Unexpected Diagnosis of Fahr’s Disease in a Patient with Severe Obesity and a Heterozygotic Variant in the TMEM67 Gene
Abstract
1. Introduction
2. Case Presentation
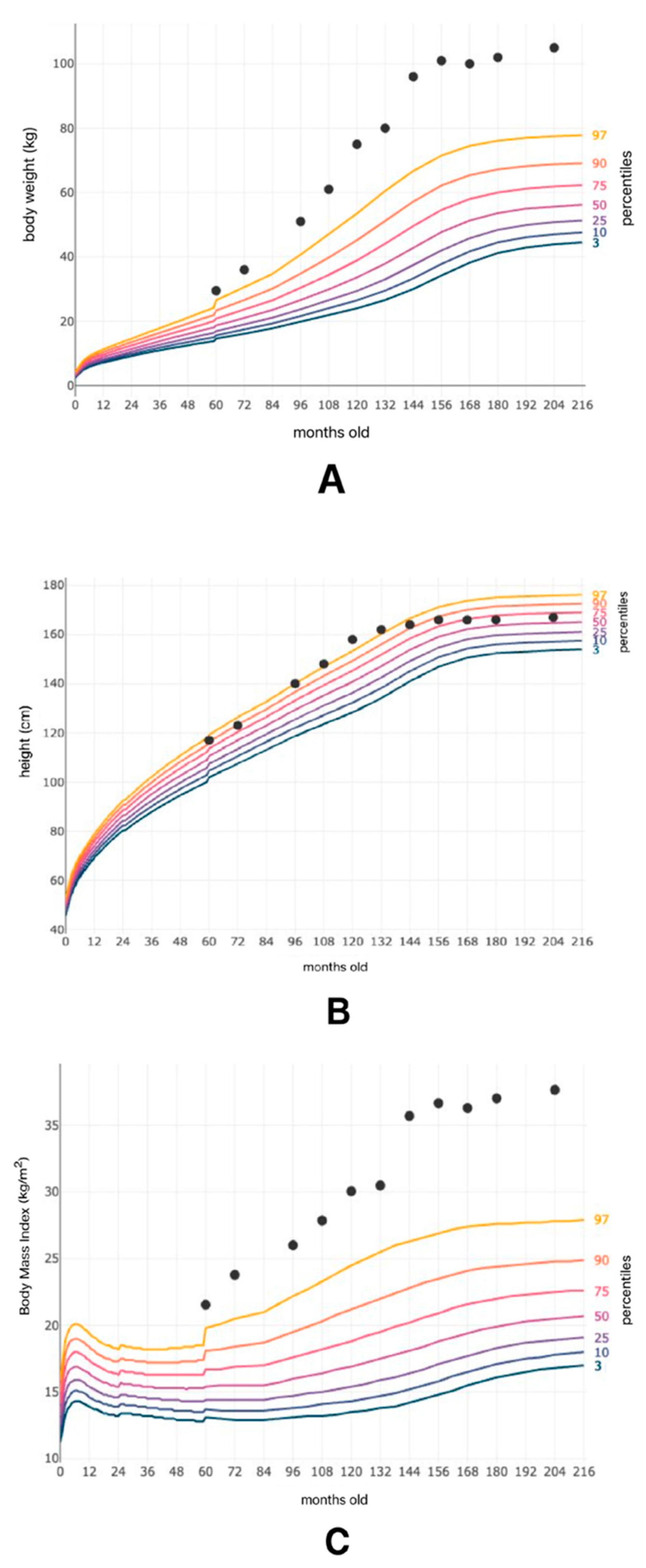
2.1. Detailed Case Description
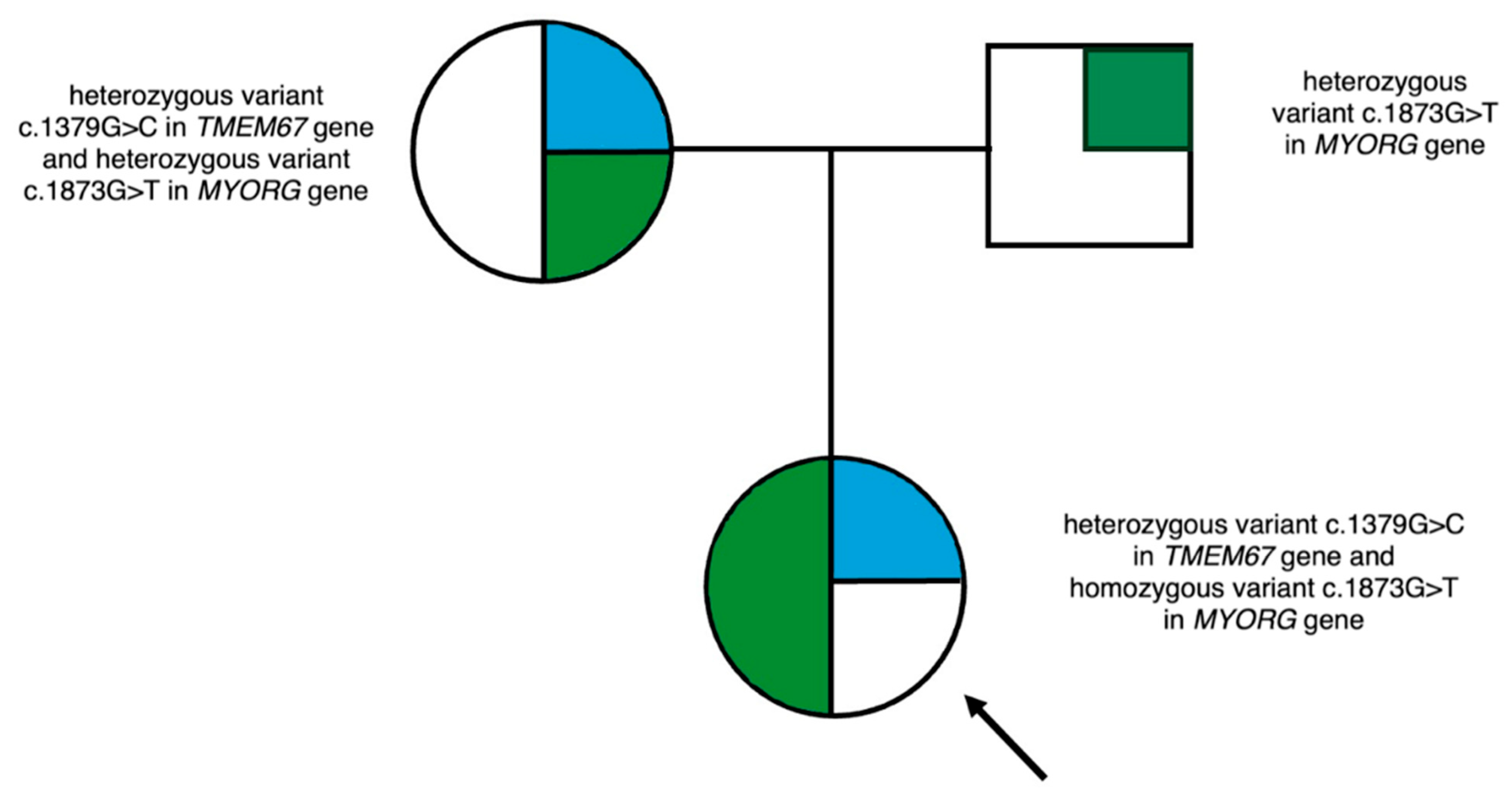
2.2. Molecular Analysis
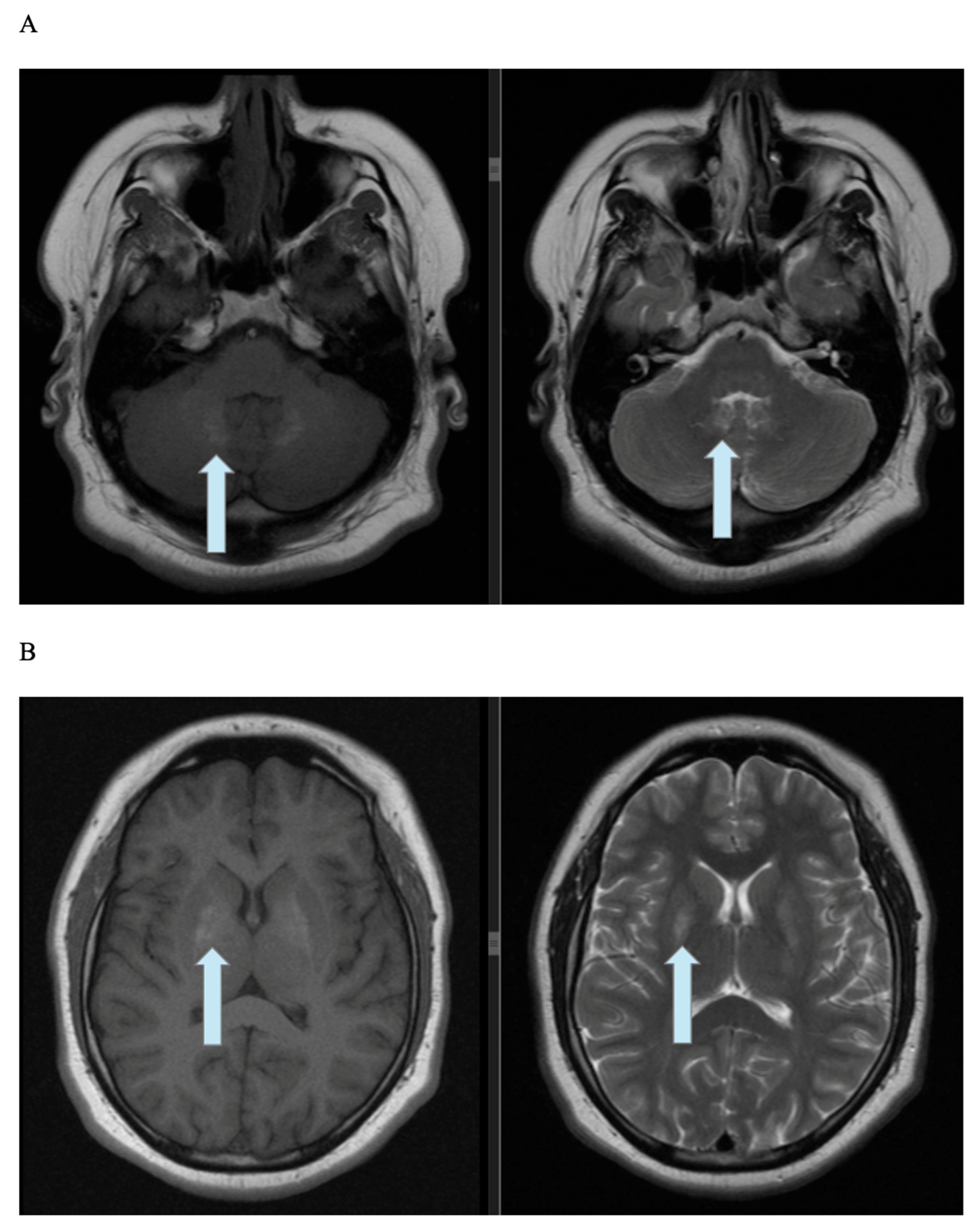
2.3. Imaging
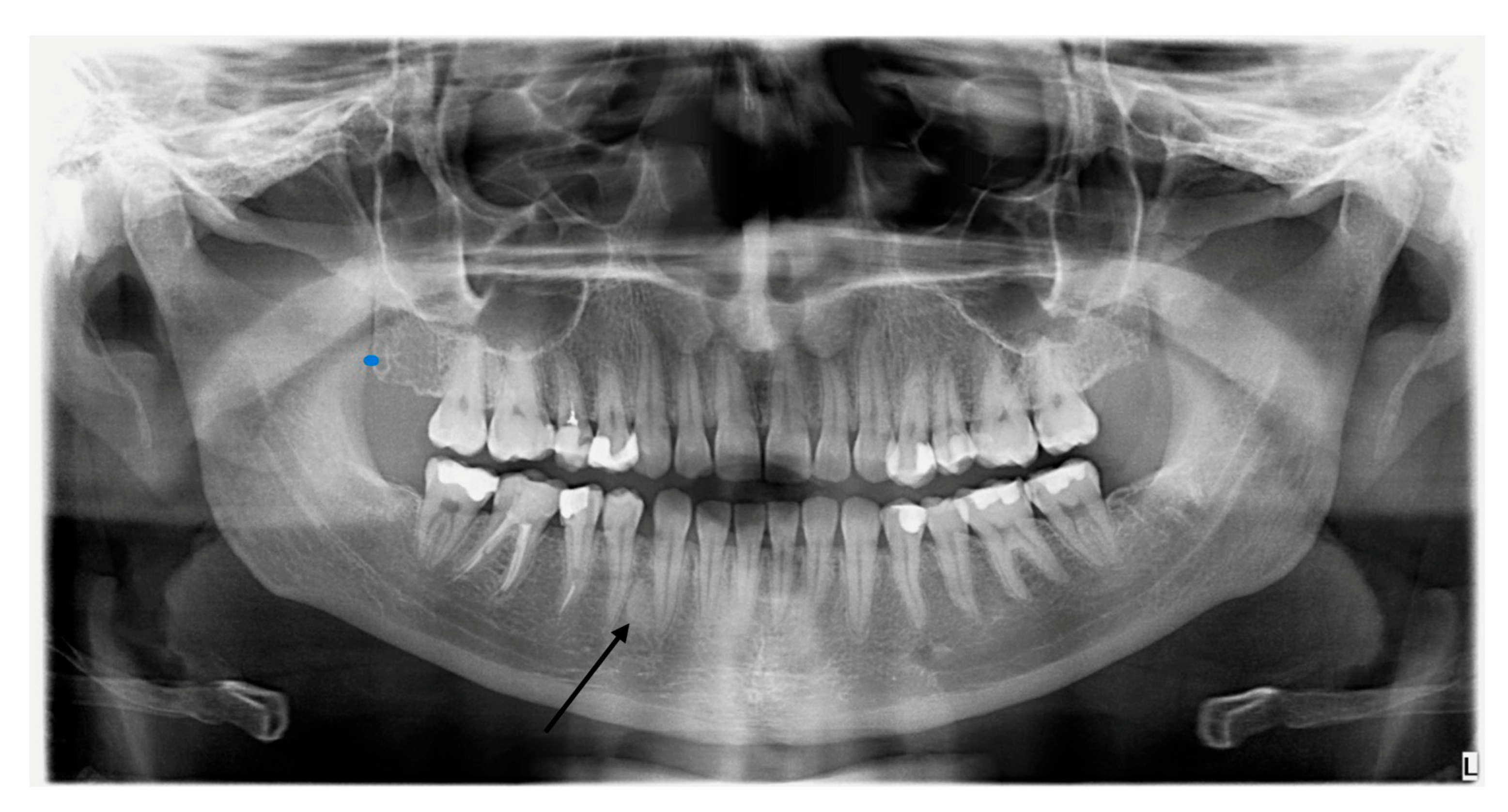
2.4. Dental Examination
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| BBS | Bardet–Biedl syndrome |
| CBCT | cone-beam computed tomography |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GLP-1 | glucagon-like peptide-1 |
| NGS | next-generation sequencing |
| HbA1c | glycated hemoglobin |
| IFG | impaired fasting glycaemia |
| MRI | magnetic resonance imaging |
| OGTT | oral glucose tolerance test |
| PCOS | polycystic ovarian syndrome |
| VUS | variant of unknown significance |
References
- Tomlinson, J.W. Bardet-Biedl syndrome: A focus on genetics, mechanisms and metabolic dysfunction. Diabetes Obes. Metab. 2024, 26 (Suppl. S2), 13–24. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, A. Bardet-Biedl syndrome: A clinical overview focusing on diagnosis, outcomes and best-practice management. Diabetes Obes. Metab. 2024, 26 (Suppl. S2), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, R.; Gunay-Aygun, M. Bardet-Biedl Syndrome Overview. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2003. [Google Scholar] [PubMed]
- Abuzzahab, M.J.; Dubern, B.; Goldstone, A.P.; Haqq, A.M.; Heymsfield, S.B.; Miller, J.L.; Richards, J.; Wabitsch, M.; Yanovski, J.A. Improving the diagnosis of hyperphagia in melanocortin-4 receptor pathway diseases. Obesity 2025, 33, 1217–1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yalcin, E.D.; Ararat, E. Oral and dental findings in Bardet-Biedl syndrome: A case report. Niger. J. Clin. Pract. 2019, 22, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, U.; Arya, G.; Singh, S.; Pillai, A.; Nair, P.P. Oro-dental findings in Bardet-Biedl syndrome. BMJ Case Rep. 2012, 2012, bcr1220115320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hampl, M.; Cela, P.; Szabo-Rogers, H.L.; Kunova Bosakova, M.; Dosedelova, H.; Krejci, P.; Buchtova, M. Role of Primary Cilia in Odontogenesis. J. Dent. Res. 2017, 96, 965–974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melluso, A.; Secondulfo, F.; Capolongo, G.; Capasso, G.; Zacchia, M. Bardet-Biedl Syndrome: Current Perspectives and Clinical Outlook. Ther. Clin. Risk Manag. 2023, 19, 115–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haqq, A.M.; Chung, W.K.; Dollfus, H.; Haws, R.M.; Martos-Moreno, G.Á.; Poitou, C.; Yanovski, J.A.; Mittleman, R.S.; Yuan, G.; Forsythe, E.; et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022, 10, 859–868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomlinson, J.W. Should GLP-1 receptor agonist therapy be used to treat obesity in Bardet-Biedl syndrome? J. Clin. Investig. 2025, 135, e191822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dollfus, H.; Lilien, M.R.; Maffei, P.; Verloes, A.; Muller, J.; Bacci, G.M.; Cetiner, M.; van den Akker, E.L.T.; Grudzinska Pechhacker, M.; Testa, F.; et al. Bardet-Biedl syndrome improved diagnosis criteria and management: Inter European Reference Networks consensus statement and recommendations. Eur. J. Hum. Genet. 2024, 32, 1347–1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Supit, V.D.; Kurniawan, D.; Fatimah, E. Fahr syndrome and neurological manifestations in hypoparathyroidism patients. Radiol. Case Rep. 2024, 19, 1248–1253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.Y.; Ho, C.J.; Lu, Y.T.; Lin, C.H.; Lan, M.Y.; Tsai, M.H. The Genetics of Primary Familial Brain Calcification: A Literature Review. Int. J. Mol. Sci. 2023, 24, 10886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, D.; Lu, Y.; Huang, H.; Chen, Y.; Jiang, Z.; Yao, R.; Bu, Y.; Li, Y.; Cen, Z.; Luo, W. Gene Variants Related to Primary Familial Brain Calcification: Perspectives from Bibliometrics and Meta-Analysis. eNeuro 2025, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Xu, T.; Zhu, Y.; Duan, X. Fahr’s Syndrome with Pseudohypoparathyroidism: Oral Features and Genetic Insights. Int. J. Mol. Sci. 2024, 25, 11611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amisha, F.; Munakomi, S. Fahr Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Balck, A.; Schaake, S.; Kuhnke, N.S.; Domingo, A.; Madoev, H.; Margolesky, J.; Dobricic, V.; Alvarez-Fischer, D.; Laabs, B.H.; Kasten, M.; et al. Genotype-Phenotype Relations in Primary Familial Brain Calcification: Systematic MDSGene Review. Mov. Disord. 2021, 36, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Mufaddel, A.A.; Al-Hassani, G.A. Familial idiopathic basal ganglia calcification (Fahr’s disease). Neurosciences 2014, 19, 171–177. [Google Scholar] [PubMed] [PubMed Central]
- Perugula, M.L.; Lippmann, S. Fahr’s Disease or Fahr’s Syndrome? Innov. Clin. Neurosci. 2016, 13, 45–46. [Google Scholar] [PubMed] [PubMed Central]
- Bekiesinska-Figatowska, M.; Mierzewska, H.; Jurkiewicz, E. Basal ganglia lesions in children and adults. Eur. J. Radiol. 2013, 82, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Spadaro, L.; Marra, A.; Bramanti, P. Fahr’s disease presenting with dementia at onset: A case report and literature review. Behav. Neurol. 2014, 2014, 750975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avrahami, E.; Cohn, D.F.; Feibel, M.; Tadmor, R. MRI demonstration and CT correlation of the brain in patients with idiopathic intracerebral calcification. J. Neurol. 1994, 241, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Yan, Y.; Mao, J.X.; Gao, R.T. Cone-beam computed tomography assessment of the prevalence and distribution of bone islands of the jaws in a group of chinese patients. BMC Oral Health 2025, 25, 1443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forsythe, E.; Kenny, J.; Bacchelli, C.; Beales, P.L. Managing Bardet-Biedl Syndrome-Now and in the Future. Front. Pediatr. 2018, 6, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forsythe, E.; Beales, P.L. Bardet–Biedl syndrome. Eur. J. Hum. Genet. 2013, 21, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Croft, J.B.; Morrell, D.; Chase, C.L.; Swift, M. Obesity in heterozygous carriers of the gene for the Bardet-Biedl syndrome. Am. J. Med. Genet. 1995, 55, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Beales, P.L.; Reid, H.A.; Griffiths, M.H.; Maher, E.R.; Flinter, F.A.; Woolf, A.S. Renal cancer and malformations in relatives of patients with Bardet-Biedl syndrome. Nephrol. Dial. Transplant. 2000, 15, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Chen, I.C.; Yang, H.W.; Yen, H.C.; Huang, Y.C.; Hsu, C.C.; Chen, Y.M.; Ke, Y.Y. The characterization and comorbidities of heterozygous Bardet-Biedl syndrome carriers. Int. J. Med. Sci. 2024, 21, 784–794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roberts, K.J.; Ariza, A.J.; Selvaraj, K.; Quadri, M.; Mangarelli, C.; Neault, S.; Davis, E.E.; Binns, H.J. Testing for rare genetic causes of obesity: Findings and experiences from a pediatric weight management program. Int. J. Obes. 2022, 46, 1493–1501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Day, M.L.; Avila, C.C.; Novak, D.L. Hydrometrocolpos and postaxial polydactyly in a girl newborn: A case report. Clin. Case Rep. 2022, 10, e05453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashkinadze, E.; Rosen, T.; Brooks, S.S.; Katsanis, N.; Davis, E.E. Combining fetal sonography with genetic and allele pathogenicity studies to secure a neonatal diagnosis of Bardet-Biedl syndrome. Clin. Genet. 2013, 83, 553–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoskins, B.E.; Thorn, A.; Scambler, P.J.; Beales, P.L. Evaluation of multiplex capillary heteroduplex analysis: A rapid and sensitive mutation screening technique. Hum. Mutat. 2003, 22, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rustad, C.F.; Bragadottir, R.; Tveten, K.; Nordgarden, H.; Miller, J.U.; Åsten, P.M.; Vasconcelos, G.; Kulseth, M.A.; Holla, Ø.L.; Olsen, H.G.; et al. Clinical and genetic aspects of Bardet-Biedl syndrome in adults in Norway. Orphanet J. Rare Dis. 2025, 20, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shamseldin, H.E.; Shaheen, R.; Ewida, N.; Bubshait, D.K.; Alkuraya, H.; Almardawi, E.; Howaidi, A.; Sabr, Y.; Abdalla, E.M.; Alfaifi, A.Y.; et al. The morbid genome of ciliopathies: An update. Genet Med. 2020, 22, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarska, K.; Oczoś, P.; Grzybowska-Adamowicz, J.; Zmysłowska-Polakowska, E.; Pietrusiński, M.; Zmysłowska, A. Unexpected Diagnosis of Fahr’s Disease in a Patient with Severe Obesity and a Heterozygotic Variant in the TMEM67 Gene. Genes 2025, 16, 1406. https://doi.org/10.3390/genes16121406
Piekarska K, Oczoś P, Grzybowska-Adamowicz J, Zmysłowska-Polakowska E, Pietrusiński M, Zmysłowska A. Unexpected Diagnosis of Fahr’s Disease in a Patient with Severe Obesity and a Heterozygotic Variant in the TMEM67 Gene. Genes. 2025; 16(12):1406. https://doi.org/10.3390/genes16121406
Chicago/Turabian StylePiekarska, Katarzyna, Paulina Oczoś, Julia Grzybowska-Adamowicz, Ewa Zmysłowska-Polakowska, Michał Pietrusiński, and Agnieszka Zmysłowska. 2025. "Unexpected Diagnosis of Fahr’s Disease in a Patient with Severe Obesity and a Heterozygotic Variant in the TMEM67 Gene" Genes 16, no. 12: 1406. https://doi.org/10.3390/genes16121406
APA StylePiekarska, K., Oczoś, P., Grzybowska-Adamowicz, J., Zmysłowska-Polakowska, E., Pietrusiński, M., & Zmysłowska, A. (2025). Unexpected Diagnosis of Fahr’s Disease in a Patient with Severe Obesity and a Heterozygotic Variant in the TMEM67 Gene. Genes, 16(12), 1406. https://doi.org/10.3390/genes16121406





