Exploring the Dietary Strategies of Coated Sodium Butyrate: Improving Antioxidant Capacity, Meat Quality, Fatty Acid Composition, and Gut Health in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Sample Collection
2.3. Plasma Biochemical Indexes
2.4. Oxidative Stress Biomarkers
2.5. Meat Quality Traits
2.6. Inosine 5′-Monophosphate (IMP)
2.7. Free Fatty Acid (FFA) Analysis
2.8. Intramuscular Fat
2.9. 16s rRNA Sequencing
2.10. Statistical Analysis
3. Results
3.1. Biochemical Parameters
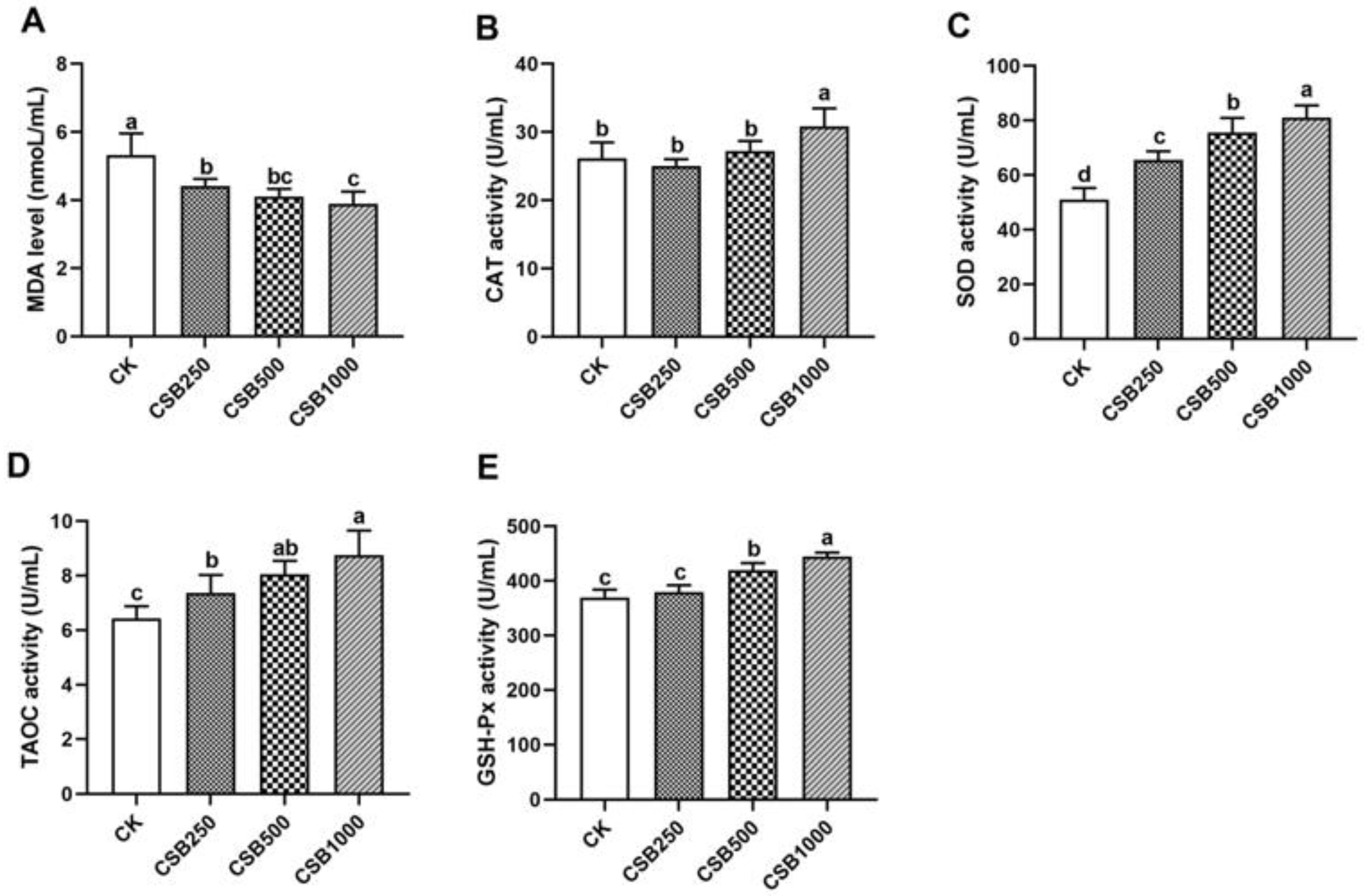
3.2. Antioxidant Capacity
3.3. Meat Quality
3.4. Inosinic Acid and Intramuscular Fat Content
3.5. Free Fatty Acid Composition
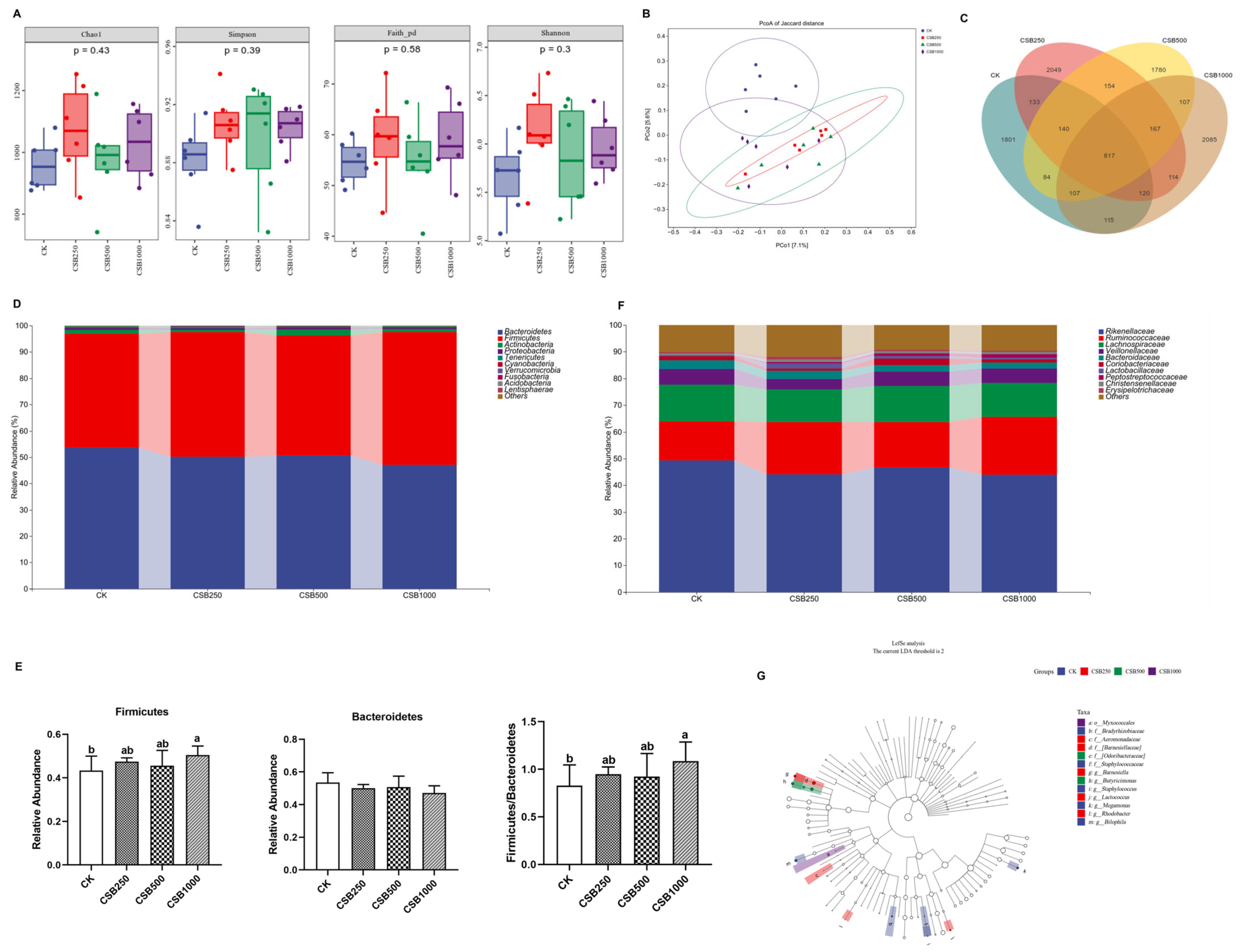
3.6. Gut Microbiota Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, X.; Liu, R.; Zhao, D.; He, Z.; Li, W.; Zheng, M.; Li, Q.; Wang, Q.; Liu, D.; Feng, F.; et al. Large-scale genomic and transcriptomic analyses elucidate the genetic basis of high meat yield in chickens. J. Adv. Res. 2024, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Pereira, L.; Barnaba, C.; de Los Campos, G.; Medina-Meza, I.G. Evaluation of the nutritional quality of ultra-processed foods (ready to eat + fast food): Fatty acids, sugar, and sodium. J. Food Sci. 2022, 87, 3659–3676. [Google Scholar] [PubMed]
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [PubMed]
- Rubin, C.J.; Zody, M.C.; Eriksson, J.; Meadows, J.R.; Sherwood, E.; Webster, M.T.; Jiang, L.; Ingman, M.; Sharpe, T.; Ka, S.; et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 2010, 464, 587–591. [Google Scholar]
- Muir, W.M.; Wong, G.K.; Zhang, Y.; Wang, J.; Groenen, M.A.; Crooijmans, R.P.; Megens, H.J.; Zhang, H.; Okimoto, R.; Vereijken, A.; et al. Genome-wide assessment of worldwide chicken SNP genetic diversity indicates significant absence of rare alleles in commercial breeds. Proc. Natl. Acad. Sci. USA 2008, 105, 17312–17317. [Google Scholar]
- Sell-Kubiak, E.; Wimmers, K.; Reyer, H.; Szwaczkowski, T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: A review. J. Appl. Genet. 2017, 58, 487–498. [Google Scholar]
- Meng, Q.; Sun, S.; Bai, Y.; Luo, Z.; Li, Z.; Shi, B.; Shan, A. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. 2020, 167, 108176. [Google Scholar]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Ropy, A.; Abdel-Razik, A.H. Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Anim. Int. J. Anim. Biosci. 2019, 13, 1234–1244. [Google Scholar]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar]
- Lin, F.; Li, X.; Wen, J.; Wang, C.; Peng, Y.; Feng, J.; Hu, C. Effects of coated sodium butyrate on performance, diarrhea, intestinal microflora and barrier function of pigs during the first 2-week post-weaning. Anim. Feed. Sci. Technol. 2020, 263, 114464. [Google Scholar] [CrossRef]
- Jang, Y.D.; Lindemann, M.D.; Monegue, H.J.; Monegue, J.S. The effect of coated sodium butyrate supplementation in sow and nursery diets on lactation performance and nursery pig growth performance. Livest. Sci. 2017, 195, 13–20. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, X.; Guo, Y.; Li, Z.; Zhou, Z. Coated sodium butyrate and vitamin D(3) supplementation improve gut health through influencing intestinal immunity, barrier, and microflora in early-stage broilers. J. Sci. Food Agric. 2024, 104, 4058–4069. [Google Scholar] [CrossRef] [PubMed]
- Moquet, P.C.A.; Salami, S.A.; Onrust, L.; Hendriks, W.H.; Kwakkel, R.P. Butyrate presence in distinct gastrointestinal tract segments modifies differentially digestive processes and amino acid bioavailability in young broiler chickens. Poult. Sci. 2018, 97, 167–176. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Dai, Z.; Liu, J.; Xiao, S.; Liu, Y.; Wang, X.; Yang, S.; Zhang, R.; Yang, C.; et al. Microencapsulated Sodium Butyrate Alleviates Immune Injury and Intestinal Problems Caused by Clostridium Perfringens through Gut Microbiota. Animals 2023, 13, 3784. [Google Scholar] [CrossRef]
- Zeng, T.; Sun, H.; Huang, M.; Guo, R.; Gu, T.; Cao, Y.; Li, C.; Tian, Y.; Chen, L.; Li, G.; et al. Dietary supplementation of coated sodium butyrate improves growth performance of laying ducks by regulating intestinal health and immunological performance. Front. Immunol. 2023, 14, 1142915. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Han, Y.; Garcia-Ruiz, A.I.; Rodiles, A. Hydroxychloride trace minerals have a positive effect on growth performance, carcass quality and impact ileal and cecal microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 38. [Google Scholar] [CrossRef]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Richards, J.D.; de Lange, J.G.F.M. The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: Current understanding, possible modulations, and new technologies for ecological studies. Can. J. Anim. Sci. 2005, 85, 421–435. [Google Scholar] [CrossRef]
- Muiños-Bühl, A.; González-Recio, O.; Muñoz, M.; Óvilo, C.; García-Casco, J.; Fernández, A.I. Evaluating Protocols for Porcine Faecal Microbiome Recollection, Storage and DNA Extraction: From the Farm to the Lab. Curr. Microbiol. 2018, 75, 651–657. [Google Scholar]
- Chen, B.; Li, D.; Leng, D.; Kui, H.; Bai, X.; Wang, T. Gut microbiota and meat quality. Front. Microbiol. 2022, 13, 951726. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Si, C.; Zhao, Z.T.; Meng, Z.; Yi, H.; Ye, X.M.; Qi, A.; Ouyang, K.H.; Wang, W.J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020, 155, 61–70. [Google Scholar]
- Wu, T.; Wang, P.; Fu, Q.; Xiao, H.; Zhao, Y.; Li, Y.; Song, X.; Xie, H.; Song, Z. Effects of dietary supplementation of Anoectochilus roxburghii extract (ARE) on growth performance, abdominal fat deposition, meat quality, and gut microbiota in broilers. Poult. Sci. 2023, 102, 102842. [Google Scholar] [PubMed]
- Han, J.F.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Tang, L.; Li, S.W.; Zhong, C.B.; Zhou, X.Q. Exploring the dietary strategies of phenylalanine: Improving muscle nutraceutical quality as well as muscle glycogen and protein deposition in adult grass carp (Ctenopharyngodon idella). Food Chem. X 2024, 22, 101421. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Wang, J.; Bai, S.; Zeng, Q.; Peng, H.; Zhang, B.; Xuan, Y.; Ding, X. Effects of coated sodium butyrate on performance, egg quality, nutrient digestibility, and intestinal health of laying hens. Poult. Sci. 2022, 101, 102020. [Google Scholar]
- Gao, H.; Zhang, Y.; Liu, K.; Fan, R.; Li, Q.; Zhou, Z. Dietary sodium butyrate and/or vitamin D3 supplementation alters growth performance, meat quality, chemical composition, and oxidative stability in broilers. Food Chem. 2022, 390, 133138. [Google Scholar]
- Zhang, C.; Razafindrabe, R.H.A.K.; Chen, K.; Zhao, X.; Yang, L.; Wang, L.; Chen, X.; Jin, S.; Geng, Z. Effects of different rearing systems on growth performance, carcass traits, meat quality and serum biochemical parameters of Chaohu ducks. Anim. Sci. J. Nihon Chikusan Gakkaiho 2018, 89, 672–678. [Google Scholar] [CrossRef]
- Patton, H.M. Nutritional assessment of patients with chronic liver disease. Gastroenterol. Hepatol. 2012, 8, 687–690. [Google Scholar]
- Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef]
- Hashash, J.G.; Koutroumpakis, F.; Anderson, A.M.; Rivers, C.R.; Hosni, M.; Koutroubakis, I.E.; Ahsan, M.; Gkiaouraki, E.; Dunn, M.A.; Schwartz, M.; et al. Elevated serum globulin fraction as a biomarker of multiyear disease severity in inflammatory bowel disease. Ann. Gastroenterol. 2022, 35, 609–617. [Google Scholar]
- Liu, C.; Wang, W.; Meng, X.; Sun, B.; Cong, Y.; Liu, J.; Wang, Q.; Liu, G.; Wu, S. Albumin/globulin ratio is negatively correlated with PD-1 and CD25 mRNA levels in breast cancer patients. OncoTargets Ther. 2018, 11, 2131–2139. [Google Scholar]
- Wang, J.; Liu, F.; Kong, R.; Han, X. Association Between Globulin and Diabetic Nephropathy in Type2 Diabetes Mellitus Patients: A Cross-Sectional Study. Front. Endocrinol. 2022, 13, 890273. [Google Scholar]
- Morling, J.R.; Fallowfield, J.A.; Guha, I.N.; Nee, L.D.; Glancy, S.; Williamson, R.M.; Robertson, C.M.; Strachan, M.W.; Price, J.F. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: The Edinburgh type 2 diabetes study. J. Hepatol. 2014, 60, 384–391. [Google Scholar] [PubMed]
- Liu, J.D.; Lumpkins, B.; Mathis, G.; Williams, S.M.; Fowler, J. Evaluation of encapsulated sodium butyrate with varying releasing times on growth performance and necrotic enteritis mitigation in broilers. Poult. Sci. 2019, 98, 3240–3245. [Google Scholar]
- Miao, S.; Li, Y.; Mu, T.; Wang, X.; Zhao, W.; Li, R.; Dong, X.; Zou, X. Dietary Coated Sodium Butyrate Ameliorates Hepatic Lipid Accumulation and Inflammation via Enhancing Antioxidative Function in Post-Peaking Laying Hens. Metabolites 2023, 13, 650. [Google Scholar] [CrossRef]
- Guo, Z.; Li, X.; Xie, X.; Yi, J.; Lei, M.; Ren, Y.; Zheng, J.; Kuang, L.; Li, C. Effects of Lysozyme on Growth Performance and Meat Quality of 2–3 Month-old Meat Rabbits. Southwest China J. Agric. Sci. 2010, 23, 1298–1302. [Google Scholar]
- Zhao, W.; Tian, Y.; Wang, Y.; Du, J.; Chen, L.; Gu, T.; Song, M.; Lu, L.; Sun, C. Dietary effect of Dendrobium officinale leaves on chicken meat quality, fatty acid composition, and volatile compounds profile. Food Chem. X 2024, 22, 101330. [Google Scholar]
- Dou, L.; Liu, C.; Yang, Z.; Su, R.; Chen, X.; Hou, Y.; Hu, G.; Yao, D.; Zhao, L.; Su, L.; et al. Effects of oxidative stability variation on lamb meat quality and flavor during postmortem aging. J. Food Sci. 2022, 87, 2578–2594. [Google Scholar]
- Gao, C.Q.; Shi, H.Q.; Xie, W.Y.; Zhao, L.H.; Zhang, J.Y.; Ji, C.; Ma, Q.G. Dietary supplementation with acidifiers improves the growth performance, meat quality and intestinal health of broiler chickens. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2021, 7, 762–769. [Google Scholar]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Anim. Int. J. Anim. Biosci. 2010, 4, 303–319. [Google Scholar]
- Raes, K.; De Smet, S.; Demeyer, D. Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb, beef and pork meat: A review. Anim. Feed Sci. Technol. 2004, 113, 199–221. [Google Scholar]
- Woods, V.B.; Fearon, A.M. Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Petrotos, K.; Goulas, P.; Kouretas, D. Effects of Dietary Grape Pomace Supplementation on Performance, Carcass Traits and Meat Quality of Lambs. Vivo 2018, 32, 807–812. [Google Scholar]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste enhancements between various amino acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [PubMed]
- Yang, C.; Qiu, M.; Zhang, Z.; Song, X.; Yang, L.; Xiong, X.; Hu, C.; Pen, H.; Chen, J.; Xia, B.; et al. Galacto-oligosaccharides and xylo-oligosaccharides affect meat flavor by altering the cecal microbiome, metabolome, and transcriptome of chickens. Poult. Sci. 2022, 101, 102122. [Google Scholar] [PubMed]

| Items | 1~42 d | 43~70 d |
|---|---|---|
| Ingredient (%) | ||
| Corn | 53.7 | 35.6 |
| Soybean meal | 23 | 8.2 |
| Extruded soybean | 6 | 2 |
| Rice bran | 6.5 | 6 |
| Soybean oil | 0.8 | 1.4 |
| Corn gluten meal | 3 | 4 |
| Limestone | 1.33 | 1.3 |
| Fermented soybean meal | 1.67 | |
| Wheat grain | 18 | |
| Rice bran | 6 | |
| DDGS (corn) 1 | 10 | |
| Wheat red dog | 0.3 | |
| Premix 2 | 4 | 3.2 |
| Total | 100 | 100 |
| Nutrient composition, calculated | ||
| Metabolizable energy (Kcal/kg) | 2950 | 2997 |
| Crude protein (%) | 21.1 | 16.7 |
| Crude fat (%) | 4.8 | 5.5 |
| Calcium (%) | 0.87 | 0.70 |
| Total phosphorus (%) | 0.63 | 0.58 |
| Lysine (%) | 1.22 | 0.95 |
| Tryptophan (%) | 0.22 | 0.19 |
| Methionine (%) | 0.54 | 0.40 |
| Threonine (%) | 0.85 | 0.67 |
| Methionine and cysteine (%) | 0.88 | 0.72 |
| Analyzed nutrient components | ||
| Crude protein | 21.12 | 16.34 |
| Crude fat | 4.89 | 5.58 |
| Crude ash | 5.04 | 5.53 |
| Dry matter | 89.75 | 90.24 |
| Items | CK | CSB250 | CSB500 | CSB1000 |
|---|---|---|---|---|
| TP (g/L) | 10.92 ± 2.96 | 13.60 ± 10.57 | 9.05 ± 3.13 | 15.17 ± 9.05 |
| ALB (g/L) | 5.94 ± 1.57 | 7.68 ± 6.23 | 4.97 ± 2.10 | 9.14 ± 6.63 |
| GLB (g/L) | 4.98 ± 1.44 | 5.93 ± 4.37 | 4.08 ± 1.06 | 6.03 ± 2.54 |
| AST (U/L) | 102.05 ± 32.77 | 100.50 ± 76.15 | 66.80 ± 19.98 | 106.74 ± 49.36 |
| ALT (U/L) | 5.61 ± 0.42 | 5.82 ± 1.01 | 4.89 ± 0.68 | 5.37 ± 0.74 |
| TC (mmol/L) | 1.19 ± 0.31 | 1.43 ± 0.98 | 1.10 ± 0.47 | 1.77 ± 0.94 |
| TG (mmol/L) | 0.10 ± 0.03 b | 0.16 ± 0.09 ab | 0.14 ± 0.04 ab | 0.20 ± 0.12 a |
| HDL (mmol/L) | 0.70 ± 0.18 | 0.83 ± 0.49 | 0.62 ± 0.27 | 0.91 ± 0.36 |
| LDL (mmol/L) | 0.35 ± 0.14 | 0.37 ± 0.32 | 0.27 ± 0.14 | 0.54 ± 0.41 |
| Items | CK | CSB250 | CSB500 | CSB1000 |
|---|---|---|---|---|
| IMP (mg/g) | 1.33 ± 0.11 b | 1.42 ± 0.06 ab | 1.44 ± 0.09 a | 1.45 ± 0.09 a |
| Intramuscular fat (%) | 1.97 ± 0.13 | 2.01 ± 0.08 | 2.11 ± 0.11 | 2.04 ± 0.15 |
| FFA (g/kg) | CK | CSB250 | CSB500 | CSB1000 |
|---|---|---|---|---|
| C14:0 | 4.03 ± 0.13 | 3.98 ± 0.09 | 3.93 ± 0.09 | 3.93 ± 0.04 |
| C16:0 | 199.89 ± 2.88 | 198.89 ± 4.34 | 201.83 ± 6.06 | 201.42 ± 7.04 |
| C16:1 | 24.74 ± 0.57 b | 25.62 ± 0.70 a | 24.34 ± 0.52 b | 24.36 ± 0.43 b |
| C18:0 | 89.10 ± 1.25 a | 89.56 ± 1.08 a | 86.36 ± 1.92 b | 87.37 ± 0.65 b |
| C18:1 | 259.06 ± 1.64 | 260.56 ± 2.75 | 260.98 ± 4.30 | 261.28 ± 4.01 |
| C18:2 | 163.83 ± 1.44 b | 165.06 ± 2.37 ab | 166.25 ± 1.21 a | 165.99 ± 1.80 a |
| C18:3 | 8.33 ± 0.11 | 8.45 ± 0.12 | 8.34 ± 0.08 | 8.40 ± 0.17 |
| C22:4 | 11.84 ± 0.24 b | 12.07 ± 0.07 a | 12.21 ± 0.12 a | 12.18 ± 0.16 a |
| C22:6 | 14.12 ± 0.15 b | 14.22 ± 0.08 ab | 14.34 ± 0.22 a | 14.32 ± 0.06 a |
| SFA | 293.02 ± 2.80 | 292.42 ± 3.56 | 292.12 ± 7.14 | 292.72 ± 7.18 |
| MUFA | 283.80 ± 1.57 | 286.18 ± 2.31 | 285.32 ± 4.42 | 285.64 ± 3.70 |
| PUFA | 198.11 ± 1.36 b | 199.79 ± 2.44 ab | 201.13 ± 1.34 a | 200.88 ± 1.98 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Xu, W.; Gu, T.; Lu, L.; Chen, G. Exploring the Dietary Strategies of Coated Sodium Butyrate: Improving Antioxidant Capacity, Meat Quality, Fatty Acid Composition, and Gut Health in Broilers. Genes 2025, 16, 433. https://doi.org/10.3390/genes16040433
Gu Z, Xu W, Gu T, Lu L, Chen G. Exploring the Dietary Strategies of Coated Sodium Butyrate: Improving Antioxidant Capacity, Meat Quality, Fatty Acid Composition, and Gut Health in Broilers. Genes. 2025; 16(4):433. https://doi.org/10.3390/genes16040433
Chicago/Turabian StyleGu, Zhuoya, Wenwu Xu, Tiantian Gu, Lizhi Lu, and Guohong Chen. 2025. "Exploring the Dietary Strategies of Coated Sodium Butyrate: Improving Antioxidant Capacity, Meat Quality, Fatty Acid Composition, and Gut Health in Broilers" Genes 16, no. 4: 433. https://doi.org/10.3390/genes16040433
APA StyleGu, Z., Xu, W., Gu, T., Lu, L., & Chen, G. (2025). Exploring the Dietary Strategies of Coated Sodium Butyrate: Improving Antioxidant Capacity, Meat Quality, Fatty Acid Composition, and Gut Health in Broilers. Genes, 16(4), 433. https://doi.org/10.3390/genes16040433





