Epigenetic Modulation and Neuroprotective Effects of Neurofabine-C in a Transgenic Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Models
2.2. Biochemical Characterization of Neurofabine-C Extract
2.3. Blood Sample Preparation
2.4. Brain Sample Preparation
2.5. Epigenetic Expression Analysis
2.6. Immunohistochemistry
2.7. Imaging
2.8. Statistical Analysis
3. Results
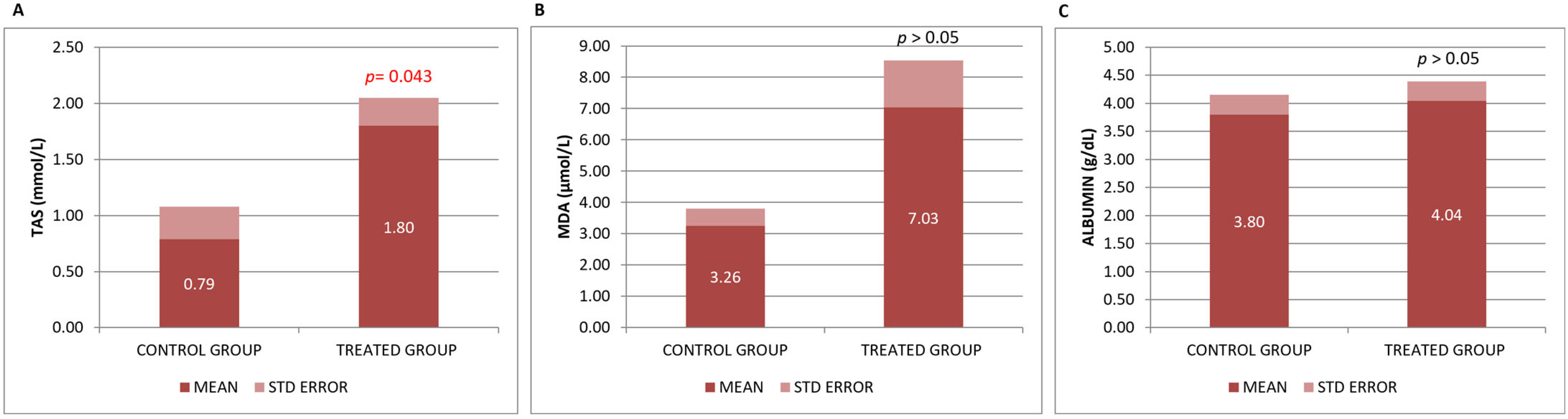
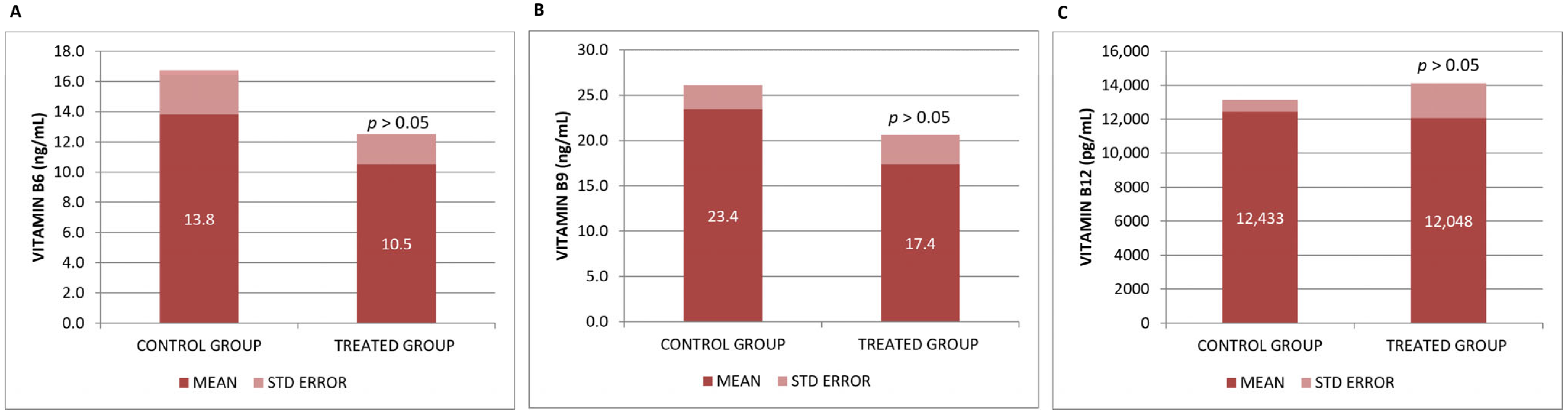
3.1. Biochemical Effects of the Neurofabine-C on Metabolic and Neurodegeneration Response
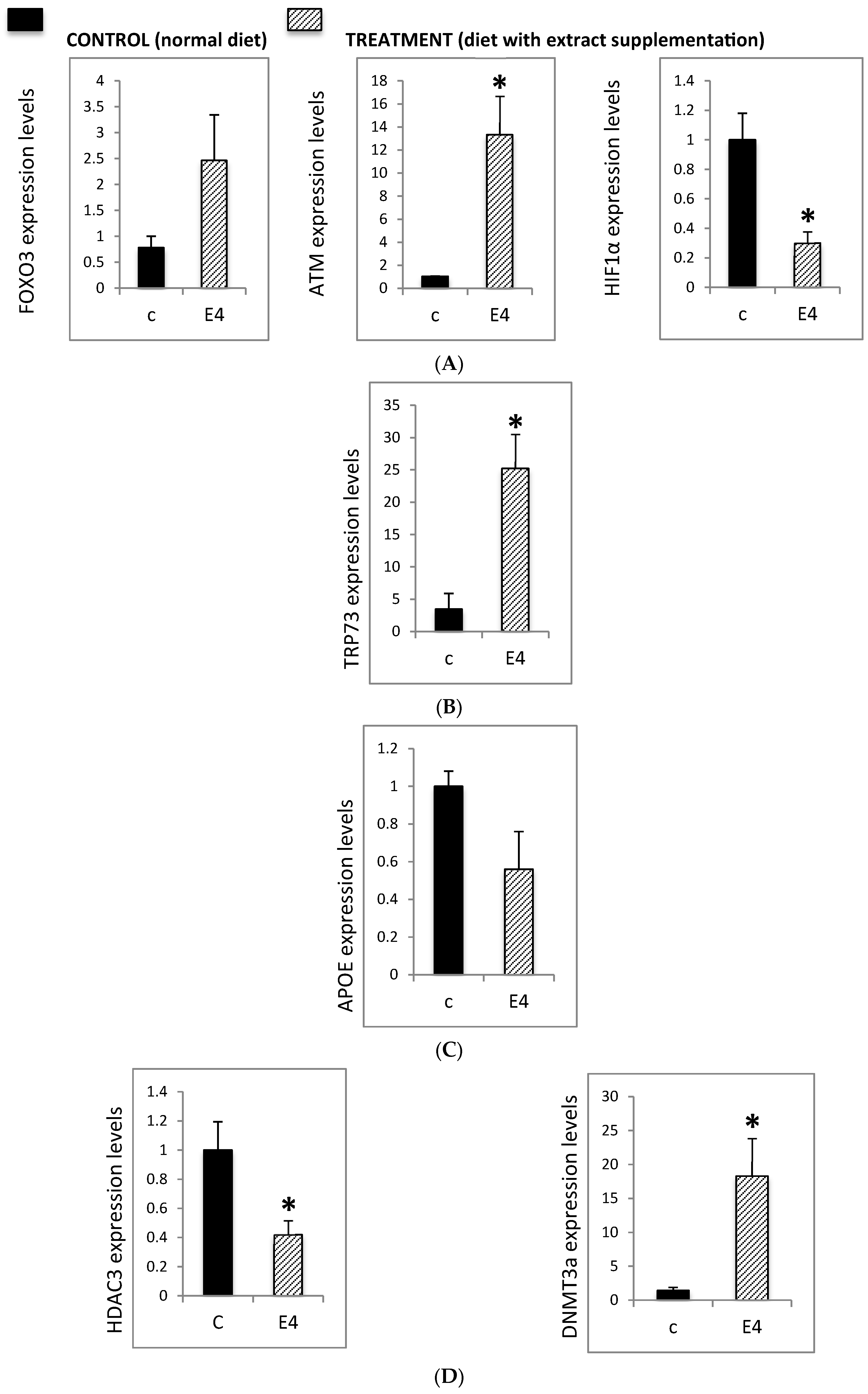
3.2. Epigenetic Modulation of Neurodegeneration-Related Pathways
3.2.1. Neurodegenerative-Related Gene Expression in the Hippocampus of AD Mice
3.2.2. Effect of Neurofabine-C on Global Methylation Levels
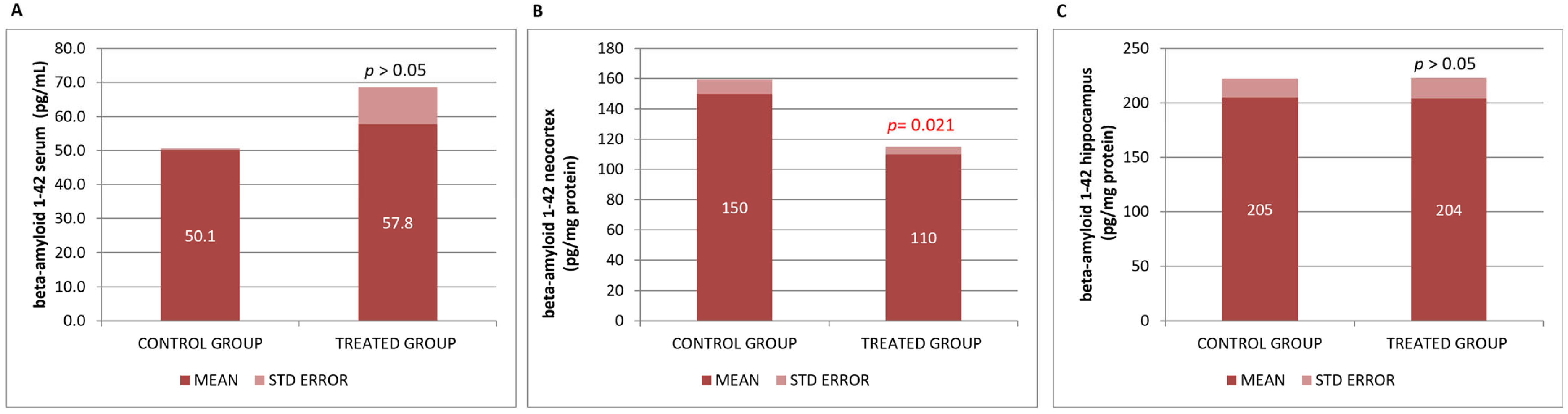
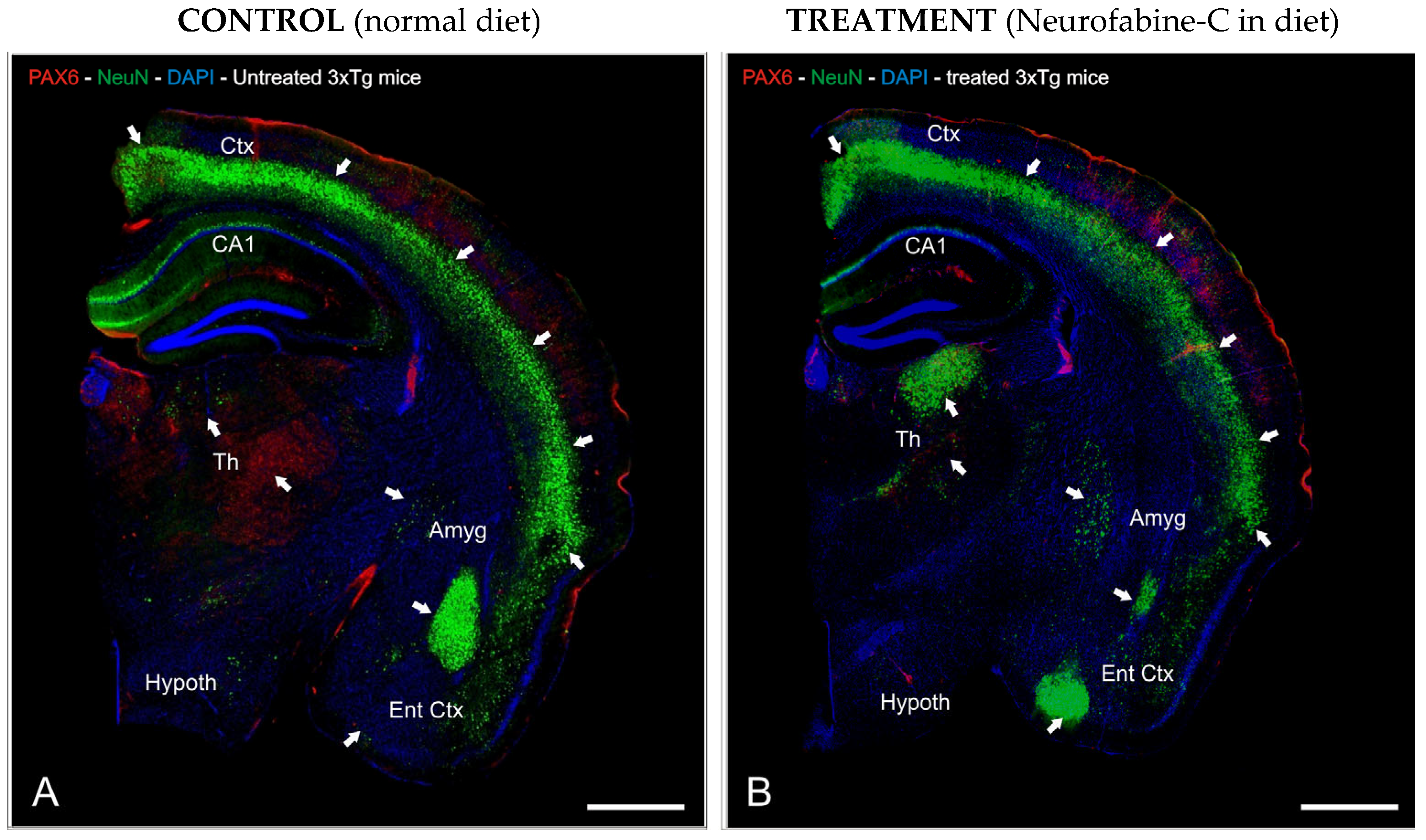
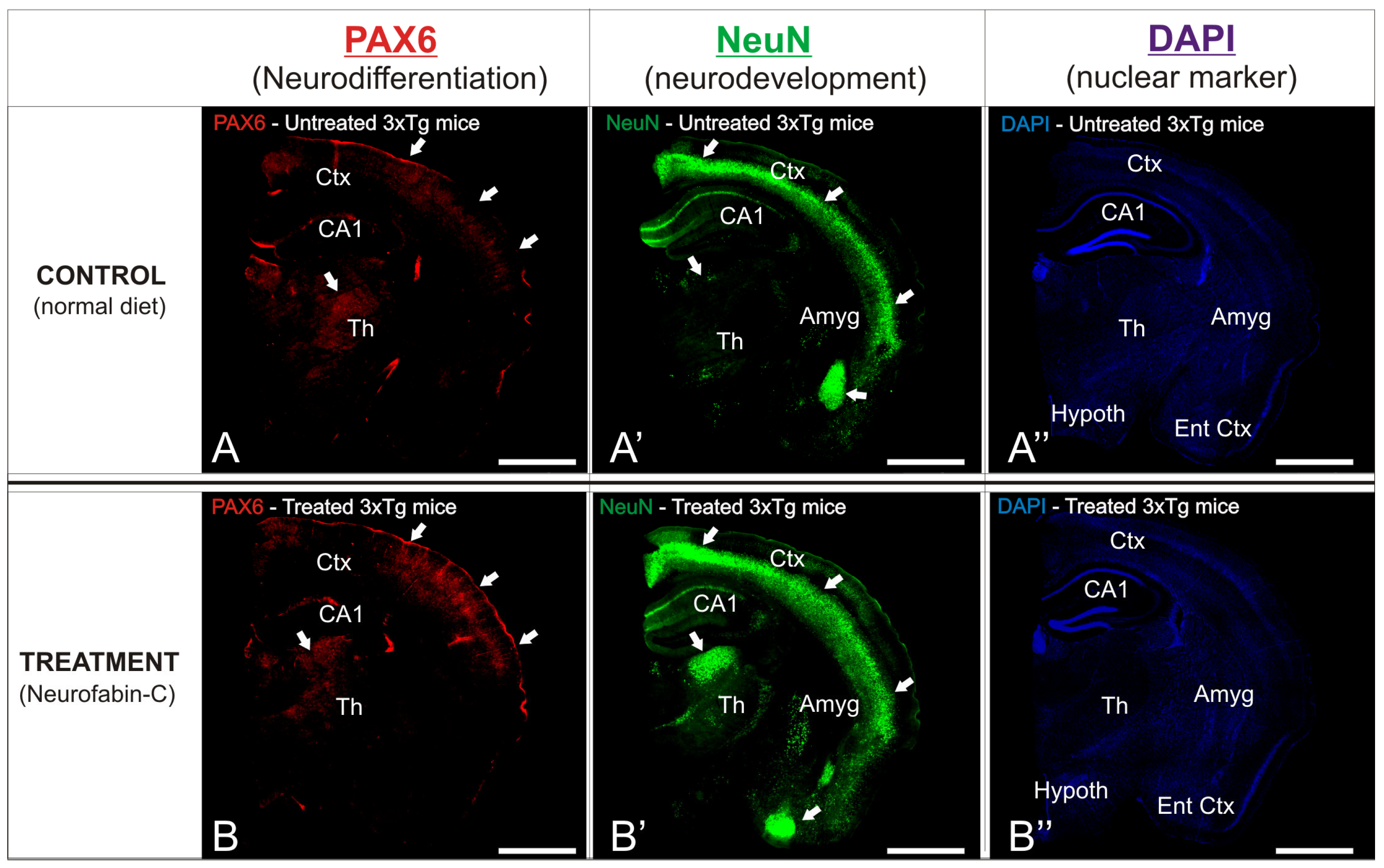
3.3. Preventive Effect of Neurofabine-C on Neurodegeneration in AD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Lai, P.-L.; Naidu, M.; Sabaratnam, V.; Wong, K.-H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N.A. Neurotrophic properties of the Lion’s Mane medicinal mushroom, Hericium erinaceus (higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms 2013, 15, 539–554. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Panaro, M.A.; Porro, C. Bioactive Natural Compounds for Therapeutic and Nutraceutical Applications in Neurodegeneration. Nutrients 2022, 14, 2216. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.-K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2024, 45, e15765. [Google Scholar] [CrossRef]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Zięba, K.; Makarewicz-Wujec, M.; Kozłowska-Wojciechowska, M. Cardioprotective Mechanisms of Cocoa. J. Am. Coll. Nutr. 2019, 38, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.; Paglieroni, T.G.; Wun, T.; Pearson, D.A.; Schmitz, H.H.; Gosselin, R.; Keen, C.L. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr. 2000, 72, 30–35. [Google Scholar] [CrossRef]
- Ludovici, V.; Barthelmes, J.; Nagele, M.P.; Flammer, A.J.; Sudano, I. Polyphenols: Anti-Platelet Nutraceutical? Curr. Pharm. Des. 2018, 24, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Schroeter, H.; Balzer, J.; Kleinbongard, P.; Matern, S.; Sies, H.; Kelm, M. Endothelial function, nitric oxide, and cocoa flavanols. J. Cardiovasc. Pharmacol. 2006, 47, S128–S135. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.D.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef]
- Fraga, C.G.; Litterio, M.C.; Prince, P.D.; Calabró, V.; Piotrkowski, B.; Galleano, M. Cocoa flavanols: Effects on vascular nitric oxide and blood pressure. J. Clin. Biochem. Nutr. 2011, 48, 63–67. [Google Scholar] [CrossRef]
- Ferri, C.; Desideri, G.; Ferri, L.; Proietti, I.; Di Agostino, S.; Martella, L.; Mai, F.; Di Giosia, P.; Grassi, D. Cocoa, blood pressure, and cardiovascular health. J. Agric. Food Chem. 2015, 63, 9901–9909. [Google Scholar] [CrossRef]
- Simpson, E.J.; Mendis, B.; Dunlop, M.; Schroeter, H.; Kwik-Uribe, C.; Macdonald, I.A. Cocoa Flavanol Supplementation and the Effect on Insulin Resistance in Females Who Are Overweight or Obese: A Randomized, Placebo-Controlled Trial. Nutrients 2023, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, M.; Martorell, M.; Sharopov, F.; Atanassov, L.; Kumar, P.; Sharifi-Rad, J.; Tejada-Gavela, S.; Iriti, M.; Pezzani, R.; Varoni, E.M. Cocoa as immunomodulatory agent: An update. Nat. Prod. Res. 2023, 1, 4196–4207. [Google Scholar] [CrossRef]
- Ellinger, S.; Stehle, P. Impact of Cocoa Consumption on Inflammation Processes—A Critical Review of Randomized Controlled Trials. Nutrients 2016, 8, 321. [Google Scholar] [CrossRef]
- Keen, C.L.; Holt, R.R.; Oteiza, P.I.; Fraga, C.G.; Schmitz, H.H. antioxidants and cardiovascular health. Am. J. Clin. Nutr. 2005, 81, 298S–303S. [Google Scholar] [CrossRef]
- Sudano, I.; Flammer, A.J.; Roas, S. Cocoa, blood pressure, and vascular function. Curr. Hypertens. Rep. 2012, 14, 279–284. [Google Scholar] [CrossRef]
- Friedrichs, B.; Toborek, M.; Hennig, B.; Heinevetter, L.; Müller, C.; Brigelius-Flohé, R. 13-HPODE and 13-HODE modulate cytokine-induced expression of endothelial cell adhesion molecules differently. Biofactors 1999, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals-just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef]
- Romero, A.; Parada, E.; González-Lafuente, L.; Farré-Alins, V.; Ramos, E.; Cacabelos, R.; Egea, J. Neuroprotective effects of E-PodoFavalin-15999 (Atremorine®). CNS Neurosci. Ther. 2017, 23, 450–452. [Google Scholar] [CrossRef]
- Carrera, I.; Fernandez-Novoa, L.; Sampedro, C.; Cacabelos, R. Neuroprotective Effect of Atremorine in an Experimental Model of Parkinson’s Disease. Curr. Pharm. Des. 2017, 23, 2673–2684. [Google Scholar] [CrossRef]
- Cacabelos, R.; Fernández-Novoa, L.; Alejo, R.; Corzo, L.; Alcaraz, M.; Nebril, L.; Cacabelos, P.; Fraile, C.; Carrera, I.; Carril, J.C. E-PodoFavalin-15999 (Atremorine®)-induced dopamine response in Parkinson’s Disease: Pharmacogenetics-related effects. J. Genom. Med. Pharmacogenom. 2016, 1, 1–26. [Google Scholar]
- Cacabelos, R.; Fernández-Novoa, L.; Alejo, L.; Corzo, L.; Rodríguez, S.; Alcaraz, M.; Nebril, L.; Cacabelos, P.; Fraile, C.; Carrera, I.; et al. E-PodoFavalin-15999 (Atremorine®)-Induced Neurotransmitter and Hormonal Response in Parkinson’s Disease. J. Expl Res. Pharmacol. 2016, 1, 1–12. [Google Scholar]
- Ceramella, J.; La Torre, C.; De Luca, M.; Iacopetta, D.; Fazio, A.; Catalano, A.; Ragno, G.; Longo, P.; Sinicropi, M.S.; Rosano, C. Exploring the anticancer and antioxidant properties of Vicia faba L. pods extracts, a promising source of nutraceuticals. PeerJ 2022, 10, e13683. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of broad bean seed extract and its phenolic composition. J. Funct. Foods 2017, 38, 656–662. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free. Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’hocine, L. Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Cicero, A.F.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Della Cuna, F.S.R.; Russo, G.I.; Stamatiou, K.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 2, 91. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Termini, D.; Albano, A.; Tsiani, E. Anti-Cancer Properties of Theaflavins. Molecules 2021, 26, 987. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 3, S112–S120. [Google Scholar] [CrossRef]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U.; Baraniak, B.; Bochnak, J. Peptides obtained from fermented faba bean seeds (Vicia faba) as potential inhibitors of an enzyme involved in the pathogenesis of metabolic syndrome. LWT 2019, 105, 306–313. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Mlouka, M.A.B.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F.; et al. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia faba). Nutrients 2018, 10, 671. [Google Scholar] [CrossRef]
- Dugardin, C.; Cudennec, B.; Tourret, M.; Caron, J.; Guérin-Deremaux, L.; Behra-Miellet, J.; Lefranc-Millot, C.; Ravallec, R. Explorative Screening of Bioactivities Generated by Plant-Based Proteins after In Vitro Static Gastrointestinal Digestion. Nutrients 2020, 12, 3746. [Google Scholar] [CrossRef]
- León-Espinosa, E.B.; Sánchez-Chino, X.; Garduño-Siciliano, L.; Álvarez-González, R.I.; Dávila-Ortiz, G.; Madrigal-Bujaidar, E.; Téllez-Medina, D.I.; Jiménez-Martínez, C. Hypocholesterolemic and Anticarcinogenic Effect of Vicia faba Protein Hydrolyzates. Nutr. Cancer 2016, 68, 856–864. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Çalışkantürk Karataş, S.; Günay, D.; Sayar, S. In vitro evaluation of whole faba bean and its seed coat as a potential source of functional food components. Food Chem. 2017, 230, 182–188. [Google Scholar] [CrossRef]
- Purves, R.W.; Zhang, H.; Khazaei, H.; Vandenberg, A. Rapid analysis of medically relevant compounds in faba bean seeds using FAIMS and mass spectrometry. Int. J. Ion Mobil. Spectrom. 2017, 20, 125–135. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT Food Sci. Technol. 2017, 82, 296–302. [Google Scholar]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Osborne, T.B. The Proteins of The Wheat Kernel; Carnegie institution of Washington: Washington, DC, USA, 1907; p. 119. [Google Scholar]
- Alghamdi, S.S. Chemical composition of faba bean (Vicia faba L.) genotypes under various water regimes. Pak. J. Nutr. 2009, 8, 477–482. [Google Scholar] [CrossRef]
- Utsumi, S. Plant Food Protein Engineering. In Advances in Food and Nutrition Research; Kinsella, J.E., Ed.; Academic Press: Kyoto, Japan, 1992; Volume 36, pp. 89–208. [Google Scholar]
- Müntz, K.; Horstmann, C.; Schlesier, B. Vicia globulins. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 259–284. [Google Scholar]
- Vitrac, X.; Monti, J.-P.; Vercauteren, J.; Deffieux, G.; Mérillon, J.-M. Direct liquid chromatography analysis of resveratrol derivatives and flavanonols in wines with absorbance and fluorescence detection. Anal. Chim. Acta 2002, 458, 103–110. [Google Scholar] [CrossRef]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Protective effect of resveratrol on markers of oxidative stress in human erythrocytes subjected to in vitro oxidative insult. Phytother. Res. 2010, 1, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and vegetable juices and Alzheimer’s disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Rotilio, G.; Ciriolo, M.R. Role of nitric oxide synthases in Parkinson’s disease: A review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res. 2008, 33, 2416–2426. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef]
- Perry, G.; Nunomura, A.; Jones, P.K.; Avila, J.; Drew, K.; Perry, G.; Smith, M.A. Oxidative imbalance is a major feature of Alzheimer disease. Curr. Biochem. Res. 2003, 3, 151–156. [Google Scholar]
- Corzo, L.; Zas, R.; Rodríguez, S.; Fernández-Novoa, L.; Cacabelos, R. Decreased levels of serum nitric oxide in different forms of dementia. Neurosci. Lett. 2007, 420, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bradley-Whitman, M.A.; Lovell, M.A. Biomarkers of lipid peroxidation in Alzheimer disease (AD): An update. Arch. Toxicol. 2015, 89, 1035–1044. [Google Scholar] [CrossRef]
- Carru, C.; Da Boit, M.; Paliogiannis, P.; Zinellu, A.; Sotgia, S.; Sibson, R.; Meakin, J.R.; Aspden, R.M.; Mangoni, A.A.; Gray, S.R. Markers of oxidative stress, skeletal muscle mass and function, and their responses to resistance exercise training in older adults. Exp. Gerontol. 2018, 103, 101–106. [Google Scholar] [CrossRef]
- Stedile, N.; Canuto, R.; de Col, C.D.; de Sene, J.S.; Stolfo, A.; Wisintainer, G.N.d.S.; Henriques, J.A.P.; Salvador, M. Dietary total antioxidant capacity is associated with plasmatic antioxidant capacity, nutrient intake and lipid and DNA damage in healthy women. Int. J. Food Sci. Nutr. 2016, 67, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.P. Effectiveness of crowberry on plasma antioxidant status, lipid profile and homocysteine. J. Food Nutr. Res. 2013, 1, 37–41. [Google Scholar]
- Liu, Y.; Li, L.; An, S.; Zhang, Y.; Feng, S.; Zhao, L.; Teng, L.; Wang, D. Antifatigue effects of Antrodia cinnamomea cultured mycelium via modulation of oxidative stress signaling in a mouse model. BioMed Res. Int. 2017, 2017, 9374026. [Google Scholar] [CrossRef]
- McGrath, L.; McGleenon, B.; Brennan, S.; McColl, D.; McILroy, S.; Passmore, A. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJM 2001, 94, 485–490. [Google Scholar] [CrossRef]
- Sahathevan, S.; Se, C.-H.; Ng, S.; Khor, B.-H.; Chinna, K.; Goh, B.L.; Gafor, H.A.; Bavanandan, S.; Ahmad, G.; Karupaiah, T. Clinical efficacy and feasibility of whey protein isolates supplementation in malnourished peritoneal dialysis patients: A multicenter, parallel, open-label randomized controlled trial. Clin. Nutr. ESPEN 2018, 25, 68–77. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Ochalek, A.; Mihalik, B.; Avci, H.X.; Chandrasekaran, A.; Téglási, A.; Bock, I.; Giudice, M.L.; Táncos, Z.; Molnár, K.; László, L.; et al. Neurons derived from sporadic Alzheimer’s disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimer’s Res. Ther. 2017, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Verberk, I.M.W.; Slot, R.E.; Verfaillie, S.C.J.; Heijst, H.; Prins, N.D.; van Berckel, B.N.M.; Scheltens, P.; Teunissen, C.E.; Van der Flier, W.M. Plasma Amyloid as Prescreener for the Earliest Alzheimer Pathological Changes. Ann. Neurol. 2018, 84, 648–658. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Hehir, C.A.T.; Baker, D.; et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Lee, C.; Park, G.H.; Jang, J.-H. Neuroprotective effect of epigallocatechin-3-gallate against beta-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity. Arch. Pharm. Res. 2009, 32, 869–881. [Google Scholar] [CrossRef]
- Guo, J.U.; Ma, D.K.; Mo, H.; Ball, M.P.; Jang, M.-H.; Bonaguidi, M.A.; Balazer, J.A.; Eaves, H.L.; Xie, B.; Ford, E.; et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat. Neurosci. 2011, 14, 1345–1351. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Hemstedt, T.J.; Bading, H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat. Neurosci. 2012, 15, 1111–1113. [Google Scholar] [CrossRef]
- McQuown, S.C.; Barrett, R.M.; Matheos, D.P.; Post, R.J.; Rogge, G.A.; Alenghat, T.; Mullican, S.E.; Jones, S.; Rusche, J.R.; Lazar, M.A.; et al. HDAC3 is a critical negative regulator of long-term memory formation. J. Neurosci. 2011, 31, 764–774. [Google Scholar] [CrossRef]
- Kwapis, J.L.; Alaghband, Y.; López, A.J.; Long, J.M.; Li, X.; Shu, G.; Bodinayake, K.K.; Matheos, D.P.; Rapp, P.R.; Wood, M.A. HDAC3-mediated repression of the Nr4a family contributes to age-related impairments in long-term memory. J. Neurosci. 2019, 39, 4999–5009. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Pai, S.; Koncevicius, K.; Pal, M.; Kriukiene, E.; Liutkeviciute, Z.; Irimia, M.; Jia, P.; Ptak, C.; Xia, M.; et al. 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat. Struct. Mol. Biol. 2012, 19, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef]
- Szulwach, K.E.; Li, X.; Li, Y.; Song, C.-X.; Wu, H.; Dai, Q.; Irier, H.; Upadhyay, A.K.; Gearing, M.; Levey, A.I.; et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011, 14, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Keown, C.L.; Kurihara, L.; Zhou, J.; He, Y.; Li, J.; Castanon, R.; Lucero, J.; Nery, J.R.; Sandoval, J.P.; et al. Single-cell methylomes identify neuronal subtypes and regulatory elements in mammalian cortex. Science 2017, 357, 600–604. [Google Scholar] [CrossRef]
- McKinnon, P.J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu. Rev. Pathol. 2012, 7, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Herrup, K. Cell division in the CNS: Protective response or lethal event in post-mitotic neurons? Biochim. Biophys. Acta 2007, 1772, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Walker, N.; Bronson, R.; Kaghad, M.; Oosterwegel, M.; Bonnin, J.; Vagner, C.; Bonnet, H.; Dikkes, P.; Sharpe, A.; et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 2000, 404, 99–103. [Google Scholar] [CrossRef]
- Pozniak, C.D.; Radinovic, S.; Yang, A.; McKeon, F.; Kaplan, D.R.; Miller, F.D. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 2000, 289, 304–306. [Google Scholar] [CrossRef]
- Daitoku, H.; Fukamizu, A. FOXO transcription factors in the regulatory networks of longevity. J. Biochem. 2007, 141, 769–774. [Google Scholar] [CrossRef]
- Carter, M.E.; Brunet, A. FOXO transcription factors. Curr. Biol. 2007, 17, R113–R114. [Google Scholar] [CrossRef] [PubMed]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef]
- Soerensen, M.; Dato, S.; Christensen, K.; McGue, M.; Stevnsner, T.; Bohr, V.A.; Christiansen, L. Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell 2010, 9, 1010–1017. [Google Scholar] [CrossRef]
- Sun, X.; He, G.; Qing, H.; Zhou, W.; Dobie, F.; Cai, F.; Staufenbiel, M.; Huang, L.E.; Song, W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18727–18732. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Wang, R.; Cui, J.; Lipton, S.A.; Liao, F.; Xu, H.; Zhang, Y. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J. Biol. Chem. 2007, 282, 10873–10880. [Google Scholar] [CrossRef]
- Peers, C.; Dallas, M.L.; Boycott, H.E.; Scragg, J.L.; Pearson, H.A.; Boyle, J.P. Hypoxia and neurodegeneration. Ann. N. Y. Acad. Sci. 2009, 1177, 169–177. [Google Scholar] [CrossRef]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis. 2014, 72, 3–12. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 2017, 47, 566–581. [Google Scholar] [CrossRef]
- van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Choi, S.W.; Friso, S. Epigenetics: A new bridge between nutrition and health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef]
- Carrera, I.; Fernandez-Novoa, L.; Sampedro, C.; Tarasov, V.V.; Aliev, G.; Cacabelos, R. Dopaminergic Neuroprotection with Atremorine in Parkinson’s Disease. Curr. Med. Chem. 2018, 25, 5372–5388. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioactive Products as Epigenetic Modulators for Treating Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Corzo, L.; Fernández-Novoa, L.; Carrera, I.; Martínez, O.; Rodríguez, S.; Alejo, R.; Cacabelos, R. Nutrition, Health, and Disease: Role of Selected Marine and Vegetal Nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar] [CrossRef] [PubMed]

| Weeks of Treatment | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Gr A (Regular Diet) | RD | RD | RD | RD | RD |
| Gr B (Neurofabine-C + Regular Diet) | RD + CES | RD + CES | RD + CES | RD + CES | RD + CES |
| PARAMETER | UNITS | GROUP | N | MEAN | STANDARD DEVIATION | STANDARD ERROR | T-TEST Sig (p) |
|---|---|---|---|---|---|---|---|
| IMMUNE EFFECT/ENERGETIC EFFECT/ANTIANEMIC EFFECT | |||||||
| LEUCOCYTES | ×109/L | CONTROL | 3 | 2.0767 | 0.76422 | 0.44122 | |
| TREATMENT | 5 | 16,160 | 0.85949 | 0.38438 | p > 0.05 | ||
| RED BLOOD CELLS | ×1012/L | CONTROL | 3 | 9.5533 | 0.83722 | 0.48337 | |
| TREATMENT | 5 | 9.6320 | 0.32729 | 0.14637 | p > 0.05 | ||
| HEMOGLOBIN | g/dL | CONTROL | 3 | 14.4333 | 1.34288 | 0.77531 | |
| TREATMENT | 5 | 14.3600 | 0.54589 | 0.24413 | p > 0.05 | ||
| HEMATOCRIT | % | CONTROL | 3 | 50.1000 | 4.66047 | 2.69072 | |
| TREATMENT | 5 | 49.4400 | 2.46739 | 1.10345 | p > 0.05 | ||
| MCV | fL | CONTROL | 3 | 52.4333 | 0.68069 | 0.39299 | |
| TREATMENT | 5 | 51.3200 | 1.65136 | 0.73851 | p > 0.05 | ||
| MCH | pg | CONTROL | 3 | 15.1000 | 0.34641 | 0.20000 | |
| TREATMENT | 5 | 14.9200 | 0.24900 | 0.11136 | p > 0.05 | ||
| MCHC | g/dL | CONTROL | 3 | 28.8000 | 0.26458 | 0.15275 | |
| TREATMENT | 5 | 29.0800 | 0.40866 | 0.18276 | p > 0.05 | ||
| PLATELETS | ×109/L | CONTROL | 3 | 871.0000 | 58.94913 | 34.03430 | |
| TREATMENT | 5 | 932.6000 | 189.22685 | 84.62482 | p > 0.05 | ||
| MPV | fL | CONTROL | 3 | 5.4000 | 0.10000 | 0.05774 | |
| TREATMENT | 5 | 5.6600 | 0.08944 | 0.04000 | p > 0.05 | ||
| PCT | % | CONTROL | 3 | 0.4700 | 0.02700 | 0.01559 | |
| TREATMENT | 5 | 0.5290 | 0.11495 | 0.05141 | p > 0.05 | ||
| NUTRITIONAL STATUS | |||||||
| VITAMIN B6 | ng/mL | CONTROL | 3 | 13.8250 | 5.07250 | 2.92861 | |
| TREATMENT | 5 | 10.5234 | 4.48124 | 2.00407 | p > 0.05 | ||
| VITAMIN B9 | ng/mL | CONTROL | 3 | 23.4408 | 4.61265 | 2.66311 | |
| TREATMENT | 5 | 17.3630 | 7.30684 | 3.26772 | p > 0.05 | ||
| VITAMIN B12 | pg/mL | CONTROL | 2 | 12,433.12 | 1004.97551 | 710.62500 | |
| TREATMENT | 5 | 12,047.75 | 4632.17184 | 2071.57022 | p > 0.05 | ||
| ALB | g/dL | CONTROL | 3 | 3.8000 | 0.60828 | 0.35119 | |
| TREATMENT | 5 | 4.0400 | 0.77974 | 0.34871 | p > 0.05 | ||
| ANTIOXIDANT EFFECT | |||||||
| TAS SERUM | mmol/L | CONTROL | 3 | 0.7867 | 0.50362 | 0.29077 | |
| TREATMENT | 5 | 1.7960 | 0.55851 | 0.24977 | p = 0.043 | ||
| MDA PLASMA | µmol/L | CONTROL | 3 | 3.2633 | 0.94214 | 0.54395 | |
| TREATMENT | 5 | 7.0280 | 3.26481 | 1.46007 | p > 0.05 | ||
| COGNITIVE STATUS (ALZHEIMER BIOMARKER) | |||||||
| βA 1-42 SERUM | pg/mL | CONTROL | 3 | 50.100 | 0.90067 | 0.52000 | |
| TREATMENT | 5 | 57.756 | 24.44359 | 10.93151 | p > 0.05 | ||
| βA 1-42 NEOCORTEX | pg/mg protein | CONTROL | 3 | 150.166 | 16.26012 | 9.38779 | |
| TREATMENT | 3 | 110.510 | 9.06759 | 5.23517 | p = 0.021 | ||
| βA 1-42 HIPPOCAMPUS | pg/mg protein | CONTROL | 3 | 205.253 | 30.19689 | 17.43418 | |
| TREATMENT | 3 | 203.680 | 33.23732 | 19.18958 | p > 0.05 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera, I.; Naidoo, V.; Corzo, L.; Martínez-Iglesias, O.; Cacabelos, R. Epigenetic Modulation and Neuroprotective Effects of Neurofabine-C in a Transgenic Model of Alzheimer’s Disease. Genes 2025, 16, 1214. https://doi.org/10.3390/genes16101214
Carrera I, Naidoo V, Corzo L, Martínez-Iglesias O, Cacabelos R. Epigenetic Modulation and Neuroprotective Effects of Neurofabine-C in a Transgenic Model of Alzheimer’s Disease. Genes. 2025; 16(10):1214. https://doi.org/10.3390/genes16101214
Chicago/Turabian StyleCarrera, Ivan, Vinogran Naidoo, Lola Corzo, Olaia Martínez-Iglesias, and Ramón Cacabelos. 2025. "Epigenetic Modulation and Neuroprotective Effects of Neurofabine-C in a Transgenic Model of Alzheimer’s Disease" Genes 16, no. 10: 1214. https://doi.org/10.3390/genes16101214
APA StyleCarrera, I., Naidoo, V., Corzo, L., Martínez-Iglesias, O., & Cacabelos, R. (2025). Epigenetic Modulation and Neuroprotective Effects of Neurofabine-C in a Transgenic Model of Alzheimer’s Disease. Genes, 16(10), 1214. https://doi.org/10.3390/genes16101214









