Longitudinal Multimodal Assessment of Structure and Function in INPP5E-Related Retinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. Molecular Genetics
2.3. Whole-Genome Sequencing (WGS) and Data Analysis
2.4. Sanger Sequencing
3. Results
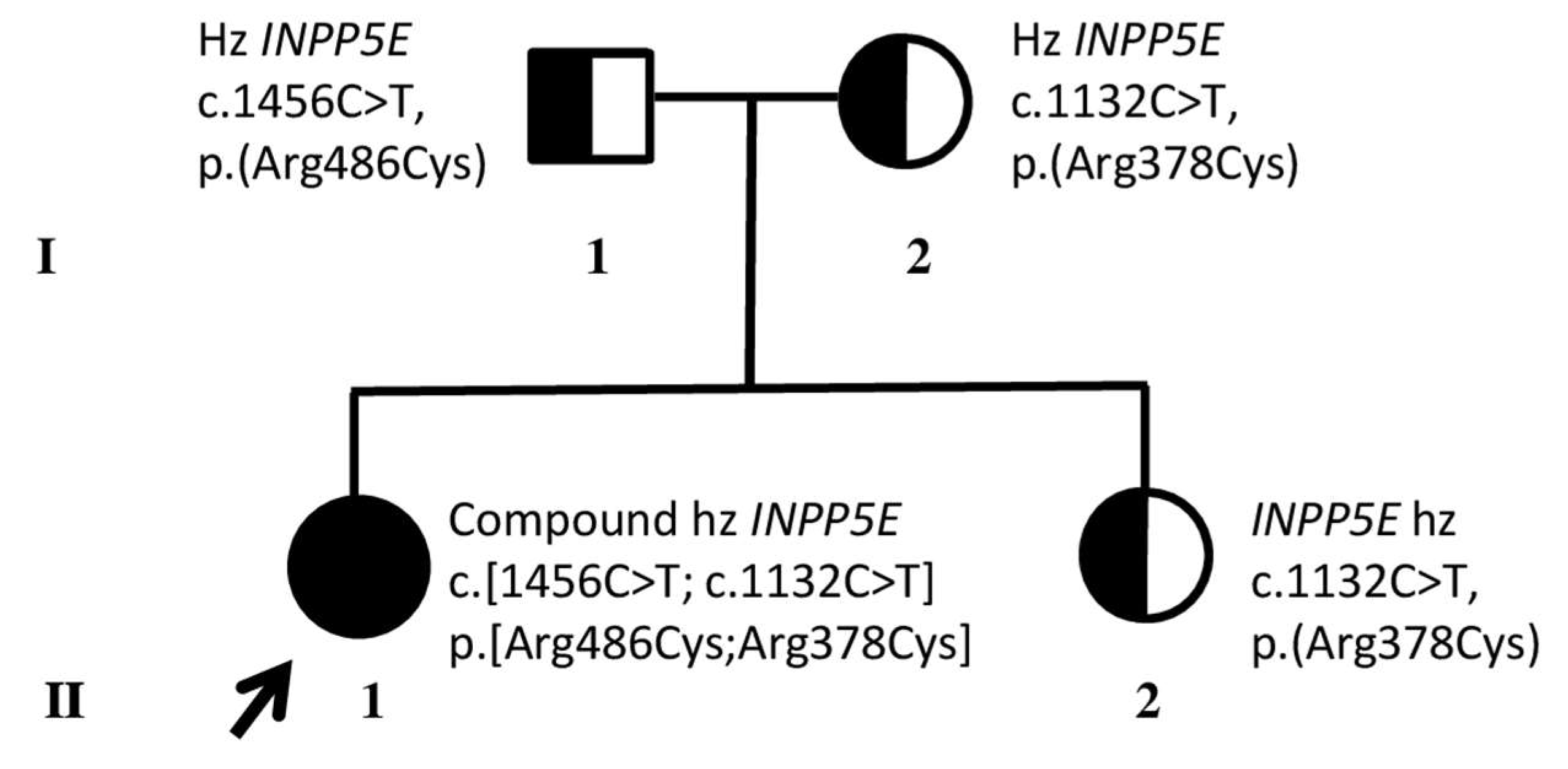
3.1. Identification of Compound Heterozygous INPP5E Pathogenic Variants
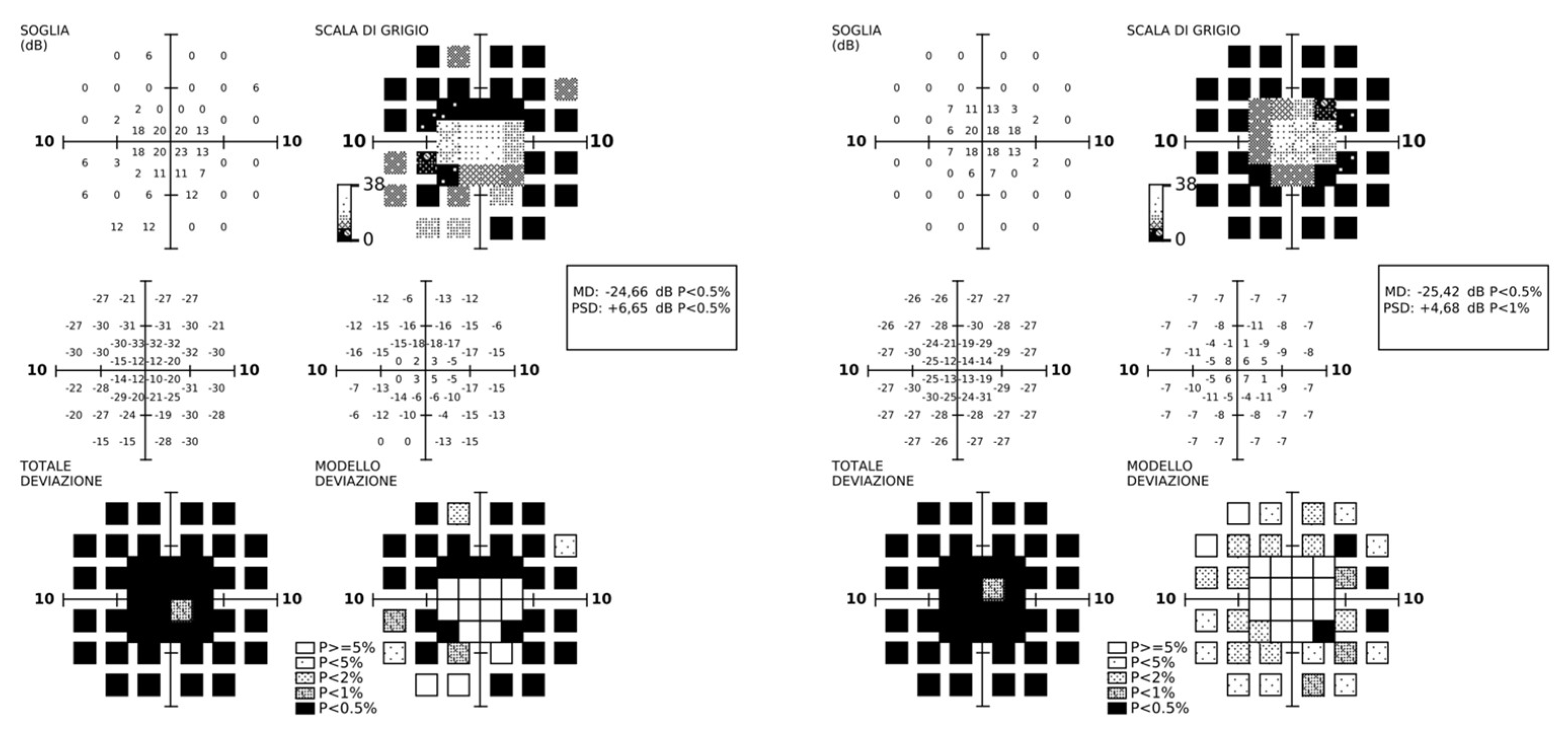
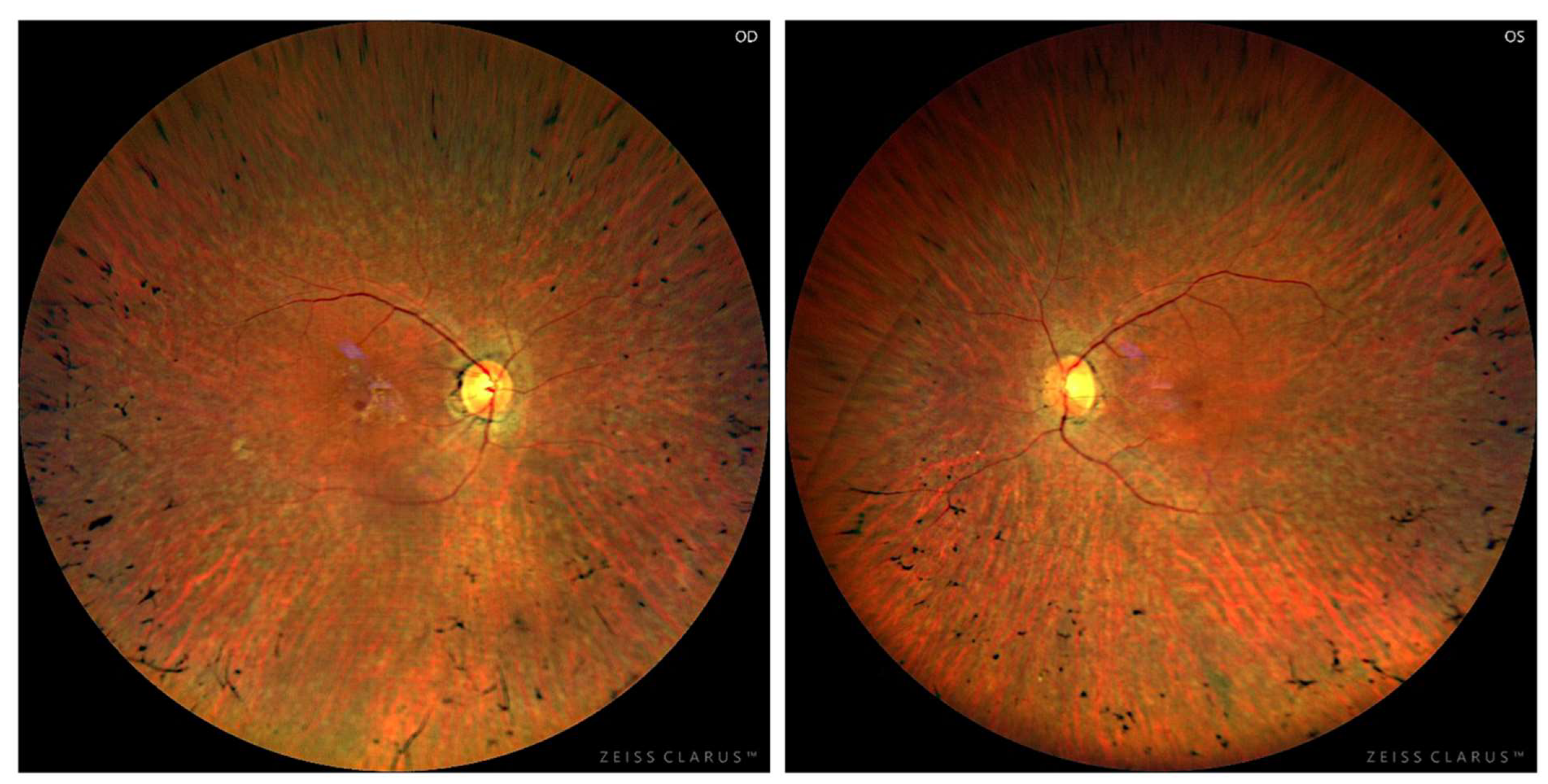
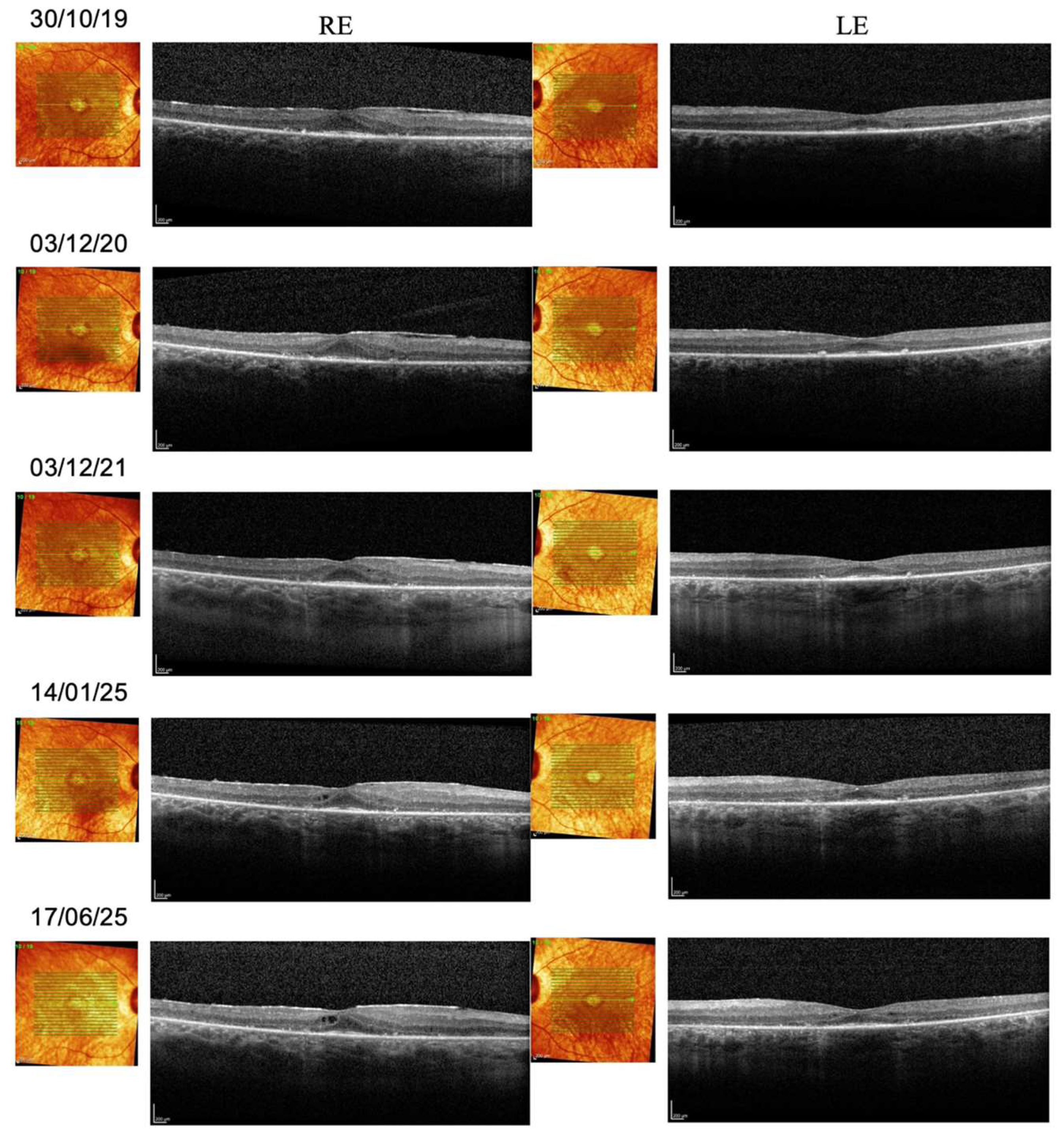
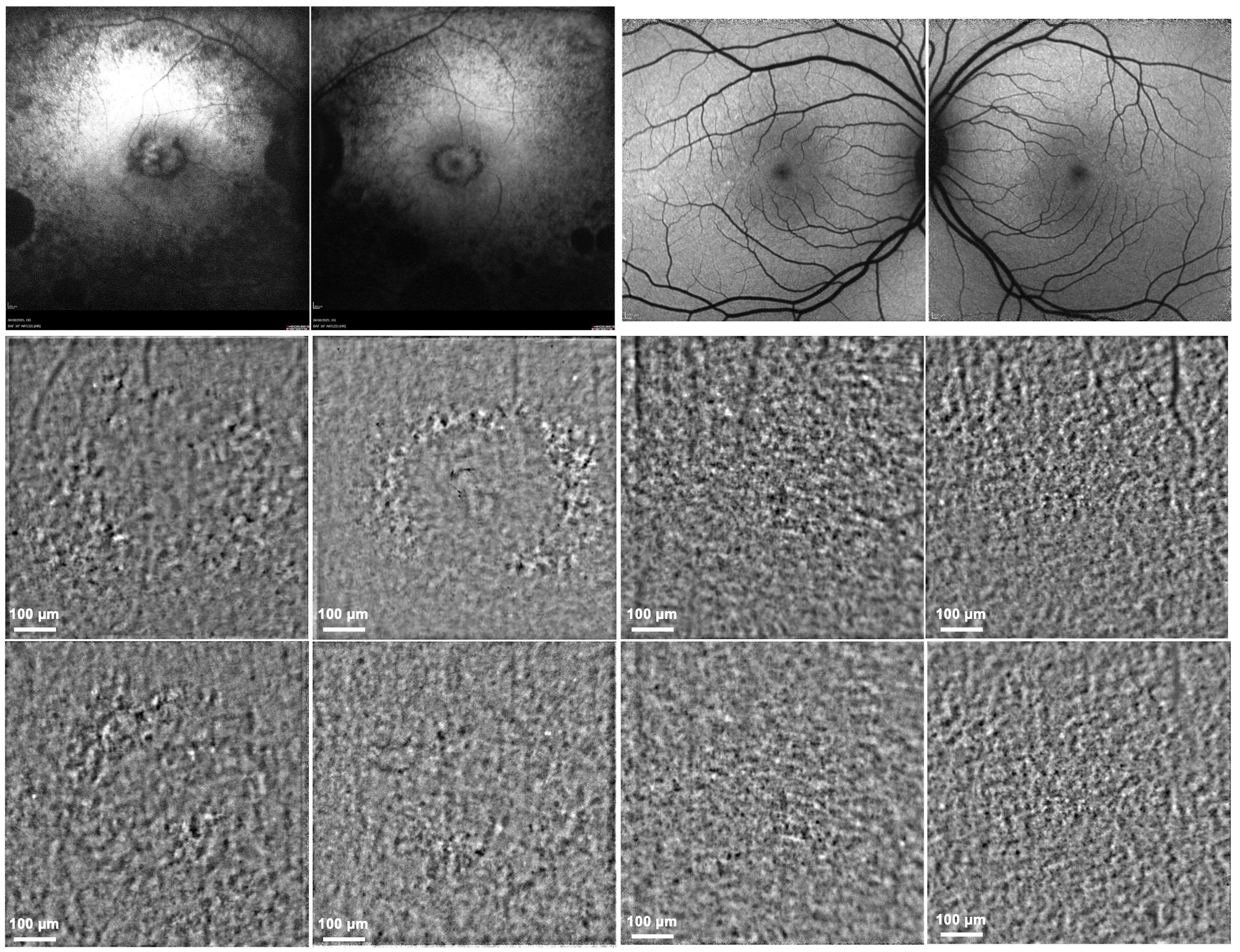
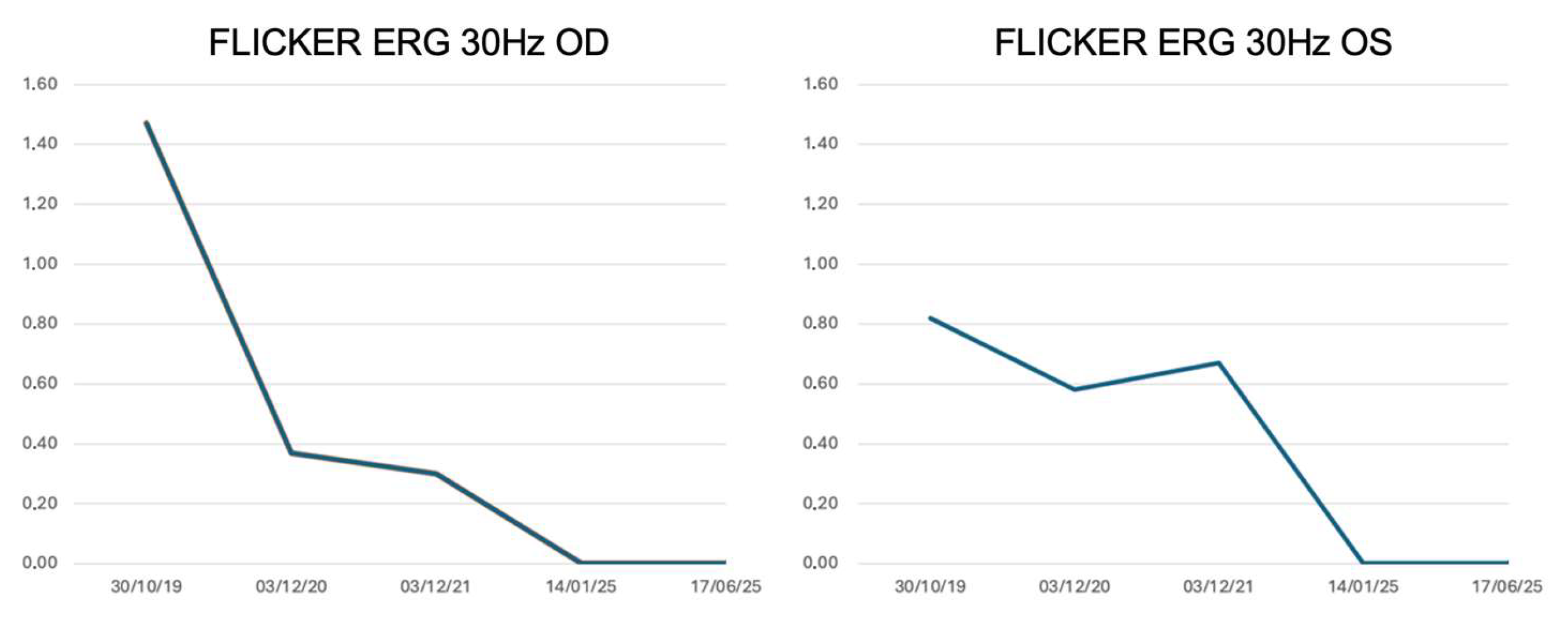
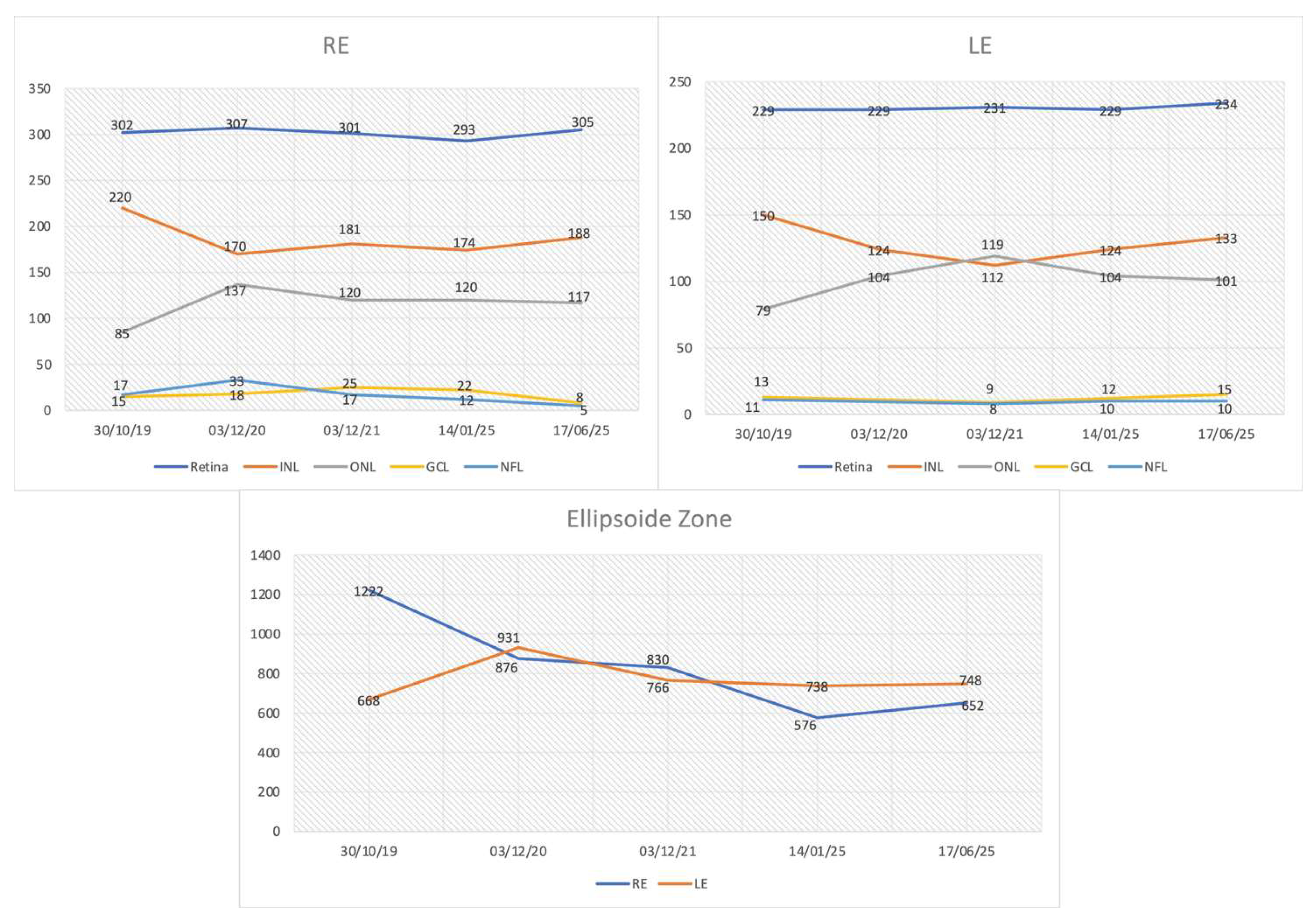
3.2. Longitudinal Structural and Functional Retinal Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| JBTS1 | Joubert Syndrome type 1 |
| MORMS | Impaired Intellectual Development, Truncal Obesity, Retinal Dystrophy, and Micropenis Syndrome |
| IRD | Inherited retinal disease |
| OCT | Optical coherence tomography |
| FAF | Fundus autofluorescence |
| EZ | Ellipsoid zone |
| ERG | Electroretinography |
| FDT | Frequency-doubling technology |
| AO-TFI | Adaptive optics transscleral flood illumination |
| PR | Photoreceptor |
| RPE | Retinal pigment epithelium |
| WGS | Whole-genome sequencing |
| ONL | Outer nuclear layer |
| INL | Inner nuclear layer |
| GCL | Ganglion cell layer |
| RE | Right eye |
| LE | Left eye |
References
- Hakeem, A.; Yang, S. Regulation of INPP5E in Ciliogenesis, Development, and Disease. Int. J. Biol. Sci. 2025, 21, 579–594. [Google Scholar] [CrossRef]
- Dyson, J.M.; Conduit, S.E.; Feeney, S.J.; Hakim, S.; DiTommaso, T.; Fulcher, A.J.; Sriratana, A.; Ramm, G.; Horan, K.A.; Gurung, R.; et al. INPP5E Regulates Phosphoinositide-Dependent Cilia Transition Zone Function. J. Cell Biol. 2017, 216, 247–263. [Google Scholar] [CrossRef]
- Sangermano, R.; Deitch, I.; Peter, V.G.; Ba-Abbad, R.; Place, E.M.; Zampaglione, E.; Wagner, N.E.; Fulton, A.B.; Coutinho-Santos, L.; Rosin, B.; et al. Broadening INPP5E Phenotypic Spectrum: Detection of Rare Variants in Syndromic and Non-Syndromic IRD. NPJ Genom. Med. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Peter, V.G.; Kaminska, K.; Santos, C.; Quinodoz, M.; Cancellieri, F.; Cisarova, K.; Pescini Gobert, R.; Rodrigues, R.; Custódio, S.; Paris, L.P.; et al. The First Genetic Landscape of Inherited Retinal Dystrophies in Portuguese Patients Identifies Recurrent Homozygous Mutations as a Frequent Cause of Pathogenesis. PNAS Nexus 2023, 2, pgad043. [Google Scholar] [CrossRef] [PubMed]
- Karali, M.; Testa, F.; Di Iorio, V.; Torella, A.; Zeuli, R.; Scarpato, M.; Romano, F.; Onore, M.E.; Pizzo, M.; Melillo, P.; et al. Genetic Epidemiology of Inherited Retinal Diseases in a Large Patient Cohort Followed at a Single Center in Italy. Sci. Rep. 2022, 12, 20815. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Martelli, F.; Zein, W.M.; Jeffrey, B.; Placidi, G.; Sieving, P.A.; Falsini, B. Statistical Evaluation of ERG Responses: A New Method to Validate Cycle-by-Cycle Recordings in Advanced Retinal Degenerations. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3. [Google Scholar] [CrossRef]
- Laforest, T.; Künzi, M.; Kowalczuk, L.; Carpentras, D.; Behar-Cohen, F.; Moser, C. Transscleral Optical Phase Imaging of the Human Retina. Nat. Photonics 2020, 14, 439–445. [Google Scholar] [CrossRef]
- Available online: http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi (accessed on 15 September 2025).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Bielas, S.L.; Silhavy, J.L.; Brancati, F.; Kisseleva, M.V.; Al-Gazali, L.; Sztriha, L.; Bayoumi, R.A.; Zaki, M.S.; Abdel-Aleem, A.; Rosti, R.O.; et al. Mutations in INPP5E, Encoding Inositol Polyphosphate-5-Phosphatase E, Link Phosphatidyl Inositol Signaling to the Ciliopathies. Nat. Genet. 2009, 41, 1032–1036. [Google Scholar] [CrossRef]
- Benkirane, M.; Marelli, C.; Guissart, C.; Roubertie, A.; Ollagnon, E.; Choumert, A.; Fluchère, F.; Magne, F.O.; Halleb, Y.; Renaud, M.; et al. High Rate of Hypomorphic Variants as the Cause of Inherited Ataxia and Related Diseases: Study of a Cohort of 366 Families. Genet. Med. 2021, 23, 2160–2170. [Google Scholar] [CrossRef]
- Walia, S.; Fishman, G.A.; Hajali, M. Prevalence of Cystic Macular Lesions in Patients with Usher II Syndrome. Eye 2009, 23, 1206–1209. [Google Scholar] [CrossRef]
- Ben-Avi, R.; Rivera, A.; Hendler, K.; Sharon, D.; Banin, E.; Khateb, S.; Yahalom, C. Prevalence and Associated Factors of Cystoid Macular Edema in Children with Early Onset Inherited Retinal Dystrophies. Eur. J. Ophthalmol. 2023, 33, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.H.; Zhang, H.Y.; Ho, A.; Francis, P.; Weleber, R.G.; Birch, D.G.; Ferris, F.L.; Sadda, S.R.; for the Trial of Oral Valproic Acid for Retinitis Pigmentosa Group. Quantification of Ellipsoid Zone Changes in Retinitis Pigmentosa Using En Face Spectral Domain–Optical Coherence Tomography. JAMA Ophthalmol. 2016, 134, 628. [Google Scholar] [CrossRef] [PubMed]
- Zada, M.; Cornish, E.E.; Fraser, C.L.; Jamieson, R.V.; Grigg, J.R. Natural History and Clinical Biomarkers of Progression in X--linked Retinitis Pigmentosa: A Systematic Review. Acta Ophthalmol. 2021, 99, 499–510. [Google Scholar] [CrossRef]
- Heyang, M.; Warren, J.L.; Ocieczek, P.; Duncan, J.L.; Moosajee, M.; Del Priore, L.V.; Shen, L.L. Long-Term Natural History of Ellipsoid Zone Width in USH2A-Retinopathy. Br. J. Ophthalmol. 2025, 109, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Gersch, J.; Hufendiek, K.; Delarocque, J.; Framme, C.; Jacobsen, C.; Stöhr, H.; Kellner, U.; Hufendiek, K. Investigation of Structural Alterations in Inherited Retinal Diseases: A Quantitative SD-OCT-Analysis of Retinal Layer Thicknesses in Light of Underlying Genetic Mutations. Int. J. Mol. Sci. 2022, 23, 16007. [Google Scholar] [CrossRef]
- Cusumano, A.; Falsini, B.; D’Apolito, F.; D’Ambrosio, M.; Sebastiani, J.; Cascella, R.; Barati, S.; Giardina, E. Longitudinal Structure–Function Evaluation in a Patient with CDHR1-Associated Retinal Dystrophy: Progressive Visual Function Loss with Retinal Remodeling. Diagnostics 2023, 13, 392. [Google Scholar] [CrossRef]
- Arsiwalla, T.A.; Cornish, E.E.; Nguyen, P.V.; Korsakova, M.; Ali, H.; Saakova, N.; Fraser, C.L.; Jamieson, R.V.; Grigg, J.R. Assessing Residual Cone Function in Retinitis Pigmentosa Patients. Transl. Vis. Sci. Technol. 2020, 9, 29. [Google Scholar] [CrossRef]
- Comander, J.; Weigel DiFranco, C.; Sanderson, K.; Place, E.; Maher, M.; Zampaglione, E.; Zhao, Y.; Huckfeldt, R.M.; Bujakowska, K.M.; Pierce, E. Natural History of Retinitis Pigmentosa Based on Genotype, Vitamin A/E Supplementation, and an Electroretinogram Biomarker. JCI Insight 2023, 8, e167546. [Google Scholar] [CrossRef]
- Cai, C.X.; Locke, K.G.; Ramachandran, R.; Birch, D.G.; Hood, D.C. A Comparison of Progressive Loss of the Ellipsoid Zone (EZ) Band in Autosomal Dominant and X-Linked Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7417. [Google Scholar] [CrossRef]
- Sather, R.; Ihinger, J.; Simmons, M.; Khundkar, T.; Lobo, G.P.; Montezuma, S.R. Clinical Characteristics and Genetic Variants of a Large Cohort of Patients with Retinitis Pigmentosa Using Multimodal Imaging and Next Generation Sequencing. Int. J. Mol. Sci. 2023, 24, 10895. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, A.J.; Raji, S.; Cehajic Kapetanovic, J. Visualising Treatment Effects in Low-Vision Settings: Proven and Potential Endpoints for Clinical Trials of Inherited Retinal Disease Therapies. Gene Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusumano, A.; Lombardo, M.; Falsini, B.; D’Ambrosio, M.; Sebastiani, J.; Marchionni, E.; D’Apice, M.R.; Rizzacasa, B.; Martelli, F.; Novelli, G. Longitudinal Multimodal Assessment of Structure and Function in INPP5E-Related Retinopathy. Genes 2025, 16, 1407. https://doi.org/10.3390/genes16121407
Cusumano A, Lombardo M, Falsini B, D’Ambrosio M, Sebastiani J, Marchionni E, D’Apice MR, Rizzacasa B, Martelli F, Novelli G. Longitudinal Multimodal Assessment of Structure and Function in INPP5E-Related Retinopathy. Genes. 2025; 16(12):1407. https://doi.org/10.3390/genes16121407
Chicago/Turabian StyleCusumano, Andrea, Marco Lombardo, Benedetto Falsini, Michele D’Ambrosio, Jacopo Sebastiani, Enrica Marchionni, Maria Rosaria D’Apice, Barbara Rizzacasa, Francesco Martelli, and Giuseppe Novelli. 2025. "Longitudinal Multimodal Assessment of Structure and Function in INPP5E-Related Retinopathy" Genes 16, no. 12: 1407. https://doi.org/10.3390/genes16121407
APA StyleCusumano, A., Lombardo, M., Falsini, B., D’Ambrosio, M., Sebastiani, J., Marchionni, E., D’Apice, M. R., Rizzacasa, B., Martelli, F., & Novelli, G. (2025). Longitudinal Multimodal Assessment of Structure and Function in INPP5E-Related Retinopathy. Genes, 16(12), 1407. https://doi.org/10.3390/genes16121407








