ROGDI-Related Disorder Resulting from Disruption of Complex Interactive Neuro-Dental Developmental Networks: A Review and Description of the First Missense Variant
Abstract
1. Introduction
2. The Disease and the Protein
2.1. ROGDI-Related Neurodevelopmental and Dental Disorder (ROGDI-RD)
2.2. ROGDI Expression and Function
3. Review of the Patients with ROGDI-RD Including a New Case
3.1. Clinical Features of the New Case
3.2. Description of the Genetic Finding
3.3. Dynamic Computational Modeling of the Val87Ala Variant
4. Review of the Phenotype and Genotype of ROGDI-RD Patients
4.1. The Clinical Features
4.2. ROGDI Variants and the Phenotype
4.3. Complex Interactive Neuro-Dental Developmental Networks and ROGDI-RD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brook, A.; O’Donnell, M.B.; Hone, A.; Hart, E.; Hughes, T.; Smith, R.; Townsend, G. General and Craniofacial Development Are Complex Adaptive Processes Influenced by Diversity. Aust. Dent. J. 2014, 59, 13–22. [Google Scholar] [CrossRef]
- Brook, A.H.; O’Donnell, M.B. Complexity, Networking, and Many-Model Thinking Enhance Understanding of the Patterning, Variation, and Interactions of Human Teeth and Dental Arches. In Odontodes; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-003-43965-3. [Google Scholar]
- Liepina, L.; Kalnina, M.L.; Micule, I.; Gailite, L.; Rots, D.; Kalnina, J.; Strautmanis, J.; Celmina, M. Kohlschütter–Tönz Syndrome: Case Report with Novel Feature and Detailed Review of Features Associated with ROGDI Variants. Am. J. Med. Genet. A 2022, 188, 1263–1279. [Google Scholar] [CrossRef]
- den Hoed, J.; de Boer, E.; Voisin, N.; Dingemans, A.J.M.; Guex, N.; Wiel, L.; Nellaker, C.; Amudhavalli, S.M.; Banka, S.; Bena, F.S.; et al. Mutation-Specific Pathophysiological Mechanisms Define Different Neurodevelopmental Disorders Associated with SATB1 Dysfunction. Am. J. Hum. Genet. 2021, 108, 346–356. [Google Scholar] [CrossRef]
- Schossig, A.; Bloch-Zupan, A.; Lussi, A.; Wolf, N.I.; Raskin, S.; Cohen, M.; Giuliano, F.; Jurgens, J.; Krabichler, B.; Koolen, D.A.; et al. SLC13A5 Is the Second Gene Associated with Kohlschütter–Tönz Syndrome. J. Med. Genet. 2017, 54, 54–62. [Google Scholar] [CrossRef]
- Wright, J.T. Enamel Phenotypes: Genetic and Environmental Determinants. Genes 2023, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Mory, A.; Dagan, E.; Illi, B.; Duquesnoy, P.; Mordechai, S.; Shahor, I.; Romani, S.; Hawash-Moustafa, N.; Mandel, H.; Valente, E.M.; et al. A Nonsense Mutation in the Human Homolog of Drosophila Rogdi Causes Kohlschutter–Tonz Syndrome. Am. J. Hum. Genet. 2012, 90, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Schossig, A.; Wolf, N.I.; Kapferer, I.; Kohlschütter, A.; Zschocke, J. Epileptic Encephalopathy and Amelogenesis Imperfecta: Kohlschütter-Tönz Syndrome. Eur. J. Med. Genet. 2012, 55, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Wallrafen, R.; Dresbach, T. The Kohlschütter-Tönz Syndrome Associated Gene Rogdi Encodes a Novel Presynaptic Protein. Sci. Rep. 2017, 7, 15791. [Google Scholar] [CrossRef] [PubMed]
- Winkley, S.R.; Kane, P.M. The ROGDI Protein Mutated in Kohlschutter-Tonz Syndrome Is a Novel Subunit of the Rabconnectin-3 Complex Implicated in V-ATPase Assembly. J. Biol. Chem. 2025, 301, 108381. [Google Scholar] [CrossRef]
- Falace, A.; Volpedo, G.; Scala, M.; Zara, F.; Striano, P.; Fassio, A. V-ATPase Dysfunction in the Brain: Genetic Insights and Therapeutic Opportunities. Cells 2024, 13, 1441. [Google Scholar] [CrossRef]
- Jimenez-Armijo, A.; Morkmued, S.; Ahumada, J.T.; Kharouf, N.; de Feraudy, Y.; Gogl, G.; Riet, F.; Niederreither, K.; Laporte, J.; Birling, M.C.; et al. The Rogdi Knockout Mouse Is a Model for Kohlschütter-Tönz Syndrome. Sci. Rep. 2024, 14, 445. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Otte, E.D.; Folsom, T.D.; Eschenlauer, A.C.; Roper, R.J.; Aman, J.W.; Thuras, P.D. Early Chronic Fluoxetine Treatment of Ts65Dn Mice Rescues Synaptic Vesicular Deficits and Prevents Aberrant Proteomic Alterations. Genes 2024, 15, 452. [Google Scholar] [CrossRef]
- Kohlschütter, A. Familial Epilepsy and Yellow Teeth--a Disease of the CNS Associated with Enamel Hypoplasia. Helvetica Paediatr. Acta 1974, 294, 283–294. [Google Scholar]
- Schossig, A.; Wolf, N.I.; Fischer, C.; Fischer, M.; Stocker, G.; Pabinger, S.; Dander, A.; Steiner, B.; Tönz, O.; Kotzot, D.; et al. Mutations in ROGDI Cause Kohlschütter-Tönz Syndrome. Am. J. Hum. Genet. 2012, 90, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, H.; Choe, J.; Jun, Y.; Lim, C.; Lee, C. The Crystal Structure of Human Rogdi Provides Insight into the Causes of Kohlschutter-Tönz Syndrome. Sci. Rep. 2017, 7, 3972. [Google Scholar] [CrossRef] [PubMed]
- Ros-Pardo, D.; Gómez-Puertas, P.; Marcos-Alcalde, Í. STAG2: Computational Analysis of Missense Variants Involved in Disease. Int. J. Mol. Sci. 2024, 25, 1280. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Akgün-Doğan, Ö.; Simsek-Kiper, P.O.; Taşkıran, E.; Schossig, A.; Utine, G.E.; Zschocke, J.; Boduroglu, K. Kohlschütter-Tönz Syndrome with a Novel ROGD1 Variant in 3 Individuals: A Rare Clinical Entity. J. Child Neurol. 2021, 36, 816–822. [Google Scholar] [CrossRef]
- Aswath, N.; Ramakrishnan, S.N.; Teresa, N.; Ramanathan, A. A Novel ROGDI Gene Mutation Is Associated with Kohlschutter-Tonz Syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, e8–e11. [Google Scholar] [CrossRef]
- Bloch-Zupan, A.; Rey, T.; Jimenez-Armijo, A.; Kawczynski, M.; Kharouf, N.; O-Rare consortium; Dure-Molla, M.; Noirrit, E.; Hernandez, M.; Joseph-Beaudin, C.; et al. Amelogenesis Imperfecta: Next-Generation Sequencing Sheds Light on Witkop’s Classification. Front. Physiol. 2023, 14, 1130175. [Google Scholar] [CrossRef]
- Essid, M.; Karoui, S.; Zribi, M.; Ben Younes, T.; Januel, L.; Lafont, E.; Labalme, A.; Ben Hafsa, M.; Hun Seo, G.; Khatrouch, S.; et al. Transcript Long-Read Sequencing Unveils the Molecular Complexity of a Novel ROGDI Splicing Variant in a Tunisian Family with Kohlschütter-Tönz Syndrome. Clin. Genet. 2025, 107, 705–707. [Google Scholar] [CrossRef]
- Gowda, V.K.; Bylappa, A.Y.; Srinivasan, V.M.; Manohar, V.; Pandey, H. Kohlschutter-Tonz Syndrome (Amelo-Cerebro-Hypohidrotic Syndrome) in an Indian Family with a Novel ROGD1 Mutation. Clin. Dysmorphol. 2023, 32, 168–171. [Google Scholar] [CrossRef]
- Huckert, M.; Mecili, H.; Laugel-Haushalter, V.; Stoetzel, C.; Muller, J.; Flori, E.; Laugel, V.; Manière, M.-C.; Dollfus, H.; Bloch-Zupan, A. A Novel Mutation in the ROGDI Gene in a Patient with Kohlschütter-Tönz Syndrome. Mol. Syndromol. 2014, 5, 293–298. [Google Scholar] [CrossRef]
- Meng, L.; Huang, D.; Xie, L.; Song, X.; Luo, H.; Gui, J.; Ding, R.; Zhang, X.; Jiang, L. Perampanel Effectiveness in Treating ROGDI-Related Kohlschütter-Tönz Syndrome: First Reported Case in China and Literature Review. BMC Med. Genom. 2023, 16, 292. [Google Scholar] [CrossRef]
- Morscher, R.J.; Rauscher, C.; Sperl, W.; Rittinger, O. Seizures, Enamel Defects and Psychomotor Developmental Delay: The First Patient with Kohlschütter-Tönz Syndrome Caused by a ROGDI-Gene Deletion. Seizure 2017, 50, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Mory, A.; Dagan, E.; Shahor, I.; Mandel, H.; Illi, B.; Zolotushko, J.; Kurolap, A.; Chechik, E.; Valente, E.M.; Amselem, S.; et al. Kohlschutter-Tonz Syndrome: Clinical and Genetic Insights Gained from 16 Cases Deriving from a Close-Knit Village in Northern Israel. Pediatr. Neurol. 2014, 50, 421–426. [Google Scholar] [CrossRef]
- Muthaffar, O.Y.; Alazhary, N.W.; Alyazidi, A.S.; Alsubaie, M.A.; Bahowarth, S.Y.; Odeh, N.B.; Bamaga, A.K. Clinical Description and Evaluation of 30 Pediatric Patients with Ultra-Rare Diseases: A Multicenter Study with Real-World Data from Saudi Arabia. PLoS ONE 2024, 19, e0307454. [Google Scholar] [CrossRef] [PubMed]
- Nerakh, G.; Koneru, S.; Dhareneni, P.R. Nephrocalcinosis, Distal Renal Tubular Acidosis and Skeletal Abnormality in Two Siblings with ROGDI-Related Kohlschütter-Tönz Syndrome. Clin. Dysmorphol. 2025, 34, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tucci, A.; Kara, E.; Schossig, A.; Wolf, N.I.; Plagnol, V.; Fawcett, K.; Paisán-Ruiz, C.; Moore, M.; Hernandez, D.; Musumeci, S.; et al. Kohlschütter-Tönz Syndrome: Mutations in ROGDI and Evidence of Genetic Heterogeneity. Hum. Mutat. 2013, 34, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.; Mountain, R.V.; Pickett, O.R.; Den Besten, P.K.; Bidlack, F.B.; Dunn, E.C. Teeth as Potential New Tools to Measure Early-Life Adversity and Subsequent Mental Health Risk: An Interdisciplinary Review and Conceptual Model. Biol. Psychiatry 2020, 87, 502–513. [Google Scholar] [CrossRef]
- Goldberg, M.; Kellermann, O.; Dimitrova-Nakov, S.; Harichane, Y.; Baudry, A. Comparative Studies between Mice Molars and Incisors Are Required to Draw an Overview of Enamel Structural Complexity. Front. Physiol. 2014, 5, 359. [Google Scholar] [CrossRef]
- Thesleff, I. Current Understanding of the Process of Tooth Formation: Transfer from the Laboratory to the Clinic. Aust. Dent. J. 2014, 59 (Suppl. 1), 48–54. [Google Scholar] [CrossRef]
- Oldham, S.; Fornito, A. The Development of Brain Network Hubs. Dev. Cogn. Neurosci. 2019, 36, 100607. [Google Scholar] [CrossRef]
- Kumasaka, S.; Shimozuma, M.; Kawamoto, T.; Mishima, K.; Tokuyama, R.; Kamiya, Y.; Davaadorj, P.; Saito, I.; Satomura, K. Possible Involvement of Melatonin in Tooth Development: Expression of Melatonin 1a Receptor in Human and Mouse Tooth Germs. Histochem. Cell Biol. 2010, 133, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, X.; Bai, Y.; Heng, B.C.; Zhang, X.; Deng, X. The Circadian Clock in Enamel Development. Int. J. Oral Sci. 2024, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Brook, A.H.; Jernvall, J.; Smith, R.N.; Hughes, T.E.; Townsend, G.C. The Dentition: The Outcomes of Morphogenesis Leading to Variations of Tooth Number, Size and Shape. Aust. Dent. J. 2014, 59 (Suppl. 1), 131–142. [Google Scholar] [CrossRef]
- Brook, A.H. Multilevel Complex Interactions between Genetic, Epigenetic and Environmental Factors in the Aetiology of Anomalies of Dental Development. Arch. Oral Biol. 2009, 54, S3–S17. [Google Scholar] [CrossRef]
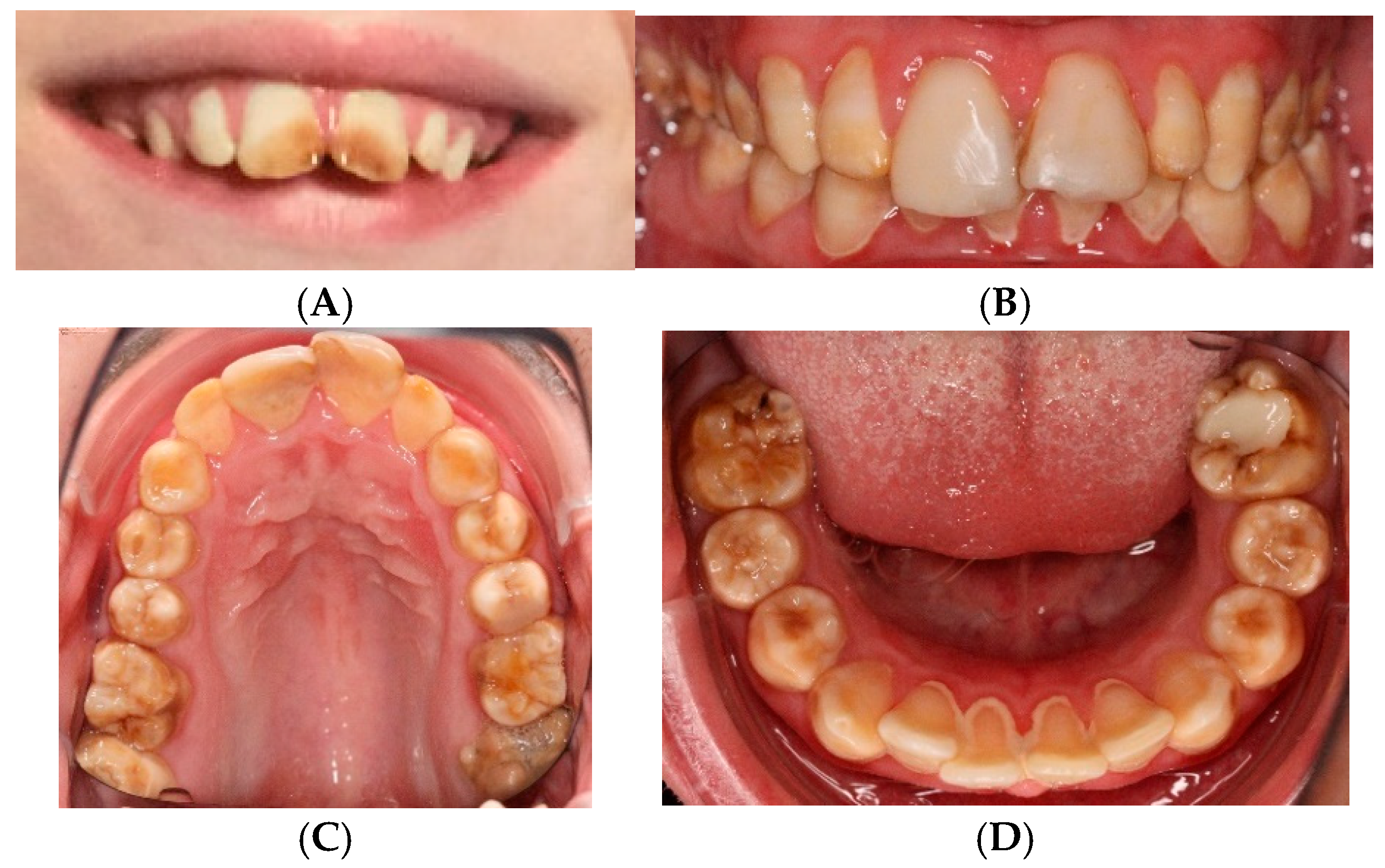
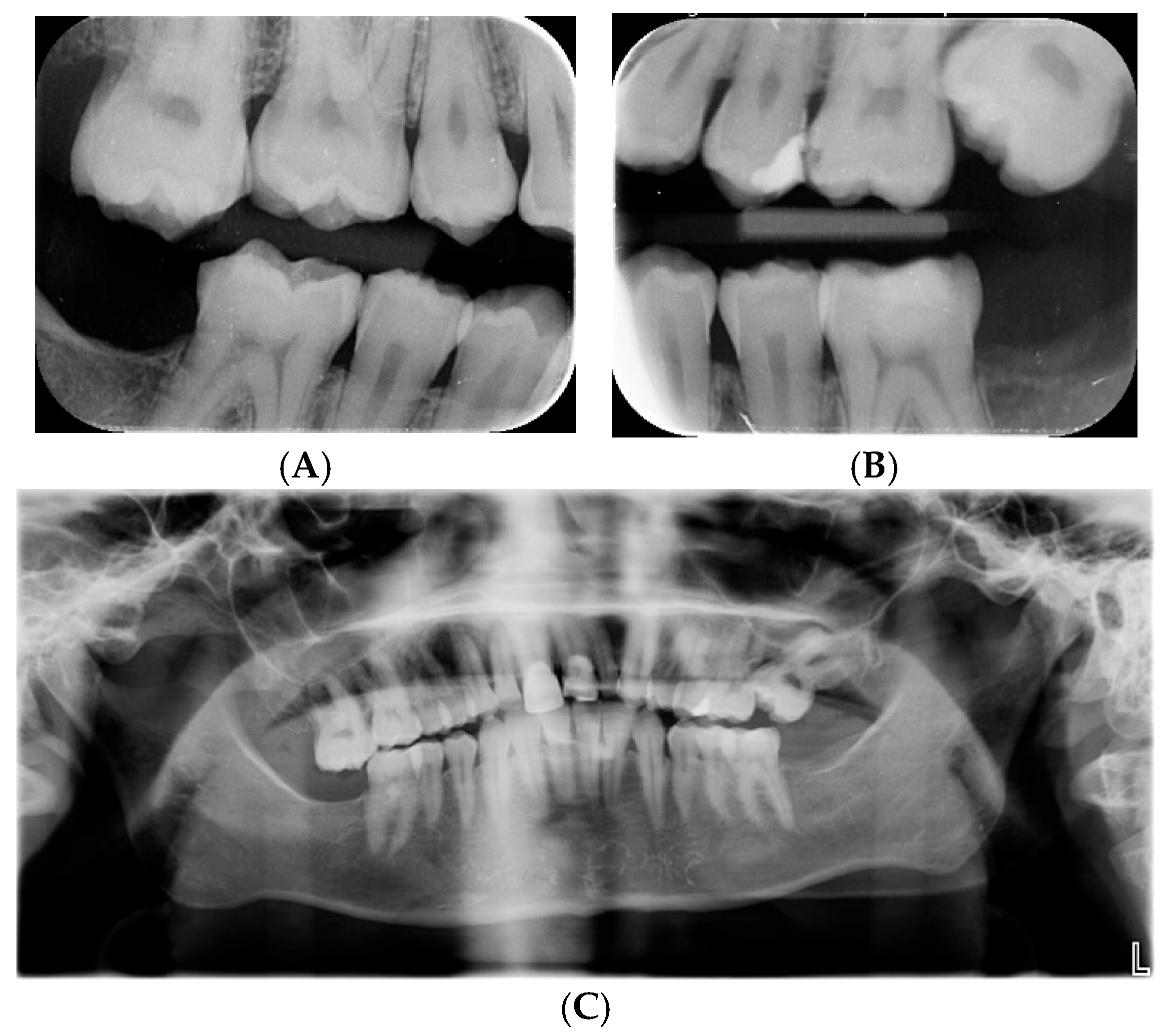
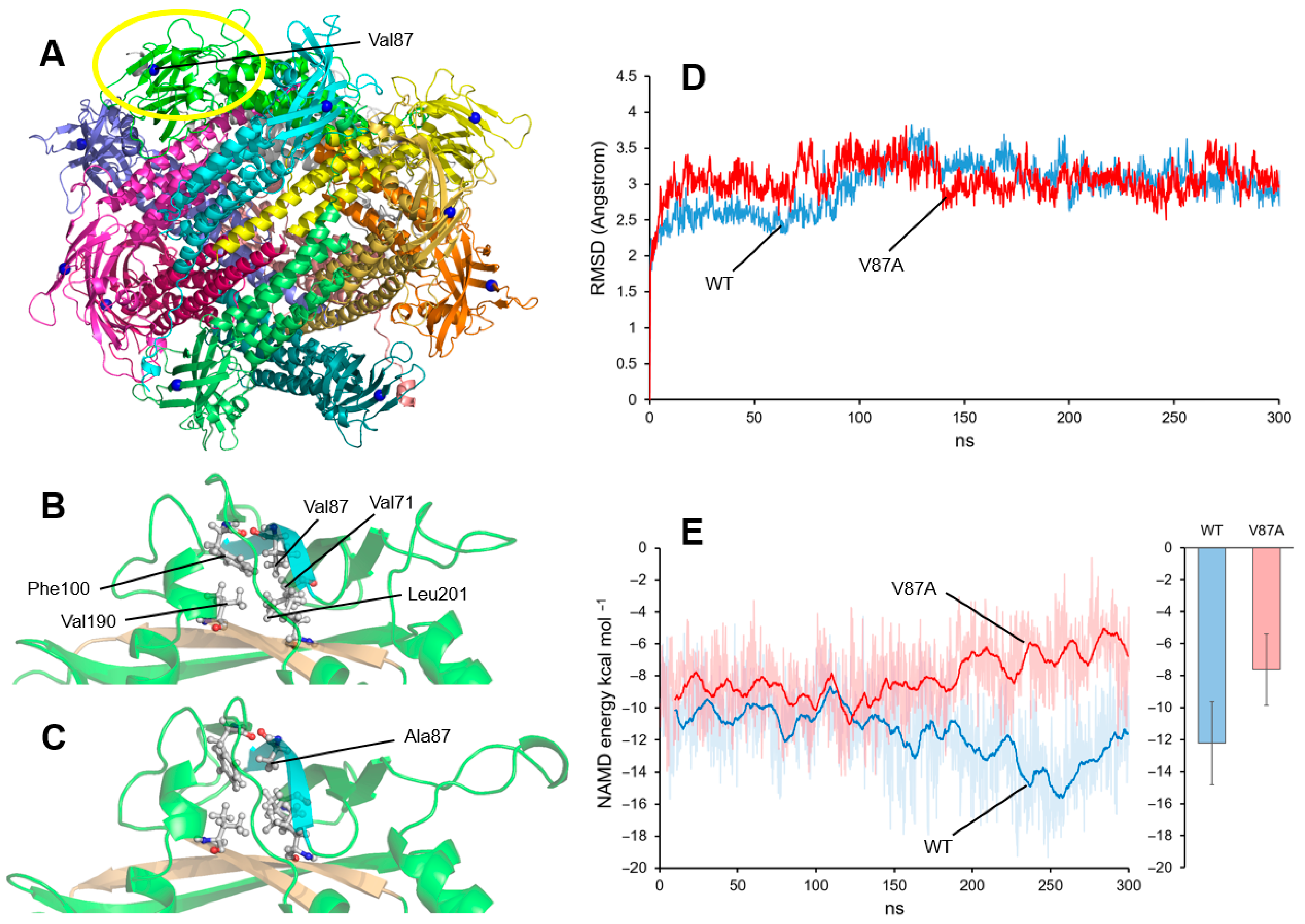
| Category | Published Cases (% of the Symptom) | Clinical Features of the Present Case |
|---|---|---|
| General information | ||
| Male | 30/54 (56%) | |
| Female | 24/54 (44%) | + |
| Age at examination | 1.5–18 years | 28 years |
| Pregnancy and Delivery | ||
| Normal | 23/33 (70%) | + |
| Abnormal | 10/33 (30%) | |
| Development | ||
| Developmental delay | 54/54 (100%) | + |
| Delay from birth | 14/33 (42%) | + |
| Delay after seizure onset | 19/33 (58%) | |
| Mild/moderate intellectual disability | 5/21 (24%) | + |
| Profound/severe intellectual disability | 16/21 (76%) | |
| Speech delay | 11/33 (33%) | |
| Absent speech | 22/33 (67%) | + |
| Ability to walk unaided | 22/29 (76%) | |
| Epilepsy | 51/51 (100%) | |
| Median age of onset (range) | 9 months (1–48 months) | 11 months |
| Febrile seizures | 10/51 (20%) | + |
| Refractory to treatment | 13/51 (25%) | + |
| Dental features | ||
| Amelogenesis imperfecta/teeth discoloration | 54/54 (100%) | + |
| Additional features | ||
| Normal head circumference | 11/18 (61%) | |
| Microcephaly | 7/18 (38%) | + |
| Dysmorphic features | 11/54 (20%) | + |
| Neuroimaging | ||
| Cerebral atrophy | 8/21 (38%) | |
| Enlarged ventricles | 7/21 (33%) | |
| Cerebellar hypoplasia | 3/21 (14%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gverdtsiteli, S.; Hammer, T.B.; Hermann, X.; Andersen, N.B.; Ros-Pardo, D.; Marcos-Alcalde, I.; Gómez-Puertas, P.; Brook, A.H.; Silahtaroglu, A.; Tümer, Z. ROGDI-Related Disorder Resulting from Disruption of Complex Interactive Neuro-Dental Developmental Networks: A Review and Description of the First Missense Variant. Genes 2025, 16, 1207. https://doi.org/10.3390/genes16101207
Gverdtsiteli S, Hammer TB, Hermann X, Andersen NB, Ros-Pardo D, Marcos-Alcalde I, Gómez-Puertas P, Brook AH, Silahtaroglu A, Tümer Z. ROGDI-Related Disorder Resulting from Disruption of Complex Interactive Neuro-Dental Developmental Networks: A Review and Description of the First Missense Variant. Genes. 2025; 16(10):1207. https://doi.org/10.3390/genes16101207
Chicago/Turabian StyleGverdtsiteli, Sopio, Trine Bjørg Hammer, Xenia Hermann, Noemi Becser Andersen, David Ros-Pardo, Iñigo Marcos-Alcalde, Paulino Gómez-Puertas, Alan Henry Brook, Asli Silahtaroglu, and Zeynep Tümer. 2025. "ROGDI-Related Disorder Resulting from Disruption of Complex Interactive Neuro-Dental Developmental Networks: A Review and Description of the First Missense Variant" Genes 16, no. 10: 1207. https://doi.org/10.3390/genes16101207
APA StyleGverdtsiteli, S., Hammer, T. B., Hermann, X., Andersen, N. B., Ros-Pardo, D., Marcos-Alcalde, I., Gómez-Puertas, P., Brook, A. H., Silahtaroglu, A., & Tümer, Z. (2025). ROGDI-Related Disorder Resulting from Disruption of Complex Interactive Neuro-Dental Developmental Networks: A Review and Description of the First Missense Variant. Genes, 16(10), 1207. https://doi.org/10.3390/genes16101207










