Abstract
Chronic inflammatory skin diseases and neurodegenerative disorders share overlapping genetic, immunologic, and metabolic pathways that may predispose individuals to cognitive decline. This review synthesizes current human genomic, transcriptomic, and bioinformatic evidence linking psoriasis, rosacea, atopic dermatitis, and bullous pemphigoid with Alzheimer’s and Parkinson’s disease. Literature from PubMed, IEEE Xplore, and Google Scholar was examined, prioritizing studies integrating genomic, transcriptomic, and proteomic analyses. Among inflammatory dermatoses, psoriasis exhibits the strongest overlap with dementia genetics, with shared susceptibility loci including APOE, IL12B, and HLA-DRB5, and transcriptional regulators such as ZNF384 that converge on IL-17/TNF signaling. Rare-variant and pleiotropy analyses further implicate SETD1A and BC070367 in psoriasis–Parkinson’s comorbidity. Rosacea demonstrates upregulation of neurodegeneration-related proteins SNCA, GSK3B, and HSPA8, together with shared regulatory hubs (PPARG, STAT4, RORA) driving NF-κB/IL-17/TNF-dependent inflammation. In atopic dermatitis, rare FLG variants interacting with BACE1 suggest a mechanistic bridge between barrier dysfunction and amyloidogenic processing. Bullous pemphigoid reveals an HLA-DQB1*03:01-mediated immunogenetic link hypothesis and cross-reactive autoantibodies targeting BP180 (collagen XVII) and BP230, highlighting an autoimmune route of neurocutaneous interaction. Other inflammatory and neurodegenerative diseases with currently weak or limited genetic evidence are also discussed, as they may represent emerging biological pathways or potential therapeutic targets within the skin–brain connection in the future. The aim of this work is to help clarify these genetic links and to advocate for the routine cognitive assessment of affected patients, enabling early detection, improved long-term quality of life, and the potential for timely therapeutic intervention.
1. Introduction
The concept of the skin–brain axis describes a complex bidirectional network of interactions between the integumentary and central nervous systems. Their relationship originates during embryogenesis, as both organs derive from the ectoderm and develop in parallel during gastrulation. Such a shared developmental link has long suggested a profound biological and functional interconnection between the two systems. Following gastrulation, the fate of ectodermal cells to differentiate into neural tissue or epidermal epithelium, is determined by a tightly regulated interplay of molecular cues, primarily involving Wnt, fibroblast growth factor (FGF), and bone morphogenetic protein (BMP) signaling pathways. Active FGF signaling promotes neural induction, whereas its inhibition, accompanied by enhanced BMP activity, drives epidermal differentiation. Wnt signaling modulates this balance by restricting the ectoderm’s responsiveness to FGFs, ensuring proper regionalization between neural and non-neural ectoderm [1]. These developmental mechanisms not only define the early divergence of the nervous system and skin but also establish the molecular basis for their lifelong physiological cross-talk. The skin and brain communicate through immune, endocrine, vascular, and neural pathways that collectively maintain systemic homeostasis and influence the pathogenesis of diverse disorders [2,3]. Hence, this shared embryological and molecular framework provides a conceptual foundation for exploring the link between cutaneous and neurological diseases, highlighting an emerging interdisciplinary field that bridges dermatology, neurobiology, and genomic medicine.
Building upon this shared developmental and structural foundation, growing evidence indicates that skin inflammation can induce systemic effects that extend well beyond the cutaneous environment, while perturbations originating in the central nervous system (CNS) can, in turn, influence skin homeostasis [4,5]. For instance, based on the frequency of depressive symptoms experienced by patients with chronic wounds, Hadian et al. [5] proposed that chronic inflammatory skin states act as reservoirs of systemic inflammatory mediators that propagate neuroinflammation and contribute to cognitive and neuropsychiatric disturbances. Sustained activation of cutaneous immune pathways appears to promote the release of pro-inflammatory cytokines and danger signals into the circulation, leading to blood–brain barrier (BBB) dysfunction, activation of central immune responses, and altered neurotransmission [5]. Conversely, disturbances within the CNS, including chronic stress, major depressive disorder, and other neuropsychiatric conditions, can feed back to the skin via neuroendocrine pathways, particularly through the hypothalamic–pituitary–adrenal (HPA) axis, thereby exacerbating immune dysregulation and inflammatory dermatoses [2].
Furthermore, a growing body of epidemiological evidence provides robust support for the association between chronic inflammatory dermatoses and cognitive decline. Large population-based cohorts have consistently demonstrated that individuals with psoriasis, atopic dermatitis, and other eczematous conditions exhibit higher rates of mild cognitive impairment (MCI) and dementia compared with unaffected controls, independent of confounding variables such as age, sex, education, and cardiovascular risk factors [6,7,8]. For what is more, in psoriasis, this association appears to follow a disease–severity gradient, suggesting a potential dose–response relationship [8]. The relationship is particularly pronounced in older adults, where impaired epidermal barrier function enhances systemic cytokine release and perpetuates a state of chronic low-grade inflammation, often referred to as “inflammaging” [8,9].
This chronic systemic inflammation, commonly seen in the elderly, is characterized by elevated circulating cytokines such as IL-6, TNF-α, and IL-1β which are implicated in both neuroinflammatory processes and neurodegenerative disorders. These cytokines, produced by adipose tissue, endothelial cells, and activated immune populations, induce oxidative stress and mitochondrial dysfunction, amplifying the generation of reactive oxygen species (ROS) and sustaining local neuroinflammation. Concurrently, cytokine-driven endothelial activation upregulates adhesion molecules including ICAM-1 and VCAM-1, facilitating leukocyte adhesion and migration into neural tissue. Within the CNS, these immune mediators activate microglia and astrocytes, leading to persistent production of inflammatory factors that impair neuronal survival and synaptic integrity. This self-perpetuating inflammatory cascade, initiated by peripheral cytokines, ultimately contributes to neurodegenerative pathology through neuronal injury, loss of homeostasis, and accumulation of pathogenic proteins such as amyloid-β and tau [10].
Epigenetic modifications act as a dynamic molecular interface connecting chronic cutaneous inflammation with neurodegenerative processes by regulating gene expression without altering the underlying DNA sequence. In inflammatory skin diseases such as psoriasis and atopic dermatitis, DNA methylation is a key determinant of immune activation and epidermal barrier integrity. Genome-wide methylation studies reveal aberrant CpG methylation in promoters of cytokine-related genes (TNF-α, IL6, IL17, IL13, IL4R), leading to their overexpression and sustaining Th1/Th17- and Th2-driven inflammation. Likewise, alterations in histone acetylation and methylation (H3K9ac, H3K27ac, H3K27me3) modify chromatin accessibility, enabling transcription of inflammatory mediators while repressing genes involved in keratinocyte differentiation and skin homeostasis. MicroRNAs such as miR-21, miR-146a, and miR-155 further amplify cytokine signaling and epidermal hyperplasia [11].
Parallel epigenetic disruptions occur in neurodegenerative diseases. In Alzheimer’s and Parkinson’s disease, dysregulated DNA methylation affects neuronal genes involved in synaptic plasticity, oxidative stress, and protein aggregation—including APP, SNCA, and MAPT—while aberrant histone deacetylation (e.g., loss of H3K27ac, H4K16ac) silences neuroprotective promoters such as BDNF and CREB. Non-coding RNAs modulate microglial activation and apoptotic signaling, linking chromatin remodeling to neuroinflammation and neuronal loss [12].
Collectively, findings from dermatologic and neurologic epigenome studies indicate that DNA methylation, histone modification, and non-coding RNA regulation form a shared epigenetic framework through which chronic inflammation, oxidative stress, and metabolic imbalance influence both cutaneous immune dysregulation and neurodegenerative pathology—providing a unifying chromatin-level mechanism that bridges skin inflammation and brain decline.
All of these reciprocal mechanisms create a biological bridge between cutaneous inflammation and cognition, with cognition being a set of higher mental processes that include perception, memory, attention, learning, and decision-making. These abilities collectively sustain autonomy and enrich quality of life. When these domains become dysregulated, the resulting cognitive impairment may manifest as subtle, transient lapses or progress insidiously toward irreversible decline. At its most severe stage lies dementia, with Alzheimer’s disease (AD) standing as its most prevalent form worldwide, though other neurodegenerative disorders, such as Parkinson’s disease (PD), likewise erode the very faculties that define human thought and selfhood [13,14].
Skin inflammatory diseases represent easily assessable factors during clinical examination, offering valuable and non-invasive information for patient evaluation. Similarly, sex constitutes an even more accessible variable in this context, with recent machine learning (ML) approaches increasingly associating sex with the prediction of neurodegenerative diseases and highlighting its role as a biological determinant of disease onset and progression. In an explainable ML study on PD, Angelini et al. [15] applied the CatBoost algorithm with SHAP analysis to data from the Parkinson’s Progression Markers Initiative, demonstrating that disease progression follows sex-specific patterns, with rigidity, autonomic dysfunction, and SNCA variants being more influential in males, whereas verbal fluency and urinary dysfunction were stronger predictors in females [15]. Likewise, D’Amore et al. [16] employed XGBoost models using Alzheimer’s Disease Neuroimaging Initiative (ADNI) data and found that women exhibited higher predictive weighting of episodic memory decline, while men showed stronger dependence on global cognition and executive function. Together, these studies highlight sex as an easily measurable yet biologically meaningful determinant of neurodegenerative trajectories [16]. When combined with cutaneous inflammatory profiles, which reflect systemic immune activation, such parameters could enhance predictive frameworks for dementia onset and progression, fostering early, personalized, and clinically accessible strategies in neurodegenerative disease surveillance.
While sex and inflammatory pathways provide a plausible biological connection between the skin and brain, the contribution of shared genetic mediators remains insufficiently defined. Recent investigations have revealed overlapping genetic signatures in psoriasis, AD and PD, and emerging links involving rosacea, atopic dermatitis, bullous pemphigoid and dementia. These findings suggest that convergent molecular mechanisms, particularly those governing immune regulation and chronic inflammation, may predispose individuals to both cutaneous and neurological pathology. Thus, genetic susceptibility represents a stable determinant of disease risk, potentially explaining why specific subsets of individuals develop both chronic inflammatory skin disorders and dementia.
In this review, we synthesize current evidence on the genetic overlap between dermatological and neurodegenerative conditions. By highlighting key molecular mediators of the skin–brain axis, we aim to provide mechanistic insights into shared pathogenic circuits and contribute to future perspectives on risk stratification and precision medicine approaches. Furthermore, through this work, we seek to establish a stronger clinical and research connection between inflammatory skin diseases and dementia, advocating that patients with chronic inflammatory dermatoses should be routinely evaluated for cognitive performance. Such early neurocognitive screening could enable timely detection of dementia-related changes and ultimately improve patients’ long-term quality of life.
3. Biological and Genetic Bridges with Other Inflammatory Dermatoses
This section extends the scope of genetic analysis beyond psoriasis to include other inflammatory and autoimmune dermatoses, mainly rosacea, atopic dermatitis, and bullous pemphigoid, that have been associated with an increased risk of neurodegenerative disease. Collectively, these conditions illustrate distinct yet convergent biological pathways through which chronic cutaneous inflammation, vascular dysregulation, and immune activation may influence central nervous system homeostasis. While psoriasis remains the most genetically substantiated model, emerging data from rosacea and atopic dermatitis highlight neurovascular and barrier-mediated mechanisms, whereas bullous pemphigoid exemplifies an autoimmune cross-reactivity model. By integrating findings from genetic, proteomic, and immunologic studies, this section aims to delineate the shared and disease-specific mechanisms underpinning the broader skin–brain axis.
3.1. Rosacea and Dementia
3.1.1. Epidemiology and Molecular Evidence
Rosacea is a chronic, relapsing inflammatory disorder of the skin with heterogeneous clinical manifestations, typically involving the central face. Globally, rosacea affects more than 5% of the population, with peak incidence between 30 and 50 years of age, a female predominance, and greater prevalence among individuals of lighter phototypes, where rates may exceed 10% [56,57]. Core features include recurrent flushing, persistent erythema, telangiectasia, and inflammatory papules or pustules, with four recognized clinical subtypes, erythematotelangiectatic, papulopustular, phymatous, and ocular, that frequently overlap and evolve over time. Ocular disease occurs in up to three-quarters of affected individuals and is characterized by irritation, foreign-body sensation, photophobia, and visual disturbance. Beyond its visible manifestations, rosacea imposes a substantial psychosocial burden, contributing to anxiety, depression, and impaired quality of life. Although traditionally regarded as a skin-limited condition, increasing evidence implicates rosacea in a spectrum of systemic comorbidities, including neurological, cardiovascular, gastrointestinal disorders, as well as dementia [56,57].
The skin–brain axis linking rosacea and dementia is supported by convergent inflammatory, proteolytic, and innate immune mechanisms. Rosacea is characterized by upregulation of matrix metalloproteinases (MMPs), notably MMP-1, MMP-3, and MMP-9, and antimicrobial peptides (AMPs) such as cathelicidin (LL-37), which exert potent pro-inflammatory and vasoactive effects within the skin microenvironment. Analogously, in neurodegenerative diseases such as AD, MMP dysregulation and aberrant adenosine monophosphate (AMP) activity contribute to neuronal injury and amyloid pathology. Elevated cerebrospinal fluid concentrations of MMP-3 and MMP-9 have been correlated with both the duration and severity of AD, suggesting their involvement in BBB disruption, amyloid precursor protein processing, and neuroinflammatory amplification. Moreover, Aβ itself functions as an AMP with intrinsic pro-inflammatory and cytotoxic properties, mirroring the innate immune activation observed in rosacea. These shared molecular mediators highlight a plausible biological continuum between cutaneous and cerebral inflammation, providing mechanistic support for epidemiological evidence linking rosacea with increased dementia risk [58].
Epidemiological evidence indicates that the prevalence of rosacea in the general population varies widely, ranging from 1% to 20%, depending on diagnostic criteria, geographic region, and study design [59]. In one of the first clinical investigations exploring the association between rosacea and PD, conducted at the University Hospital Halle in Germany, rosacea was diagnosed in 18.6% of 70 patients with PD, while 31.9% exhibited facial flushing, a neurovascular manifestation that frequently overlaps with rosacea [60]. This substantially higher occurrence relative to general population estimates suggests a potential disease-specific predisposition and supports the hypothesis that the neurogenic and inflammatory dysregulation characteristic of PD may enhance cutaneous susceptibility.
Almost 15 years later, two large nationwide Danish registry studies by Egeberg and colleagues provided robust epidemiological evidence linking rosacea with neurodegenerative diseases. In the first study, including more than 4.6 million adults, individuals with rosacea exhibited almost a twofold higher risk of developing PD compared with those without rosacea, with incidence rates of 0.12 versus 0.06 cases per 1000 person–years [61]. In the companion study, encompassing over 82,000 patients with rosacea among 5.5 million participants, the investigators reported a 25% increased risk of AD and a modest 7% increase in overall dementia, particularly among individuals older than 60 years [58]. Collectively, these findings suggest that the chronic inflammatory environment and immune dysregulation characteristic of rosacea may extend beyond the skin and contribute to neurodegenerative processes through shared pathogenic pathways.
3.1.2. Proteomic and Transcriptomic Overlaps (SNCA, GSK3B, HSPA8)
Recent studies suggest that rosacea shares biological pathways with dementia, although a direct germline genetic link has not been demonstrated [62,63,64]. An integrated proteomics–phosphoproteomics analysis of rosacea skin quantified 3874 proteins and showed that both lesional and clinically non-lesional skin are enriched for inflammatory (e.g., neutrophil activation, IL-12 signaling) and axon-extension programs versus healthy skin. Notably, proteins commonly implicated in neurodegeneration were increased in rosacea, including α-synuclein (SNCA), HSPA8, and GSK3B, while protein-level pathway enrichment flagged AD- and PD-related pathways. Clinically, SNCA positively tracked with CEA (Clinician’s Erythema Assessment), and neutrophil markers ELANE and S100A family proteins correlated with global disease severity (Investigator’s Global Assessment). Phosphoproteomics further implicated activated transcription-factor/kinase networks linked to clinical scores and showed MAPK14 phosphorylation associating with erythema and AKT1 with inflammation. These findings indicate shared protein-level pathway signatures between rosacea and dementia biology but do not demonstrate a shared germline genetic risk [62].
A complementary line of evidence comes from a Greek case–control study that explored whether the TACR3 promoter polymorphism rs3733631 (C/G), previously linked to neurogenic inflammation, might connect rosacea to neurological disease. While no overall association was found between rosacea and the variant, the G allele and G-carrying genotypes (C/G or G/G) were significantly enriched in papulopustular rosacea, particularly among male patients, whereas erythematotelangiectatic disease showed only borderline enrichment. Because neurokinin B–TACR3 signaling interacts with dopaminergic and autonomic circuits implicated in PD, the authors proposed that this pathway could represent a biological point of convergence rather than a proven shared genetic risk factor. Importantly, no PD cohort was genotyped, and issues such as small sample size and deviation from Hardy–Weinberg equilibrium in patients warrant cautious interpretation. These preliminary findings suggest that specific genetic variants may influence rosacea subtypes through neurovascular mechanisms, but they do not yet establish a direct genetic bridge to dementia or other neurodegenerative disorders [63].
3.1.3. Regulatory Hubs (PPARG, STAT4, RORA)
Cross-tissue systems biology has further advanced the connection between rosacea and AD. A bioinformatics and network-pharmacology study sought to delineate shared molecular programs between rosacea skin and AD hippocampus and to nominate candidate therapeutics acting on those programs. By integrating human gene-expression data from the GEO (Gene Expression Omnibus) database, the authors identified 747 overlapping differentially expressed genes (DEGs) enriched for immune/inflammatory, lipid-metabolic, and vascular/angiogenic pathways; transcription-factor. Network analysis highlighted hubs such as PPARG, STAT4, and RORA, which then guided drug-gene queries that prioritized melatonin and yielded 19 intersection targets with supportive docking signals [64].
To functionally explore this prediction, the investigators turned to a rosacea-like mouse model, where melatonin treatment reduced lesion area and thickness, immune-cell infiltration (CD4+ T cells, F4/80+ macrophages), keratinocyte cytokine expression, NF-κB activation, endothelial chemotaxis/migration, and microvessel density. Together, these findings suggest that rosacea and AD share convergent transcriptomic signatures, particularly NF-κB/IL-17/TNF-driven inflammation and vascular remodelling, while pointing to melatonin as a potential modulator. However, the evidence rests on expression overlap, regulatory inference, drug-target enrichment, and validation in an animal model, rather than human genomic data, and therefore does not establish a direct genetic association between rosacea and AD and thus cannot be taken as proof of a direct genetic connection between the two disorders [64]
3.1.4. Possible Disease-Specific Mechanistic Axis Linking Rosacea and Dementia
A potential disease-specific mechanistic axis linking rosacea with dementia through convergent inflammatory, neurovascular, and proteostatic pathways is supported via emerging molecular and clinical evidence. Rosacea exhibits persistent activation of innate immune and proteolytic cascades, particularly MMPs (MMP-1, MMP-3, and MMP-9) and antimicrobial peptides such as cathelicidin (LL-37), that promote chronic neurogenic inflammation, endothelial dysfunction, and oxidative stress. These same mediators are implicated in the pathophysiology of AD, where MMPs overactivity contributes to Aβ aggregation, BBB disruption, and progressive neuroinflammation [58]. Beyond these canonical inflammatory links, recent proteomic and transcriptomic studies reveal deeper biological parallels. Rosacea lesional and non-lesional skin display upregulation of neurodegeneration-related proteins, including SNCA, HSPA8, GSK3B, key regulators of protein misfolding, autophagy, and tau phosphorylation in AD and PD. These alterations, together with enrichment of MAPK14 and AKT1 phosphorylation networks, delineate a cutaneous signature of dysregulated stress-response and axonal-extension pathways that recapitulate central nervous system neurodegenerative programs [62].
Integrative bioinformatics and network pharmacology analyses combining transcriptomic datasets from AD and rosacea identified several shared regulatory hubs, including PPARG, STAT4, and RORA, which converge on NF-κB, IL-17, and TNF signaling pathways implicated in chronic inflammation and vascular remodelling. Further, experimental validation in rosacea-like murine models demonstrated that melatonin treatment significantly reduced inflammatory cell infiltration, downregulated keratinocyte cytokines (such as IL-6, IL-8, and IL-1β), and suppressed microvascular proliferation, supporting the pathogenic relevance of these shared pathways [64]. Although these findings suggest the presence of a common inflammation–vascular axis linking peripheral and central neuroinflammatory processes, several limitations must be acknowledged. The analyses were entirely in silico and cross-sectional, based on publicly available transcriptomic datasets derived from heterogeneous tissue sources and small sample sizes, and the experimental validation relied on murine models that may not fully recapitulate human neurovascular complexity. Moreover, the absence of longitudinal, proteomic, or germline genetic validation precludes causal inference and limits the ability to determine whether the observed transcriptomic convergence represents a true shared etiology or parallel downstream inflammatory responses [64].
Collectively, these data point toward a common inflammation–axon–vascular module bridging peripheral and central tissues, wherein chronic cutaneous inflammation and neurovascular dysregulation in rosacea may mirror or even propagate mechanisms of neurodegeneration. While current evidence substantiates shared molecular and transcriptomic pathways, a definitive germline genetic overlap has not yet been demonstrated, accentuating the need for cross-disciplinary genomic and mechanistic studies to clarify whether rosacea constitutes an early systemic manifestation or comorbid amplifier of dementia pathobiology.
3.2. Atopic Dermatitis and Dementia
3.2.1. Epidemiological Evidence
Atopic dermatitis is the most prevalent chronic inflammatory skin disorder and a prototypical form of eczema. It is characterized by intense pruritus, xerosis, eczematous lesions, and lichenification, typically beginning in early childhood but often persisting or recurring throughout life. Epidemiological data indicate that atopic dermatitis affects approximately 10–30% of children and 2–10% of adults in industrialized nations, with prevalence having increased two- to three-fold in recent decades. The disease results from a complex interaction between genetic predisposition and environmental triggers that disrupt epidermal barrier integrity and immune homeostasis. Loss-of-function mutations in FLG, encoding filaggrin, an essential barrier protein responsible for epidermal hydration and mechanical stability, are among the most strongly implicated genetic risk factors, present in up to 30% of patients. Such variants not only predispose to atopic dermatitis but also confer susceptibility to ichthyosis vulgaris, allergic rhinitis, and keratosis pilaris. Clinically, atopic dermatitis is part of the “atopic march,” the sequential or concurrent manifestation of atopic disorders including asthma and allergic rhinoconjunctivitis [65,66].
Earlier epidemiological investigations suggested that individuals with atopic eczema may face nearly a two-fold higher risk of developing dementia; however, these studies were limited by small samples and self-reported diagnoses [67,68]. In 2022, a large UK population-based cohort of more than 1.7 million adults aged 60–99 years found that those with eczema had a 27% higher risk of dementia than those without the condition. This association, observed for both Alzheimer’s and vascular dementia, increased with eczema severity and persisted after adjustment for comorbidities and corticosteroid use, with dementia typically diagnosed about five years after eczema onset [7].
Despite these consistent epidemiological findings, a 2025 Mendelian randomization analysis including more than 860,000 individuals found no statistically significant causal genetic relationship between atopic dermatitis and dementia [69]. This suggests that the observed epidemiological link may reflect secondary immunoinflammatory or vascular mechanisms rather than direct shared genetic susceptibility. However, the absence of a causal signal at the population level does not exclude the presence of gene or pathway-specific interactions that remain undetected in genome-wide analyses. Mendelian randomization relies primarily on common SNPs and may overlook the influence of rare or regulatory variants acting through intermediate biological networks.
3.2.2. Biological Connection: Immune-Inflammatory and Vascular Pathways
The biological connection between atopic dermatitis and dementia is increasingly understood to involve a complex interplay between systemic type 2-driven inflammation, neuroimmune activation, and vascular dysfunction. In atopic dermatitis, chronic elevation of cytokines such as IL-4, IL-13, and IL-6 perpetuates a Th2-skewed immune response that extends beyond the skin, leading to persistent systemic inflammation. Circulating cytokines and immune mediators can compromise the structural and functional integrity of the BBB, promoting leukocyte infiltration, microglial activation, and sustained neuroinflammatory signaling within the central nervous system. In particular, IL-6 has been shown to upregulate the expression of Aβ precursor protein, while persistent systemic inflammation impairs the microglial clearance of Aβ peptides, resulting in their pathological accumulation. This Aβ burden, together with chronic glial activation, enhances oxidative stress and synaptic dysfunction, linking peripheral inflammation to amyloidogenic neurodegeneration. Concomitantly, inflammatory endothelial activation and vascular remodeling, driven by cytokine and mast-cell-mediated pathways, induce endothelial dysfunction characterized by increased adhesion molecule expression, vascular permeability, and oxidative stress. These alterations impair cerebral perfusion and oxygen delivery, contributing to neuronal energy imbalance and progressive cognitive decline. Together, these findings suggest that the skin–brain axis in atopic dermatitis operates through an immunoinflammatory–vascular continuum, wherein chronic peripheral inflammation propagates cerebrovascular injury, Aβ accumulation, and neurodegenerative mechanisms underlying dementia [69].
3.2.3. FLG Rare Variants and Barrier Dysfunction
Adding to these epidemiological and biological observations, recent genetic evidence suggests that atopic dermatitis and AD may share susceptibility factors. Xiong et al. [70] aimed to explore whether rare genetic variants contribute to AD risk beyond the well-established common loci. Using data from the ADNI, the investigators applied a rare variant association framework to systematically scan for genes carrying rare exonic variants enriched in AD cases compared with controls. Among the top signals, they identified FLG, the gene encoding filaggrin, a key epidermal barrier protein and a well-known risk factor for atopic dermatitis, as a candidate gene in AD. The identification of FLG in this context was unexpected, as it is classically associated with skin barrier dysfunction, yet its emergence in dementia genetics suggests that genes central to cutaneous biology may also contribute to neurodegenerative processes [70].
3.2.4. Filaggrin–BACE1 Interaction and Amyloid Biology
To investigate how FLG variants might contribute to AD, the study incorporated computational modeling. Docking and molecular dynamics simulations showed that filaggrin, the protein encoded by FLG, can bind to the β-site APP-cleaving enzyme 1 (the protein encoded by the BACE1 gene), a key enzyme responsible for generating Aβ. Importantly, a rare FLG variant, Ser742Tyr, demonstrated stronger binding to the BACE1-encoded enzyme than the wild-type protein, raising the possibility that altered filaggrin–BACE1 interactions could modulate amyloidogenic processing. Bioinformatics analyses further linked FLG to pathways involved in nervous system development and axonal regeneration, lending additional plausibility to its role as a cross-disease mediator. While these findings remain preliminary and do not establish a direct genetic association between atopic dermatitis and AD, they nominate FLG as a potential biological bridge between skin-barrier dysfunction and neurodegeneration [70].
This study has several notable strengths. By applying a rare-variant association framework to the ADNI cohort, the authors moved beyond conventional GWAS approaches and identified FLG as a novel candidate gene in AD. The detection of a classical epidermal-barrier gene in the context of dementia represents an original and provocative finding. Importantly, the study complemented statistical evidence with mechanistic exploration, using molecular docking and dynamics simulations to show that filaggrin interacts with the BACE1-encoded β-site APP-cleaving enzyme 1, the central enzyme in amyloid-β generation. Pathway enrichment further linked FLG to nervous-system development and axonal regeneration, providing additional biological plausibility [70].
However, several limitations must be considered. The analysis did not directly test whether atopic dermatitis and AD co-occur genetically, but instead focused on FLG as an overlapping locus. The findings rely on computational predictions and statistical associations, without functional validation in cellular or animal models. The study cohort, drawn from ADNI, is relatively small and largely European, which restricts power and generalizability across populations. Furthermore, much of the mechanistic inference centered on a single variant (Ser742Tyr), and it remains unclear whether other FLG variants exert similar effects. Thus, while the work highlights FLG as a potential biological bridge between barrier dysfunction and neurodegeneration, replication in larger, multi-ethnic cohorts and experimental validation will be essential to clarify its role [70].
Exploring the potential link between atopic eczema and dementia is of clinical importance, as it could enable earlier identification of individuals at elevated risk for cognitive decline and inform strategies to reduce long-term disease burden. In conclusion, converging epidemiological and genetic data support a possible link between atopic dermatitis and dementia. While epidemiological studies point to an elevated risk of cognitive decline in patients with eczema, emerging rare variant analyses nominate FLG as a potential genetic mediator connecting skin barrier dysfunction to AD biology. These findings remain preliminary but raise important questions about shared mechanisms that could, in the future, inform risk stratification and novel therapeutic strategies.
3.2.5. Possible Disease-Specific Mechanistic Axis Linking Atopic Dermatitis and Dementia
Integrating epidemiological, genetic, and mechanistic evidence, the connection between atopic dermatitis and dementia appears to involve immune-inflammatory, vascular, and barrier-gene pathways rather than direct inherited causality. Chronic Th2-skewed inflammation in atopic dermatitis, characterized by sustained IL-4, IL-13, and IL-6 signaling, can disrupt the BBB, promote microglial activation, and induce neuroinflammatory cascades that impair Aβ clearance. Endothelial activation and oxidative stress further compromise cerebral perfusion, fostering neurovascular dysfunction [69]. At the molecular level, rare-variant bioinformatics analysis identified FLG, with variant-induced strengthening of filaggrin–BACE1 interactions that may subtly promote amyloidogenic processing [70]. Although Mendelian randomization studies [69] do not confirm a causal genetic overlap, these emerging data suggest that skin-barrier genes, chronic systemic inflammation, and cerebrovascular stress may intersect along a shared biological continuum, the skin–brain axis, linking cutaneous barrier failure with neurodegenerative vulnerability.
3.2.6. Genetic and Biological Evidence Linking Rosacea and Atopic Dermatitis to Dementia
Viewed together, the current evidence for rosacea and atopic dermatitis highlights a continuum of support for a biological rather than a strictly genetic bridge with dementia. Rosacea exhibits strong proteomic and transcriptomic overlap with neurodegenerative pathways; however, no validated germline associations have been established to date. Instead, several shared regulatory hubs have been identified, converging on signaling axes. In contrast, atopic dermatitis introduces preliminary genetic insights through rare FLG variants, suggesting a potential mechanistic connection to amyloidogenic processing and thus a more direct interface with Alzheimer’s disease biology.
These findings are summarized in Table 3, which outlines the type of evidence, key molecular players, and the qualitative strength of support across inflammatory skin diseases studied in relation to dementia.

Table 3.
Biological and genetic evidence linking inflammatory skin diseases to dementia.
3.3. Bullous Pemphigoid and Emerging Neurogenic Link with Dementia
3.3.1. Epidemiology and Clinical Evidence
Bullous pemphigoid (BP), an autoimmune blistering disease predominantly affecting the elderly, has been increasingly linked to neurodegenerative disorders, particularly Alzheimer’s and Parkinson’s disease. Epidemiologic studies demonstrate that individuals with BP have a significantly higher risk of neurological comorbidities, including dementia, which frequently precedes the onset of cutaneous disease. A large Taiwanese population-based cohort found that 17.7% of BP patients had a prior diagnosis of dementia compared with 4.6% of controls, corresponding to an adjusted odds ratio of 3.04 (95% CI 2.67–3.46) [71]. Similarly, a 50-year population study from Olmsted County, Minnesota, reported that 10% of BP patients had dementia at the time of diagnosis versus 2% of controls, yielding an odds ratio of 6.75 (95% CI 2.08–21.92), which increased to 9.00 (95% CI 2.44–33.24) in those with generalized BP [72]. In both studies, dementia typically preceded BP by several years, suggesting that neurodegenerative processes may initiate or enhance autoimmune reactivity through antigen exposure and immune cross-reactivity, ultimately linking neurological degeneration with subsequent blistering skin disease.
3.3.2. Shared Autoantigens (BP180, BP230)
Mechanistically, BP autoantigens, BP180 (collagen XVII, also known as BPAG2) and BP230, are not restricted to the skin but are also expressed in neurons of several brain regions, including the cerebral cortex, hippocampus, basal nuclei, and substantia nigra. Neurological diseases may lead to neuronal injury and compromise of the BBB, exposing these normally sequestered antigens to the immune system and triggering an autoimmune response that subsequently manifests as cutaneous disease. Supporting this neurocutaneous model, antibodies against collagen XVII have been detected in the serum of patients with dementia and other neurological disorders, and sera from BP patients with neurological comorbidities recognize both BP180 and BP230 in human brain extracts. These findings strengthen the hypothesis that collagen XVII serves as a shared antigen bridging the central nervous system and skin, supporting the epidemiological association between BP and neurodegenerative disease [73].
3.3.3. Immunogenetic Susceptibility (HLA-DQB1*03:01)
To date, very limited studies have investigated the genetic connections between BP and neurodegeneration. Amber et al. [74] proposed a multi-hit hypothesis to explain the frequent co-occurrence of BP with neurological disease, focusing on the immunogenetic role of the HLA-DQB1*03:01 allele. Drawing on prior immunogenetic, histopathologic, and neuroimmunological data, the authors suggested that this allele enhances T-cell binding affinity for epitopes within BP180, particularly the NC16a domain, predisposing carriers to loss of tolerance once neuronal injury exposes normally sequestered antigens. This model elegantly integrates the well-documented neuronal expression of BP180 and BP230 with epidemiologic observations showing that neurological disease, most commonly dementia, PD, or stroke, precedes BP onset in approximately 70% of cases, often by several years. The findings, supported by earlier evidence that these autoantigens are expressed in neurons of the cortex, hippocampus, and substantia nigra and that sera from patients with BP or neurodegenerative disease can recognize brain-derived BP180, reinforce a neurocutaneous immune axis whereby neurodegeneration may act as the initiating event in cutaneous autoimmunity [74].
A key strength of Amber et al.’s [74] work lies in its conceptual synthesis of molecular immunology, neurobiology, and clinical epidemiology, providing a coherent mechanistic framework that explains both the directionality and antigenic specificity of the BP–neurodegeneration association. However, the hypothesis remains theoretical and indirect, derived largely from immunogenetic correlations, in silico modeling, and extrapolation from animal studies rather than direct functional or longitudinal patient data. The absence of prospective genetic validation and the limited assessment of non-HLA immune or environmental modifiers constrain causal inference. Nevertheless, the study represents a significant conceptual advance, uniting dermatologic and neurologic autoimmunity under a shared antigen hypothesis and generating testable predictions for future multi-omic and translational research.
3.4. Hidradenitis Suppurativa and the γ-Secretase Pathway
Hidradenitis suppurativa (HS, acne inversa) is a chronic, relapsing inflammatory skin disease affecting approximately 1% of adults and characterized by painful nodules, abscesses, and sinus tracts in apocrine-gland–bearing areas [75]. Beyond its cutaneous manifestations, HS has been examined for potential links to neurodegenerative disorders, particularly AD, owing to shared alterations in the γ-secretase complex, a transmembrane protease responsible for the intramembranous cleavage of multiple substrates, including Notch and amyloid precursor protein (APP) [76].
Familial HS most commonly involves heterozygous loss-of-function variants in γ-secretase subunit genes such as NCSTN (nicastrin), PSENEN (presenilin enhancer 2), and PSEN1 (presenilin-1) [77]. These truncating or frameshift mutations reduce γ-secretase activity and impair Notch signaling, leading to follicular occlusion, epidermal hyperplasia, and chronic inflammation. In contrast, autosomal-dominant familial AD arises primarily from gain-of-function missense or in-frame mutations in PSEN1 or PSEN2, which alter γ-secretase cleavage of APP and increase production of the neurotoxic Aβ42 peptide [78]. Thus, although HS and AD converge mechanistically on γ-secretase dysfunction, their molecular outcomes diverge: reduced enzymatic activity underlies HS pathogenesis, whereas aberrant APP processing promotes amyloid deposition and neurodegeneration in AD. Together, these findings highlight γ-secretase as a shared mechanistic node linking epithelial and neuronal homeostasis through distinct modes of dysregulation rather than a single causal genetic bridge [79].
This mechanistic overlap was further explored at the genetic level in the only human study, to the best of our knowledge, to date, investigating a possible connection between HS and AD. In a genetic linkage and sequencing analysis of six unrelated Han Chinese families with autosomal-dominant HS, Wang et al. [76] identified heterozygous loss-of-function variants in PSENEN, NCSTN, and PSEN1 [76]. Because PSEN1 and PSEN2 mutations also underlie early-onset familial AD, these findings suggest a shared molecular pathway linking cutaneous and neurodegenerative biology through disrupted γ-secretase activity. However, the overlap appears mechanistic rather than allelic since AD-associated PSEN1 mutations are typically missense or in-frame substitutions that alter substrate processing, whereas HS-associated variants are truncating or frameshift mutations leading to haploinsufficiency and loss of function. Clinically, none of the 50 affected individuals studied, including 15 over the age of 50, showed evidence of dementia or cognitive decline, although a comprehensive neurological evaluation was not performed. Consequently, while HS and AD both implicate γ-secretase dysfunction, current data do not support a direct genetic overlap. Major limitations include the small familial sample size, lack of longitudinal neurocognitive assessment, and absence of functional validation to determine whether HS-linked PSEN1 mutations exert amyloidogenic effects in neural tissue [76].
Epidemiological data examining the HS and AD relationship remain inconclusive. A large retrospective population-based cohort study conducted in 2017 compiles longitudinal clinical data from 27 U.S. health systems and approximately 48 million unique patients, identified 28,755 individuals with HS [80]. Diagnoses of HS and AD were obtained using standardized clinical coding, and analyses were adjusted for age and sex. The absolute risk of AD among patients with HS was 0.2% (65 of 28,755). While crude analysis suggested a lower AD risk in HS (odds ratio [OR] = 0.34; 95% CI 0.26–0.43), adjusted analysis rendered the association modest and statistically nonsignificant (adjusted OR = 1.23; 95% CI 0.96–1.56). These findings were consistent with prior Danish registry data [81] reporting no significant relationship between the two conditions. The authors concluded that, despite overlapping γ-secretase biology, HS does not appear to confer an increased epidemiologic risk of AD, though their analysis could not account for genetically defined familial HS subgroups carrying PSEN or NCSTN mutations—an important limitation [80].
In contrast, a later Turkish cross-sectional study involving 192 HS patients [82] assessed family history of both HS and AD. None of the participants had AD themselves, but 9.4% reported a family history of the disorder. The prevalence of AD family history was significantly higher among patients with a family history of HS (25.8%) compared with those without (6.2%), corresponding to a 4.5-fold increased risk; this rose to an 8.8-fold increase among patients aged 40 years or older. The authors contrasted their findings with earlier large-scale cohort studies, suggesting that the absence of an observed association in those investigations may reflect the limitations of retrospective, code-based analyses with short follow-up durations, which could miss familial or long-term trends [82].
Overall, while γ-secretase biology provides a plausible molecular intersection between HS and AD, current evidence does not support a consistent or causal genetic link. Future prospective, longitudinal, and genotype-stratified studies are warranted to clarify how specific γ-secretase variants influence disease expression and whether they modulate risk for neurodegenerative comorbidity. Such investigations could also elucidate how genetic, environmental, and hormonal factors interact over time, ultimately advancing a more comprehensive understanding of HS pathogenesis and informing personalized therapeutic strategies [83].
3.5. Seborrheic Dermatitis, Vitiligo, and Prurigo Nodularis
A recent review addressing the relationship between skin inflammation and dementia also discusses several less-studied inflammatory dermatoses, such as seborrheic dermatitis, prurigo nodularis, and vitiligo, which may be associated with cognitive dysfunction and neurodegenerative disease. These conditions share systemic inflammatory characteristics, barrier impairment, and immune dysregulation, factors increasingly recognized as relevant to neuroinflammation and neuronal decline [6]. For example, in a case report study from Italy, patients with prurigo nodularis have been reported to exhibit varying degrees of cognitive impairment, ranging from psychiatric symptoms and mild cognitive dysfunction to AD [84]. Similarly, vitiligo, an inflammatory skin disorder marked by melanocyte loss and impaired epidermal barrier recovery, has been linked to a higher incidence of dementia (5.0 vs. 1.0 per 1000 person–years; adjusted HR = 5.3), although long-term follow-up data suggest that the risk of AD may not differ significantly from controls [85,86]. Evidence on seborrheic dermatitis remains limited but points toward a possible association with systemic comorbidities and neurodegenerative changes [6].
At present, there is no confirmed genetic association between these inflammatory skin diseases and dementia. The observed relationships likely reflect immune-mediated, inflammatory, and metabolic mechanisms rather than shared heritable susceptibility. Nonetheless, the inclusion of these disorders is important from a holistic perspective, as they accentuate the potential influence of chronic peripheral inflammation on central nervous system health. Future studies integrating immunogenetic, transcriptomic, and clinical data may help elucidate how inflammatory skin processes contribute to or mirror neurodegenerative pathways.
4. Huntington’s Disease and the Peripheral Mirror of Neurodegeneration
HD is a genetic neurodegenerative disorder caused by an expanded CAG trinucleotide repeat in the HTT gene, leading to production of mutant huntingtin protein (mHTT). This mutation induces mitochondrial dysfunction and disrupts the ubiquitin–proteasome system in neurons, resulting in progressive motor, cognitive, and psychiatric impairment. Although rare, HD is a globally distributed neurodegenerative disorder, with prevalence approaching 5 per 100,000 worldwide, highest in populations of European ancestry, and stable to slightly increasing rates over recent decades, largely due to diagnostic and survival improvements rather than a true rise in new cases [87].
Peripheral manifestations of HD have been increasingly recognized with recent transcriptomic analyses identifying significant upregulation of PLCB4, UBE2D3, APC, and ROCK1 in fibroblasts derived from HD patients compared with controls. These genes are involved in RNA processing, cellular signaling, and cytoskeletal organization, mirroring molecular disruptions seen in neuronal tissue [88]. Moreover, increased expression of parkin protein in juvenile HD fibroblasts appears to exert a protective role by enhancing proteasome activity and maintaining mitochondrial integrity [89]. Together, these findings highlight the potential of skin fibroblasts as accessible peripheral models for exploring systemic molecular alterations in HD and for identifying novel diagnostic and therapeutic targets; however, to the best of our knowledge, there are no current genetic studies directly associating inflammatory skin diseases with HD [50].
Although these findings do not implicate inflammatory skin diseases directly in HD genetics, they provide compelling evidence that the skin reflects systemic molecular changes associated with neurodegeneration. Given this overlap, the skin serves as a valuable peripheral model for studying HD and may, in the future, reveal novel genetic or inflammatory interactions bridging cutaneous and neural pathology. Therefore, it is important to acknowledge this emerging skin–brain connection within the context of neurogenetic research, as it may guide future studies toward identifying shared biomarkers and therapeutic targets [50].
5. Integrative Perspective
As schematically illustrated in Figure 1, this review consolidates current evidence implicating genetic mediators at the interface of cutaneous inflammation and dementia, revealing both convergent mechanisms and unresolved controversies. Converging genetic, immunologic, and transcriptomic evidence supports the existence of a shared skin–brain axis connecting inflammatory dermatoses and neurodegenerative disorders. Among these disease pairings, psoriasis and AD currently represent the most substantiated model. Susceptibility at APOE, IL12B, and variants near HLA-DRB5, together with transcriptomic regulators such as ZNF384, delineate a molecular bridge linking cutaneous inflammation with neurodegenerative vulnerability through dysregulated IL-17, IL-23, and TNF signaling and impaired antigen presentation [27,38,40,43,44].
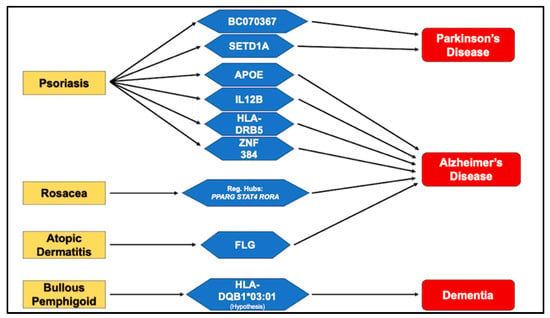
Figure 1.
Genes Bridging Cutaneous Inflammation and Cognitive Decline.
Complementary pleiotropy-informed analyses have identified additional loci, including SETD1A and BC070367, connecting psoriasis and Parkinson’s disease via immune–epigenetic interactions [55]. Clinically, ongoing research by Levitt et al. [35] demonstrated that psoriasis patients treated with biologic agents targeting IL-17, IL-23, IL-12/23, or TNF-α exhibited a nearly 50% reduction in dementia incidence compared with those receiving conventional systemic therapy, suggesting that sustained cytokine inhibition may mitigate systemic and neuroinflammatory cascades contributing to cognitive decline [35].
Beyond psoriasis, other chronic inflammatory dermatoses illustrate distinct yet convergent mechanisms along this axis. In rosacea, proteomic and transcriptomic investigations have revealed enrichment of neurodegeneration-related proteins such as SNCA, GSK3B, and HSPA8, as well as regulatory hubs including PPARG, STAT4, and RORA, which converge on NF-κB, IL-17, and TNF signaling pathways [62,64]. These findings indicate that persistent cutaneous inflammation and vascular dysregulation may recapitulate early neurodegenerative processes. In atopic dermatitis, rare-variant analyses have highlighted FLG as a potential cross-disease gene [70]. Computational modelling suggests that the FLG Ser742Tyr variant enhances binding to BACE1, the β-site APP-cleaving enzyme critical for amyloid-β generation, supporting a barrier–amyloid axis linking epidermal dysfunction to AD [70]. BP represents a complementary autoimmune model, where shared neuronal and epidermal expression of BP180 (collagen XVII) and BP230, together with the HLA-DQB1*03:01 allele, support a neurocutaneous autoimmunity hypothesis [73,74]. Epidemiological data consistently show that dementia often precedes the onset of bullous pemphigoid, implying that neurodegeneration may expose normally sequestered neuronal antigens, thereby triggering secondary autoimmune responses in the skin [71,72].
Geographic and ethnic epidemiological differences add further complexity, as both inflammatory dermatoses and neurodegenerative diseases exhibit variable prevalence and severity across populations. Gradients in allele frequencies, such as the north–south distribution of APOE ε4 or regional variation in IL12B polymorphisms imply that heritable factors contribute to these regional disparities [14,30,31]. Such findings strengthen the argument for genetic involvement in the observed comorbidity patterns and highlight the importance of multi-ethnic, ancestry-aware genetic studies in understanding the architecture of the skin–brain axis.
Despite increasing clarity, substantial knowledge gaps remain. In BP, the biological and molecular interactions with neurodegeneration remain speculative, grounded primarily in immunogenetic and serologic observations [73,74]. Similarly, HS epidemiological associations with dementia and AD remain inconclusive, and mechanistic and genomic data are inadequate [73,74]. Conversely, HD, a neurodegenerative disease, provides an inverse example. It is closely associated with skin fibroblast dysfunction, as these cells recapitulate neurodegenerative hallmarks such as mitochondrial stress and defective autophagy, yet no evidence currently links HD to inflammatory dermatoses [88,89].
This illustrates that while the skin may mirror neural degeneration at the cellular level, inflammatory and genetic pathways do not universally overlap across all neurodegenerative disorders.
6. Strengths and Limitations
The body of current evidence exhibits several methodological strengths. Integrative multi-omic approaches combining genomic, transcriptomic, and proteomic analyses have revealed convergent immune and metabolic pathways across both systems, reinforcing biological plausibility. Large-scale, population-based cohorts from Korea, Germany, and Israel have strengthened epidemiologic validity, while the alignment between genetic discoveries, such as IL12B, and the therapeutic efficacy of corresponding biologics, such as ustekinumab, provides translational coherence linking genotype, cytokine modulation, and clinical outcomes [14,30,35,39,52].
Nevertheless, important limitations constrain interpretation. Most studies remain cross-sectional or retrospective, relying on registry-based diagnoses that may introduce misclassification bias. Genetic analyses have primarily focused on European and East Asian populations, restricting global generalizability. The strength of association for rarer dermatoses such as BP and rosacea is limited by small sample sizes, while findings for atopic dermatitis are based largely on computational models without experimental validation. Furthermore, causality is often inferred indirectly through statistical pleiotropy or expression overlap rather than demonstrated through mechanistic studies. The absence of longitudinal cognitive, neuroimaging, or biomarker endpoints also limits the ability to determine whether these associations translate into true neuroprotective benefit. Environmental and lifestyle modifiers, including microbiome composition, diet, and pharmacologic exposure, remain underexplored within genetic frameworks, further complicating causal interpretation.
Future research should focus on addressing these gaps through multi-ethnic, longitudinal, and mechanistic studies that integrate genomic, transcriptomic, and metabolomic data. Large international consortia linking dermatologic and neurologic biobanks could enable cross-phenotype genome-wide and rare-variant analyses to dissect shared and population-specific risk. Functional studies using induced pluripotent stem cell-derived keratinocytes, glia, and neurons may clarify how disease-associated variants alter cytokine networks, barrier integrity, and neuroimmune interactions. In parallel, clinical trials of biologic and systemic immunomodulators should incorporate neurocognitive and neuroimaging endpoints to determine whether early, sustained control of systemic inflammation can attenuate neurodegenerative progression.
7. Future Directions
From a clinical perspective, the emerging convergence of evidence argues for the introduction of routine MCI screening in patients with chronic inflammatory dermatoses. More broadly, recognizing inflammatory skin diseases, sex and neurodegenerative disorders as interconnected systemic processes rather than isolated organ-specific conditions may redefine future prevention and treatment paradigms. Early recognition of cognitive decline would facilitate timely therapeutic intervention, enable individualized patient management, and improve long-term quality of life. Furthermore, future studies should explore the potential neuroprotective effects of biologic and anti-inflammatory agents used in skin inflammatory diseases, currently psoriasis, as emerging evidence suggests that modulation of systemic inflammation through IL-17, IL-23, or TNF-α inhibition may attenuate neuroinflammatory pathways and reduce the risk or progression of neurodegeneration.
In summary, the cumulative data suggest that the skin–brain axis is governed by a shared immunogenetic circuitry involving cytokine signaling, lipid metabolism, antigen presentation, and neuroimmune cross-talk. Geographic variation, differential genetic predisposition, sex and environmental influences all contribute to the heterogeneity observed across populations. Bridging these findings through integrative, multi-ethnic, and mechanistic research holds the potential to establish a new precision medicine framework at the interface of dermatology and neurology, one that not only controls cutaneous inflammation but also preserves cognitive health and extends the quality and longevity of life for affected individuals.
8. Conclusions
Taken together, these insights redefine inflammatory skin diseases as systemic disorders with neurological relevance rather than purely cutaneous entities. The recognition of shared genetic and immunologic determinants between the skin and brain not only reshapes pathophysiological understanding but also opens translational opportunities for early neurocognitive screening and cytokine-targeted intervention. Integrating dermatology and neurology through precision immunogenetics could transform patient care, enabling the prevention of neurodegeneration through the timely control of systemic inflammation. Ultimately, elucidating the molecular dialogue between the epidermis and cortex may allow clinicians to treat the skin as both a diagnostic window and a therapeutic gateway to the brain.
Author Contributions
Conceptualization, E.R. and V.-S.G.; methodology, K.L.; software, K.L.; validation, E.R. and V.K.; formal analysis, V.-S.G.; investigation, E.R.; resources, V.-S.G.; data curation, K.L.; writing—original draft preparation, V.-S.G. and E.R.; writing—review and editing, E.R. and V.-S.G.; visualization, K.L. and V.K.; supervision, V.K.; project administration, E.R.; funding acquisition, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
No conflicts of interest to declare.
Abbreviations
Alzheimer’s Disease (AD), Late-Onset AD (LOAD), Parkinson’s Disease (PD), Machine Learning (ML), Mild Cognitive Impairment (MCI) Central Nervous System (CNS), Blood–Brain Barrier (BBB), Alzheimer’s Disease Neuroimaging Initiative (ADNI) Hazard Ratio (HR), Amyloid-β (Aβ) Genome-Wide Association Study (GWAS), Differentially Expressed Genes (DEGs), Matrix Metalloproteinases (MMPs), α-synuclein (SNCA) Adenosine Monophosphate (AMP), Filaggrin (FLG), Bullous pemphigoid (BP), Hidradenitis Suppurativa (HS), Amyloid Precursor Protein (APP).
References
- Dermitzakis, I.; Chatzi, D.; Kyriakoudi, S.A.; Evangelidis, N.; Vakirlis, E.; Meditskou, S.; Theotokis, P.; Manthou, M.E. Skin Development and Disease: A Molecular Perspective. Curr. Issues Mol. Biol. 2024, 46, 8239–8267. [Google Scholar] [CrossRef]
- Kim, H.s.; Jung, H.; Park, Y.H.; Heo, S.-H.; Kim, S.; Moon, M. Skin-brain axis in Alzheimer’s disease—Pathologic, diagnostic, and therapeutic implications: A Hypothetical Review. Aging Dis. 2025, 16, 901–916. [Google Scholar] [CrossRef]
- Donovan, M.F.; Cascella, M. Embryology, Weeks 6–8. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zouboulis, C.C. The skin as an endocrine organ. Dermato-endocrinology 2009, 1, 250–252. [Google Scholar] [CrossRef]
- Hadian, Y.; Fregoso, D.; Nguyen, C.; Bagood, M.D.; Dahle, S.E.; Gareau, M.G.; Isseroff, R.R. Microbiome-skin-brain axis: A novel paradigm for cutaneous wounds. Wound Repair. Regen. 2020, 28, 282–292. [Google Scholar] [CrossRef]
- Wen, S.; Elias, P.M.; Wakefield, J.S.; Mauro, T.M.; Man, M.Q. The link between cutaneous inflammation and cognitive impairment. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1705–1712. [Google Scholar] [CrossRef]
- Magyari, A.; Ye, M.; Margolis, D.J.; McCulloch, C.E.; Cummings, S.R.; Yaffe, K.; Langan, S.M.; Abuabara, K. Adult atopic eczema and the risk of dementia: A population-based cohort study. J. Am. Acad. Dermatol. 2022, 87, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Matthewman, J.; Mansfield, K.E.; Cadogan, S.L.; Abuabara, K.; Smith, C.; Bhaskaran, K.; Langan, S.M.; Warren-Gash, C. Psoriasis and dementia: A population-based matched cohort study of adults in England. Ann. Clin. Transl. Neurol. 2025, 12, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Möbus, L.; Weidinger, S.; Emmert, H. Epigenetic factors involved in the pathophysiology of inflammatory skin diseases. J. Allergy Clin. Immunol. 2020, 145, 1049–1060. [Google Scholar] [CrossRef]
- Berson, A.; Nativio, R.; Berger, S.L.; Bonini, N.M. Epigenetic Regulation in Neurodegenerative Diseases. Trends Neurosci. 2018, 41, 587–598. [Google Scholar] [CrossRef]
- Dhakal, A.; Bobrin, B.D. Cognitive Deficits. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zingel, R.; Jacob, L.; Smith, L.; Konrad, M.; Kostev, K. Association Between Psoriasis and Dementia: A Retrospective Cohort Study. J. Alzheimers Dis. Rep. 2023, 7, 41–49. [Google Scholar] [CrossRef]
- Angelini, G.; Malvaso, A.; Schirripa, A.; Campione, F.; D’Addario, S.L.; Toschi, N.; Caligiore, D. Unraveling sex differences in Parkinson’s disease through explainable machine learning. J. Neurol. Sci. 2024, 462, 123091. [Google Scholar] [CrossRef]
- D’Amore, F.M.; Moscatelli, M.; Malvaso, A.; D’Antonio, F.; Rodini, M.; Panigutti, M.; Mirino, P.; Carlesimo, G.A.; Guariglia, C.; Caligiore, D. Explainable machine learning on clinical features to predict and differentiate Alzheimer’s progression by sex: Toward a clinician-tailored web interface. J. Neurol. Sci. 2025, 468, 123361. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.A.; Badri, T. Psoriasis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Dauden, E.; Blasco, A.J.; Bonanad, C.; Botella, R.; Carrascosa, J.M.; González-Parra, E.; Jodar, E.; Joven, B.; Lázaro, P.; Olveira, A.; et al. Position statement for the management of comorbidities in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2058–2073. [Google Scholar] [CrossRef]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 44, 863–872. [Google Scholar] [CrossRef]
- Veal, C.D.; Capon, F.; Allen, M.H.; Heath, E.K.; Evans, J.C.; Jones, A.; Patel, S.; Burden, D.; Tillman, D.; Barker, J.N.; et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am. J. Hum. Genet. 2002, 71, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Tsunemi, Y.; Saeki, H.; Nakamura, K.; Sekiya, T.; Hirai, K.; Fujita, H.; Asano, N.; Kishimoto, M.; Tanida, Y.; Kakinuma, T.; et al. Interleukin-12 p40 gene (IL12B) 3′-untranslated region polymorphism is associated with susceptibility to atopic dermatitis and psoriasis vulgaris. J. Dermatol. Sci. 2002, 30, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Park, J.S.; Lee, D.Y.; Youn, J.I.; Kim, I.G. Vitamin D receptor polymorphism is associated with psoriasis. J. Investig. Dermatol. 1999, 112, 113–116. [Google Scholar] [CrossRef]
- Furumoto, H.; Nakamura, K.; Imamura, T.; Hamamoto, Y.; Shimizu, T.; Muto, M.; Asagami, C. Association of apolipoprotein allele epsilon 2 with psoriasis vulgaris in Japanese population. Arch. Dermatol. Res. 1997, 289, 497–500. [Google Scholar] [CrossRef]
- Strittmatter, W.J.; Saunders, A.M.; Schmechel, D.; Pericak-Vance, M.; Enghild, J.; Salvesen, G.S.; Roses, A.D. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 1977–1981. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Risch, N.J.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Rimmler, J.B.; Locke, P.A.; Conneally, P.M.; Schmader, K.E.; et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 1994, 7, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, T.; Lu, L. Apolipoprotein E Gene Polymorphism in Psoriasis: A Meta-analysis. Arch. Med. Res. 2013, 44, 46–53. [Google Scholar] [CrossRef]
- Association, A.S. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.E.; Lee, S.H.; Han, K.; Lee, J.H. Increased risk of Alzheimer’s disease in patients with psoriasis: A nationwide population-based cohort study. Sci. Rep. 2020, 10, 6454. [Google Scholar] [CrossRef] [PubMed]
- Pezzolo, E.; Mutlu, U.; Vernooij, M.W.; Dowlatshahi, E.A.; Gisondi, P.; Girolomoni, G.; Nijsten, T.; Ikram, M.A.; Wakkee, M. Psoriasis is not associated with cognition, brain imaging markers, and risk for dementia: The Rotterdam Study. J. Am. Acad. Dermatol. 2021, 85, 671–680. [Google Scholar] [CrossRef]
- Mohammadi Shahrokhi, V.; Ravari, A.; Mirzaei, T.; Zare-Bidaki, M.; Asadikaram, G.; Arababadi, M.K. IL-17A and IL-23: Plausible risk factors to induce age-associated inflammation in Alzheimer’s disease. Immunol. Investig. 2018, 47, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, R.; Kaelber, D.C.; Gurney, M.E. Tumor Necrosis Factor (TNF) blocking agents are associated with lower risk for Alzheimer’s disease in patients with rheumatoid arthritis and psoriasis. PLoS ONE 2020, 15, e0229819. [Google Scholar] [CrossRef]
- Barak Levitt, J.A.; Ziv, M. Dementia Risk in Psoriasis Patients Treated with Biologics: A Propensity Score-matched Population-based Cohort Study. Acta Derm. Venereol. 2025, 105, adv43243. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Kong, X.; Mu, J.; Wang, Z.; Yang, X.; Ye, J. Association between psoriasis and serum apolipoprotein A1 and B: A systematic review and meta-analysis. Heliyon 2023, 9, e21168. [Google Scholar] [CrossRef]
- Capon, F.; Di Meglio, P.; Szaub, J.; Prescott, N.J.; Dunster, C.; Baumber, L.; Timms, K.; Gutin, A.; Abkevic, V.; Burden, A.D.; et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 2007, 122, 201–206. [Google Scholar] [CrossRef]
- Cargill, M.; Schrodi, S.J.; Chang, M.; Garcia, V.E.; Brandon, R.; Callis, K.P.; Matsunami, N.; Ardlie, K.G.; Civello, D.; Catanese, J.J.; et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 2007, 80, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.L.; Kimball, A.B.; Papp, K.A.; Yeilding, N.; Guzzo, C.; Wang, Y.; Li, S.; Dooley, L.T.; Gordon, K.B. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008, 371, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Tan, L.; Jiang, T.; Tan, M.S.; Zhang, W.; Yu, J.T. Association of IL-12A and IL-12B polymorphisms with Alzheimer’s disease susceptibility in a Han Chinese population. J. Neuroimmunol. 2014, 274, 180–184. [Google Scholar] [CrossRef]
- Colquhoun, M.; Kemp, A.K. Ustekinumab. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Schneeberger, S.; Kim, S.J.; Geesdorf, M.N.; Friebel, E.; Eede, P.; Jendrach, M.; Boltengagen, A.; Braeuning, C.; Ruhwedel, T.; Hülsmeier, A.J.; et al. Interleukin-12 signaling drives Alzheimer’s disease pathology through disrupting neuronal and oligodendrocyte homeostasis. Nat. Aging 2025, 5, 622–641. [Google Scholar] [CrossRef]
- Yokoyama, J.S.; Wang, Y.; Schork, A.J.; Thompson, W.K.; Karch, C.M.; Cruchaga, C.; McEvoy, L.K.; Witoelar, A.; Chen, C.H.; Holland, D.; et al. Association Between Genetic Traits for Immune-Mediated Diseases and Alzheimer Disease. JAMA Neurol. 2016, 73, 691–697. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, X.; Su, H.; Liu, F.; Zhuang, Z.; Chen, Y. ZNF384: A Potential Therapeutic Target for Psoriasis and Alzheimer’s Disease Through Inflammation and Metabolism. Front. Immunol. 2022, 13, 892368. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Yaddanapudi, S.S. Parkinson Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, AU, USA, 2018. [Google Scholar]
- Liepelt-Scarfone, I.; Ophey, A.; Kalbe, E. Cognition in prodromal Parkinson’s disease. Prog. Brain Res. 2022, 269, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Helmer, C.; Foubert-Samier, A.; Auriacombe, S.; Dartigues, J.F.; Tison, F. Risk of dementia in an elderly population of Parkinson’s disease patients: A 15-year population-based study. Alzheimers Dement. 2012, 8, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef]
- Rallis, E.; Grech, V.-S.; Lotsaris, K.; Tertipi, N.; Sfyri, E.; Kefala, V. Skin and Induced Pluripotent Stem Cells as Biomarkers for Neurodegenerative Diseases. Genes 2024, 15, 1507. [Google Scholar] [CrossRef]
- Sánchez--Danés, A.; Richaud--Patin, Y.; Carballo-Carbajal, I.; Jiménez-Delgado, S.; Caig, C.; Mora, S.; Di Guglielmo, C.; Ezquerra, M.; Patel, B.; Giralt, A.; et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012, 4, 380–395. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, K.; Gee, H.Y. The incidence rates and risk factors of Parkinson disease in patients with psoriasis: A nationwide population-based cohort study. J. Am. Acad. Dermatol. 2020, 83, 1688–1695. [Google Scholar] [CrossRef]
- Ungprasert, P.; Srivali, N.; Kittanamongkolchai, W. Risk of Parkinson’s Disease Among Patients with Psoriasis: A Systematic Review and Meta-analysis. Indian J. Dermatol. 2016, 61, 152–156. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Lin, J.; Cui, Y.; Shang, H. Psoriasis and progression of Parkinson’s disease: A Mendelian randomization study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2401–2405. [Google Scholar] [CrossRef] [PubMed]
- Witoelar, A.; Jansen, I.E.; Wang, Y.; Desikan, R.S.; Gibbs, J.R.; Blauwendraat, C.; Thompson, W.K.; Hernandez, D.G.; Djurovic, S.; Schork, A.J.; et al. Genome-wide Pleiotropy Between Parkinson Disease and Autoimmune Diseases. JAMA Neurol. 2017, 74, 780–792. [Google Scholar] [CrossRef]
- Farshchian, M.; Daveluy, S. Rosacea. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ahn, C.S.; Huang, W.W. Rosacea Pathogenesis. Dermatol. Clin. 2018, 36, 81–86. [Google Scholar] [CrossRef]
- Egeberg, A.; Hansen, P.R.; Gislason, G.H.; Thyssen, J.P. Patients with rosacea have increased risk of dementia. Ann. Neurol. 2016, 79, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Ravn, A.H.; Thyssen, J.P.; Egeberg, A. Skin disorders in Parkinson’s disease: Potential biomarkers and risk factors. Clin. Cosmet. Investig. Dermatol. 2017, 10, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Gemende, I.; Marsch, W.C.; Fischer, P.A. Skin function and skin disorders in Parkinson’s disease. J. Neural Transm. 2001, 108, 205–213. [Google Scholar] [CrossRef]
- Egeberg, A.; Hansen, P.R.; Gislason, G.H.; Thyssen, J.P. Exploring the Association Between Rosacea and Parkinson Disease: A Danish Nationwide Cohort Study. JAMA Neurol. 2016, 73, 529–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Wang, B.; Shi, W.; Hu, X.; Wang, Y.; Guo, Y.; Xie, H.; Xiao, W.; Li, J. Integrated Omics Reveal the Molecular Characterization and Pathogenic Mechanism of Rosacea. J. Investig. Dermatol. 2024, 144, 33–42.e2. [Google Scholar] [CrossRef] [PubMed]
- Karpouzis, A.; Avgeridis, P.; Tripsianis, G.; Gatzidou, E.; Kourmouli, N.; Veletza, S. Assessment of Tachykinin Receptor 3′ Gene Polymorphism rs3733631 in Rosacea. Int. Sch. Res. Not. 2015, 2015, 469402. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, Y.; Wang, Y.; Yan, S.; Xu, S.; Deng, Z.; Yang, X.; Xie, H.; Li, J. Bioinformatics and Network Pharmacology Identify the Therapeutic Role and Potential Mechanism of Melatonin in AD and Rosacea. Front. Immunol. 2021, 12, 756550. [Google Scholar] [CrossRef]
- Kolb, L.; Ferrer-Bruker, S.J. Atopic Dermatitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef]
- Eriksson, U.K.; Gatz, M.; Dickman, P.W.; Fratiglioni, L.; Pedersen, N.L. Asthma, Eczema, Rhinitis and the Risk for Dementia. Dement. Geriatr. Cogn. Disord. 2007, 25, 148–156. [Google Scholar] [CrossRef]
- Pan, T.-L.; Bai, Y.-M.; Cheng, C.-M.; Tsai, S.-J.; Tsai, C.-F.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Chen, T.-J.; Liang, C.-S.; et al. Atopic dermatitis and dementia risk: A nationwide longitudinal study. Ann. Allergy Asthma Immunol. 2021, 127, 200–205. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Kim, S.Y.; Woo, C.E.; Shin, K.; Son, E.; Kim, J.W.; Kim, S.J.; Song, T.J.; Park, H.R.; Kim, K.; et al. Association between Atopic Dermatitis and Dementia: Evidence from Systematic Review, Meta-analysis, and Mendelian Randomization. Acta Derm. Venereol. 2025, 105, adv41321. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Cai, J.; Li, R.; Wen, C.; Tan, H.; On Behalf Of The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Database. Rare Variant Analysis and Molecular Dynamics Simulation in Alzheimer’s Disease Identifies Exonic Variants in FLG. Genes 2022, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wu, C.Y.; Lin, M.W.; Chen, T.J.; Liao, K.K.; Chen, Y.C.; Hwang, C.Y.; Chu, S.Y.; Chen, C.C.; Lee, D.D.; et al. Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br. J. Dermatol. 2011, 165, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Brick, K.E.; Weaver, C.H.; Savica, R.; Lohse, C.M.; Pittelkow, M.R.; Boeve, B.F.; Gibson, L.E.; Camilleri, M.J.; Wieland, C.N. A population-based study of the association between bullous pemphigoid and neurologic disorders. J. Am. Acad. Dermatol. 2014, 71, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, A. Collagen XVII: A shared antigen in neurodermatological interactions? Clin. Dev. Immunol. 2013, 2013, 240570. [Google Scholar] [CrossRef]
- Amber, K.T.; Zikry, J.; Hertl, M. A multi-hit hypothesis of bullous pemphigoid and associated neurological disease: Is HLA-DQB1*03:01, a potential link between immune privileged antigen exposure and epitope spreading? HLA 2017, 89, 127–134. [Google Scholar] [CrossRef]
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; Wen, W.; Sun, J.; Su, B.; Liu, B.; Ma, D.; Lv, D.; Wen, Y.; Qu, T.; et al. Gamma-secretase gene mutations in familial acne inversa. Science 2010, 330, 1065. [Google Scholar] [CrossRef]
- Ingram, J.R. The Genetics of Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 23–28. [Google Scholar] [CrossRef]
- De Strooper, B.; Iwatsubo, T.; Wolfe, M.S. Presenilins and γ-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006304. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Tang, K.; Sun, Q. The Relationship Between Alzheimer’s Disease and Skin Diseases: A Review. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1551–1560. [Google Scholar] [CrossRef]
- Garg, A.; Strunk, A. Risk of Alzheimer’s disease is not increased among patients with hidradenitis suppurativa: A retrospective population-based cohort analysis. J. Am. Acad. Dermatol. 2017, 77, 176–177. [Google Scholar] [CrossRef]
- Theut Riis, P.; Egeberg, A.; Gislason, G.H.; Jemec, G.B. Patients with hidradenitis suppurativa have no increased risk of Alzheimer disease. Br. J. Dermatol. 2017, 177, 273–275. [Google Scholar] [CrossRef]
- Esme, P.; Esme, M.; Caliskan, E. Increased prevalence of family history of Alzheimer’s disease in hidradenitis suppurativa: Cross-sectional analysis of 192 HS patients. Dermatol. Ther. 2020, 33, e14219. [Google Scholar] [CrossRef] [PubMed]
- Ames, E.; Sanders, M.; Jacobs, M.; Vida, T.A. Unlocking the Mechanisms of Hidradenitis Suppurativa: Inflammation and miRNA Insights. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2829–2846. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Cosentino, F.I.I.; Ferri, R.; Lanuzza, B.; Siragusa, M.; Tripodi, M.; Schepis, C. Cognitive Impairment in Inpatients with Prurigo Nodularis and Psychiatric Comorbidities. Int. J. Environ. Res. Public Health 2021, 18, 6265. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Tai, Y.H.; Dai, Y.X.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Association between vitiligo and subsequent risk of dementia: A population-based cohort study. J. Dermatol. 2021, 48, 28–33. [Google Scholar] [CrossRef]
- Kim, T.-G.; Shin, J.; Hwang, S.; Lee, S.-H.; Chang Kim, H.; Oh, S.H. Risk for neurodegenerative disorders in Korean patients with vitiligo: A nationwide retrospective cohort study. J. Am. Acad. Dermatol. 2019, 81, 621–623. [Google Scholar] [CrossRef]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Marchina, E.; Misasi, S.; Bozzato, A.; Ferraboli, S.; Agosti, C.; Rozzini, L.; Borsani, G.; Barlati, S.; Padovani, A. Gene expression profile in fibroblasts of Huntington’s disease patients and controls. J. Neurol. Sci. 2014, 337, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Aladdin, A.; Király, R.; Boto, P.; Regdon, Z.; Tar, K. Juvenile Huntington’s Disease Skin Fibroblasts Respond with Elevated Parkin Level and Increased Proteasome Activity as a Potential Mechanism to Counterbalance the Pathological Consequences of Mutant Huntingtin Protein. Int. J. Mol. Sci. 2019, 20, 5338. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).