expression profile in HepG2 cells reveals the potential role of long non-coding RNAs in the cholesterol metabolism. Chin. Med. J. 2015, 128, 91–97. [Google Scholar] [CrossRef]
- Eckel, R.H. Reconsidering the Importance of the Association of Egg Consumption and Dietary Cholesterol With Cardiovascular Disease Risk. JAMA 2019, 321, 1055–1056. [Google Scholar] [CrossRef]
- Pan, J.-X. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 322–328. [Google Scholar]
- Salvatori, F.; D’Aversa, E.; Serino, M.L.; Singh, A.V.; Secchiero, P.; Zauli, G.; Tisato, V.; Gemmati, D. miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach. Int. J. Mol. Sci. 2023, 24, 13268. [Google Scholar] [CrossRef]
- Fisher, J.L.; Jones, E.F.; Flanary, V.L.; Williams, A.S.; Ramsey, E.J.; Lasseigne, B.N. Considerations and challenges for sex-aware drug repurposing. Biol. Sex Differ. 2022, 13, 13. [Google Scholar] [CrossRef]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. "Bridging the Gap" Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef]

| Diseases | Type of Modification | Major Regulator | Target Gene | Mechanism | Reference |
|---|---|---|---|---|---|
| Myocardial hypertrophy | Histone methylation | Histone methyltransferase G9a | Histone 3 lysine 9 EZH2 | Promotes cardiomyocyte growth by silencing anti-hypertrophic genes | [66] |
| Vascular calcification | Histone methylation | IL-6/SIL-6R | Histone 3 lysine 9 JMJD2B | Increases osteogenic transdifferentiation of VSMCs | [67] |
| Histone methylation | EZH2 | TAGLN | Suppresses smooth muscle cell markers during calcification | [68] | |
| Histone acetylation | SIRT6 | Runx2 | Inhibits osteogenic transcription factor activation | [69] | |
| Histone acetylation | HDAC4 | Sox9, Runx2, ALP | Regulates osteogenic differentiation of VSMCs | [70] | |
| Histone acetylation | HDAC9 | Runx2 | Promotes vascular smooth muscle calcification | [71] | |
| Histone acetylation | SIRT1 | RUNX2, osteocalcin | Prevents osteogenic differentiation in diabetic conditions | [72] | |
| Atherosclerosis | Histone acetylation | SIRT1 | eNOS | Activates eNOS, improving vascular function | [22] |
| Histone acetylation | HDAC3 | eNOS | Reverses aspirin-induced eNOS acetylation | [22] | |
| Histone acetylation | SIRT1 | P65, P300, NF-kB | Suppresses inflammatory signaling in vascular cells | [73] | |
| Myocardial infarction | Histone acetylation | SIRT2 | FOXO3A | Triggers nuclear translocation, inducing apoptosis | [74] |
| Histone acetylation | SIRT3 | Cyclophilin D | Prevents mitochondrial permeability transition pore opening | [75] | |
| Histone acetylation | HDAC6 | Peroxyredoxin 1 | Modulates redox status during ischemia/reperfusion | [76] | |
| Heart failure | Histone acetylation | SIRT2 | Angiotensin II | Protects against cardiac hypertrophy and remodeling | [77] |
| Histone acetylation | SIRT3 | GSK3β, SMAD3 | Blocks aging-associated cardiac tissue fibrosis | [78] | |
| Histone acetylation | SIRT4 | Angiotensin II | Accelerates pathological cardiac hypertrophy | [79] | |
| Histone acetylation | SIRT6 | P300 | Suppresses cardiomyocyte hypertrophy | [80] | |
| Histone acetylation | SIRT1 | NOTCH1 | Regulates proliferative signaling in cardiomyocytes | [81] | |
| Histone acetylation | SIRT7 | P53 | Increases stress resistance and prevents apoptosis | [82] | |
| Hypertension | Histone acetylation | HDAC6 | CSEγ | Regulates cystathionine γ-lyase preventing degradation | [83] |
| Histone acetylation | SIRT3 | SOD2 | Modulates antioxidant enzyme activity | [19] | |
| Histone acetylation | HDAC1/2 | Npr1 | Represses natriuretic peptide receptor expression | [84] | |
| Histone acetylation | HDAC2 | KCNJ2K+ ion channel | Regulates cardiac action potential duration | [85] |
| Diseases | Type of Non-Coding RNA | Major Regulator | Target Gene | Mechanism | Reference |
|---|---|---|---|---|---|
| Coronary heart disease | miRNA | miRNA-SNP rs41291957 | miRNA-143, miRNA-145 | Alters vascular smooth muscle cell differentiation | [86] |
| Atherosclerosis | miRNA | miRNA-1 | KLF4 | Regulates endothelial cell function and inflammation | [87] |
| miRNA | miR-92a | KLF4 | Promotes endothelial dysfunction and plaque formation | [88] | |
| LncRNA | LncRNA Mexis | ABCA1 | Modulates cholesterol efflux and lipid metabolism | [89] | |
| LncRNA | LncRNA NEXN-AS1 | TLR-4 oligomer, NF-kB | Mitigates inflammatory response in vascular cells | [90] | |
| Acute myocardial infarction | miRNA | miR-125b | SIRT7 | Facilitates cardiac repair by preventing cell death | [91] |
| miRNA | miR-21a-5p | PDCD4, PTEN, Peli1, FasL | Mediates cardioprotection by mesenchymal stem cells | [92] | |
| miRNA | miR-25-3p | E2Z2 | Attenuates cardiomyocyte apoptosis | [92] | |
| miRNA | miR-144 | PTEN/AKT | Increases cardiomyocyte survival under hypoxic conditions | [93] | |
| Heart failure | miRNA | miR-425, miR-744 | TGF-β | Represents progression of fibrosis and heart failure | [94] |
| LncRNA | LncRNA Meg3 | MMP2 | Regulates cardiac fibrosis and matrix remodeling | [95] | |
| LncRNA | LncRNA Whisper | Col3a1, Fn1, TGFb2, αSma | Regulates cardiac fibroblast activation and fibrosis | [96] | |
| Vascular calcification | miRNA | miR-30b | MMPs, SOX9 | Attenuates phenotypic transformation of VSMCs | [97] |
| miRNA | miR-128-3p | Wnt-1, β-catenin, GSK-3β, Bax, Islet1 | Accelerates cardiovascular calcification in diabetes | [98] | |
| miRNA | miRNA-19A-3p | HDAC4 | Promotes osteogenic differentiation | [99] | |
| LncRNA | LncRNATUG1 | miRNA-204-5p | Increases osteoblast differentiation via Runx2 | [100] | |
| LncRNA | Lrrc75a-as1 | SRF, CREB1, STAT3 | Regulates VSMC phenotypic switching | [101] | |
| LncRNA | LncRNA-SNHG29 | miR-200b-3p | Inhibits VSMC calcification via α-Klotho/FGFR1/FGF23 axis | [102] | |
| LncRNA | Bhlhe40 lncRNA-ES3 | miR-95-5p, miR-6776-5p, miR-3620-5p, miR-4747-5p | Mediates high glucose-induced VSMC calcification | [103] | |
| Hypertension | miRNA | miR-181a-5p, miR-663 | renin | Regulates renin–angiotensin system activity | [104] |
| LncRNA | HAS2-AS1 | C/EBPβ | Promotes cell migration and inflammation | [105] | |
| LncRNA | MRAK048635_P1 | α-SMA, SM22a, calponin, osteopontin | Regulates VSMC function and phenotypic switching | [106] | |
| Metabolic cardiomyopathy | miRNA | miRNa-494-3p | JunD/PPARα | Promotes myocardial lipid accumulation | [107] |
| Cardiomyocyte differentiation | LncRNA | Linc1405 | Eomes, MesP1 | Promotes cardiac mesoderm specification | [108] |
| Cardiac regeneration | LncRNA | LncRNA CAREL | MiR-296 | Regulates cardiac regenerative capacity | [109] |
| LncRNA | LncRNA NR_045363, Sirt1 antisense LncRNA | miRNA-216a, Sirt1 mRNA | Promotes cardiomyocyte proliferation | [110,111] | |
| Myocardial infarction | LncRNA | LncRNA Gpr19 | Mir-325-5p, Mtfr1 | Regulates apoptosis and oxidative stress | [112] |
| LncRNA | LncRNA UCA1 | Mir-143, MDM2, p53 | Protects cardiomyocytes against hypoxia/reoxygenation | [113] | |
| Atrial fibrillation | LncRNA | LncRNA MIAT | miR-133a-3p | Regulates atrial fibrillation and myocardial fibrosis | [114] |
| Bioproduct | Source | Effects on DNA Methylation | Potential CVD Benefits |
|---|---|---|---|
| Polyphenols |
|
|
|
| Flavonoids |
|
|
|
| Resveratrol |
|
|
|
| Sulforaphane |
|
|
|
| Genistein |
|
|
|
| Lycopene |
|
|
|
| RCI-1502 |
|
|
|
| Folic Acid |
|
|
|
| Compound | Induced miRNAs | Inhibited miRNAs |
|---|---|---|
| Resveratrol | miR 221 miR 222 miR 15b | miR 126 miR 21 miR 155 miR 34a |
| Gallic acid | miR 145 | miR 21 |
| Garlic | miR126 miR 210 | |
| Curcumin | miR 126 | |
| Kaempferol | miR 21 | miR 15b |
| Quercetin | miR 21 | miR 155 miR 199a |
| Apigenin | miR 15b | |
| Luteolin | miR 21 | |
| Ampelopsin | miR 21 | |
| Puerarin | miR 21 miR 15b | |
| Genistein | miR 34a miR 155 | |
| Crocin | miR 126 miR 210 | miR 34a |
| Geniposide | miR 145 |
| Study Title | Study Objectives/Results |
|---|---|
| The effect of Tongguan Capsule for MicroRNA profiles in Coronary Heart Disease patients (NCT02850627: Interventional; No results posted) | To test the expression of microRNAs related to the syndromes after the intervention of Tongguan capsule. |
| Air pollution, Epigenetics, and cardiovascular health: a human intervention Trial (NCT01864824: Interventional; No results posted) | To test the effects of methyl-donors on a battery of cardiovascular endpoints highly sensitive to particle pollution. |
| Nicotinamide Riboside in Systolic Heart Failure (NCT03423342: Interventional; Recruiting) | To determine the safety and tolerability of Nicotinamide Riboside in patients with clinically stable systolic heart failure. |
| Nicotinamide Roboside treatment increased whole blood NAD+ levels and changed mitochondrial function. A significant number of patients had abnormal laboratory values and/or adverse events related to treatment. | |
| Mechanistic studies of Nicotinamide Riboside in Human Heart Failure (NCT04528004: Recruiting; Interventional) | To demonstrate the effects of increasing NAD+ levels in heart failure patients. |
| Nicotinamide Riboside in LVAD recipients (NCT03727646: Interventional) | To test the hypothesis that oral Nicotinamide Riboside supplementation increases myocardial NAD+ levels and improves cardiomyocyte mitochondrial function in individuals with advanced heart failure planned for elective left ventricular assist device (LVAD). |
| Nicotinamide Riboside administration enhanced PBMC respiration and reduced proinflammatory cytokine gene expression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioproducts with Epigenetic Properties for Treating Cardiovascular Disorders. Genes 2025, 16, 566. https://doi.org/10.3390/genes16050566
Martínez-Iglesias O, Naidoo V, Carrera I, Corzo L, Cacabelos R. Natural Bioproducts with Epigenetic Properties for Treating Cardiovascular Disorders. Genes. 2025; 16(5):566. https://doi.org/10.3390/genes16050566
Chicago/Turabian StyleMartínez-Iglesias, Olaia, Vinogran Naidoo, Iván Carrera, Lola Corzo, and Ramón Cacabelos. 2025. "Natural Bioproducts with Epigenetic Properties for Treating Cardiovascular Disorders" Genes 16, no. 5: 566. https://doi.org/10.3390/genes16050566
APA StyleMartínez-Iglesias, O., Naidoo, V., Carrera, I., Corzo, L., & Cacabelos, R. (2025). Natural Bioproducts with Epigenetic Properties for Treating Cardiovascular Disorders. Genes, 16(5), 566. https://doi.org/10.3390/genes16050566








