Fluctuating Genetic Influences at Three Different Stages of Development of Dental Arches: A Complex System
Abstract
1. Introduction
- Arch shape shows significant variation in Australian children;
- Arch shape is broadly consistent between arches within individuals, but it is anatomically conserved across time;
- An individual’s arch shape is significantly influenced by their genome;
- Sexual heterogeneity in arch shape is mediated allometrically (through differences in mature size) rather than qualitatively.
2. Materials and Methods
2.1. Data Collection
2.2. Cast Inclusion and Exclusion Criteria
2.3. Photographing Dental Casts
2.4. Landmark Digitization
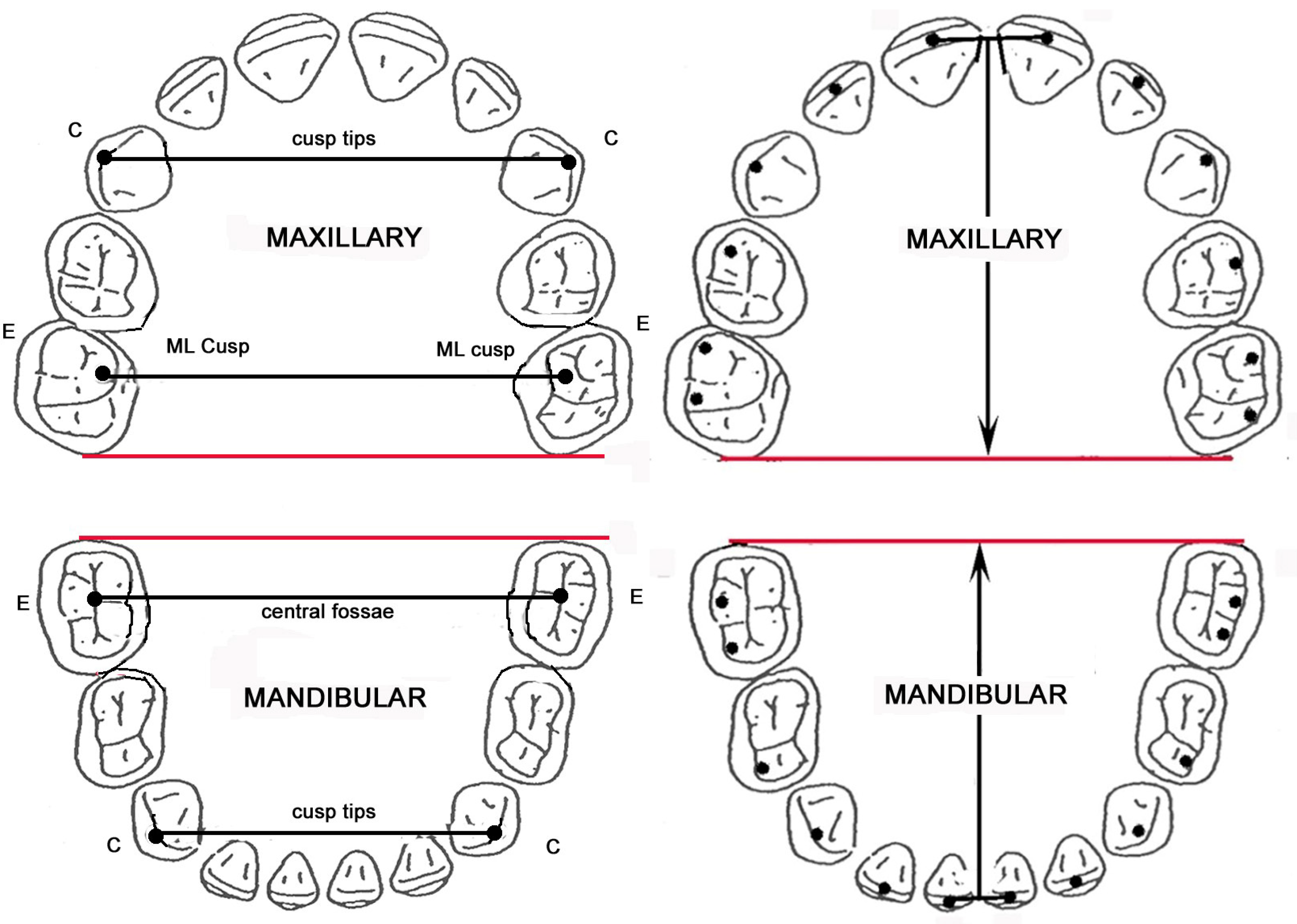
2.5. Arch Dimensions
2.6. Arch Shape
2.7. Validation and Error Study
2.8. Normality Testing and Data Cleaning
2.9. Statistical Analyses
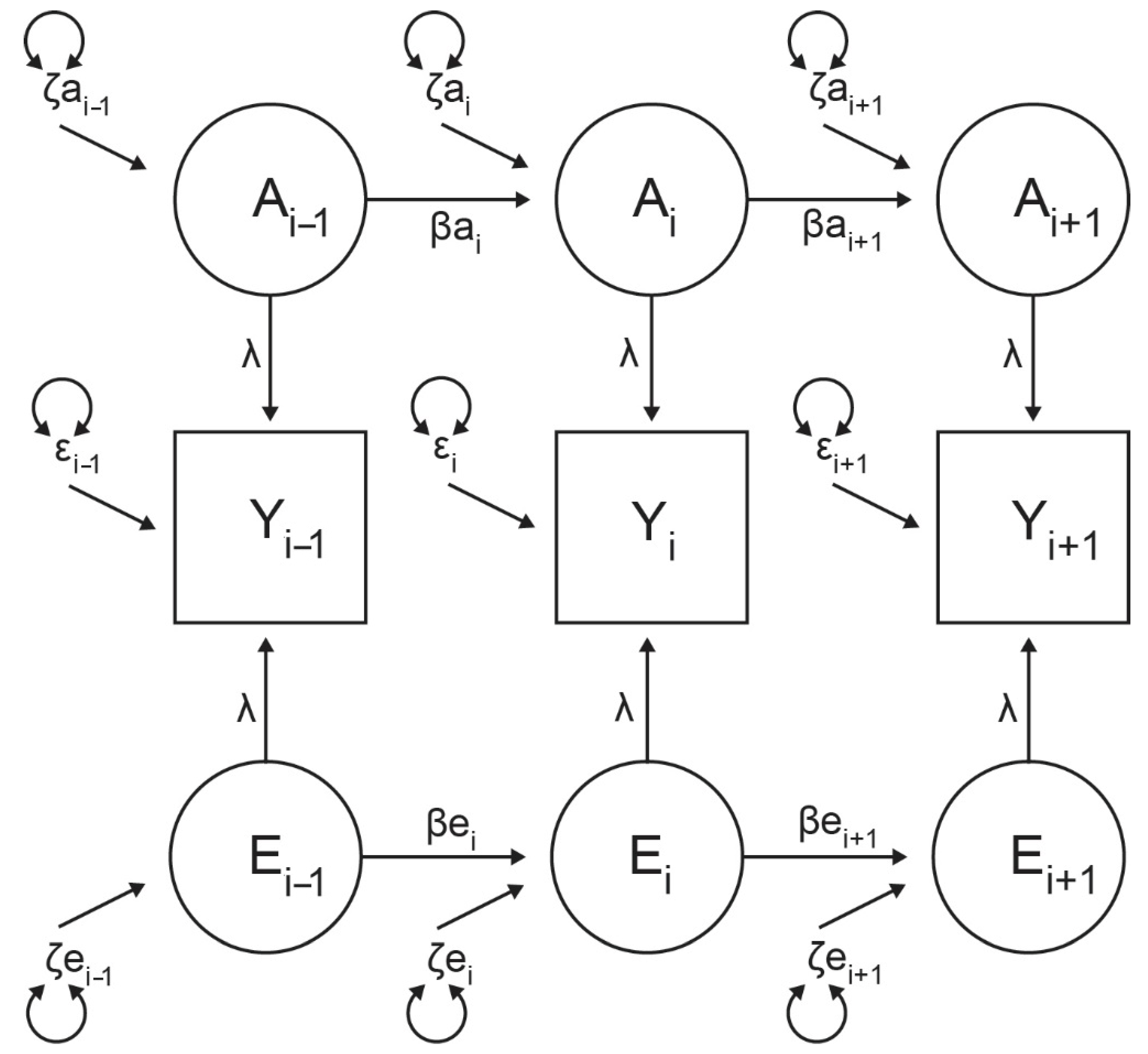
2.10. Genetic Analyses
2.11. Variance Components Analysis
3. Results
3.1. Normality, Validation, and Reliability Tests
3.2. Descriptive Statistics
3.2.1. Asymmetry
3.2.2. Shape
3.3. Phenotypic Correlations
3.4. Same-Sex Twin Intra-Class Correlations
3.5. Genetic Modeling
4. Discussion
Study Limitations and Future Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brook, A.; Brook O’Donnell, M. Complexity, networking and many model thinking enhance understanding of the patterning, variation and interactions of human teeth and dental arches. In Odontodes The Developmental and Evolutionary Building Blocks of Dentitions; Chen, D., Ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Eguchi, S.; Townsend, G.; Richards, L.; Hughes, T.; Kasai, K. Genetic contribution to dental arch size variation in Australian twins. Arch. Oral Biol. 2004, 49, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.F.; Smith, R.J. A study of occlusion and arch widths in families. Am. J. Orthod. 1980, 78, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.C.; Townsend, G.C.; Brown, T.; Burgess, V.B. Dental arch morphology in South Australian twins. Arch. Oral Biol. 1990, 35, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Corruccini, R.S.; Yap Potter, R.H. Genetic analysis of occlusal variation in twins. Am. J. Orthod. 1980, 78, 140–154. [Google Scholar] [CrossRef]
- Townsend, G.C.; Corruccini, R.S.; Richards, L.C.; Brown, T. Genetic and environmental determinants of dental occlusal variation in South Australian twins. Australas. Orthod. J. 1988, 10, 231–235. [Google Scholar] [CrossRef]
- Weaver, C.A.; Miller, S.F.; Da Fontoura, C.S.G.; Wehby, G.L.; Amendt, B.A.; Holton, N.E.; Allareddy, V.; Southard, T.E.; Moreno Uribe, L.M. Candidate gene analyses of 3-dimensional dentoalveolar phenotypes in subjects with malocclusion. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 539–558. [Google Scholar] [CrossRef]
- Hughes, T.; Richards, L.; Townsend, G. Dental arch form in young Australian twins. In Dental Morphology; Alan, B., Ed.; Sheffield Academic Press, Ltd.: Sheffield, UK, 2001; pp. 309–319. [Google Scholar]
- Brown, T.; Abbott, A.H.; Burgess, V.B. Age changes in dental arch dimensions of Australian Aboriginals. Am. J. Phys. Anthropol. 1983, 62, 291–303. [Google Scholar] [CrossRef]
- Kasai, K.; Richards, L.C.; Townsend, G.C.; Kanazawa, E.; Iwasawa, T. Fourier Analysis of Dental Arch Morphology in South Australian Twins. Anthr. Sci. 1995, 103, 39–48. [Google Scholar] [CrossRef]
- Steele-Perkins, G.; Butz, K.G.; Lyons, G.E.; Zeichner-David, M.; Kim, H.-J.; Cho, M.-I.; Gronostajski, R.M. Essential Role for NFI-C/CTF Transcription-Replication Factor in Tooth Root Development. Mol. Cell. Biol. 2003, 23, 1075–1084. [Google Scholar] [CrossRef]
- Zhao, H.; Oka, K.; Bringas, P.; Kaartinen, V.; Chai, Y. TGF-β type I receptor Alk5 regulates tooth initiation and mandible patterning in a type II receptor-independent manner. Dev. Biol. 2008, 320, 19–29. [Google Scholar] [CrossRef]
- Neale, M.; Cardon, L.R. Methodology for Genetic Studies of Twins and Families, 1st ed.; Nato Science Series D, Behavioural and Social Sciences; Springer Netherlands: Dordrecht, The Netherlands, 1992; Volume 67. [Google Scholar]
- Mossey, P.A. The heritability of malocclusion: Part 1—Genetics, principles and terminology. Br. J. Orthod. 1999, 26, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Richards, L.; Townsend, G. Form, Symmetry and Asymmetry of the Dental Arch: Orthogonal Analysis Revisited. Dent. Anthropol. 2002, 16, 3–8. [Google Scholar] [CrossRef]
- Lin, T.-H.; Meade, M.J.; Hughes, T. Dental arch shape in twins: A morphometric study of genetic influences. Am. J. Orthod. Dentofac. Orthop. 2024, 166, 583–594. [Google Scholar] [CrossRef]
- Bockmann, M.; Hughes, T.; Townsend, G. Genetic modeling of primary tooth emergence: A study of Australian twins. Twin Res. Hum. Genet. 2010, 13, 573–581. [Google Scholar] [CrossRef]
- Cheng, P.C.K. Dental-Arch Morphology of Australian Aborigines: A Metric Study of Arch Size and Shape. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 1972. [Google Scholar]
- Moorrees, C.F.A.; Reed, R.B. Changes in Dental Arch Dimensions Expressed on the Basis of Tooth Eruption as a Measure of Biologic Age. J. Dent. Res. 1965, 44, 129–141. [Google Scholar] [CrossRef]
- Kendall, D.G. A Survey of the Statistical Theory of Shape. Stat. Sci. 1989, 4, 87–99. [Google Scholar] [CrossRef]
- Lu, K.H. An orthogonal analysis of the form, symmetry and asymmetry of the dental arch. Arch. Oral Biol. 1966, 11, 1057–1069. [Google Scholar] [CrossRef]
- Robson, D.S. A simple method for constructing orthogonal polynomials when the independent variable is unequally spaced. Biometrics 1959, 15, 187–191. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Bland, M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research, R Package, Version 2.4.6; Northwestern University: Evanston, IL, USA, 2024.
- Dahlberg, G. Statistical Methods for Medical and Biological Students; George Allen and Unwin, Ltd.: London, UK, 1940. [Google Scholar]
- El-Nofely, A.; Sadek, L.; Soliman, N. Spacing in the human deciduous dentition in relation to tooth size and dental arch size. Arch. Oral Biol. 1989, 34, 437–441. [Google Scholar] [CrossRef]
- Townsend, G.C.; Brown, T. Inheritance of tooth size in Australian Aboriginals. Am. J. Phys. Anthropol. 1978, 48, 305–314. [Google Scholar] [CrossRef]
- Jinks, J.L.; Fulker, D.W. Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of human behavior. Psychol. Bull. 1970, 73, 311–349. [Google Scholar] [CrossRef] [PubMed]
- Neale, M.; Boker, S.; Xie, G.; Maes, H. Mx: Statistical Modeling, 6th ed.; Department of Psychiatry, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University: Richmond, VA, USA, 2003. [Google Scholar]
- Gillespie, N.A.; Martin, N.G. Multivariate Genetic Analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Dolan, C.V.; Molenaar, P.C.M.; Boomsma, D.I. Simultaneous genetic analysis of longitudinal means and covariance structure in the simplex model using twin data. Behav. Genet. 1991, 21, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Gillespie, N.A.; Martin, N.G. Biometrical genetics. Biol. Psychol. 2002, 61, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, N.A.; Evans, D.E.; Wright, M.M.; Martin, N.G. Genetic Simplex Modeling of Eysenck’s Dimensions of Personality in a Sample of Young Australian Twins. Twin Res. 2004, 7, 637–648. [Google Scholar] [CrossRef][Green Version]
- Massey, F.J. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Rajbhoj, A.A.; Parchake, P.; Begnoni, G.; Willems, G.; Cadenas De Llano-Pérula, M. Dental changes in humans with untreated normal occlusion throughout lifetime: A systematic scoping review. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 340–362.e343. [Google Scholar] [CrossRef]
- Bishara, S.E.; Treder, J.E.; Damon, P.; Olsen, M. Changes in the dental arches and dentition between 25 and 45 years of age. Angle Orthod. 1996, 66, 417–422. [Google Scholar] [PubMed]
- Thilander, B. Dentoalveolar development in subjects with normal occlusion. A longitudinal study between the ages of 5 and 31 years. Eur. J. Orthod. 2009, 31, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sillman, J.H. Dimensional changes of the dental arches: Longitudinal study from birth to 25 years. Am. J. Orthod. 1964, 50, 824–842. [Google Scholar] [CrossRef]
- Papagiannis, A.; Halazonetis, D.J. Shape variation and covariation of upper and lower dental arches of an orthodontic population. Eur. J. Orthod. 2016, 38, 202–211. [Google Scholar] [CrossRef]
- Wen, Y.F.; Wong, H.M.; Pei, T.; Mcgrath, C. Adolescent dental arch development among Southern Chinese in Hong Kong: A geometric morphometric approach. Sci. Rep. 2019, 9, 18526. [Google Scholar] [CrossRef]


| Ptype | Dentition | Maxilla | Mandible | ||
|---|---|---|---|---|---|
| M | F | M | F | ||
| AB1 | Primary | 27.8 (27.4, 28.1) | 27.3 (26.9, 27.7) | 22.0 (21.7, 22.4) | 21.6 (21.3, 22.0) |
| Mixed | 30.3 (30.0, 30.6) | 29.8 (29.5, 30.1) | 24.6 (24.3, 24.9) | 24.1 (23.8, 24.4) | |
| Permanent | 32.8 (32.3, 33.3) | 31.7 (31.1, 32.3) | 24.6 (24.1, 25.2) | 24.0 (24.1, 25.2) | |
| AB2 | Primary | 33.9 (33.5, 34.3) | 32.9 (32.4, 33.3) | 33.3 (32.9, 33.8) | 32.4 (32.9, 33.8) |
| Mixed | 39.5 (39.2, 39.9) | 38.7 (38.3, 39.0) | 40.2 (39.8, 40.1) | 39.1 (38.8, 39.5) | |
| Permanent | 40.7 (40.1, 41.3) | 39.3 (38.6, 39.9) | 40.4 (39.8, 41.0) | 38.7 (38.1, 39.3) | |
| AD | Primary | 27.8 (27.5, 28.1) | 27.5 (27.1, 27.8) | 24.8 (24.5, 25.1) | 24.4 (24.1, 24.7) |
| Mixed | 29.1 (28.9, 29.4) | 28.5 (28.2, 28.7) | 24.9 (24.6, 25.2) | 24.2 (23.9, 24.5) | |
| Permanent | 26.2 (25.7, 26.6) | 25.6 (25.2, 26.1) | 22.2 (21.7, 22.6) | 21.7 (21.3, 22.2) | |
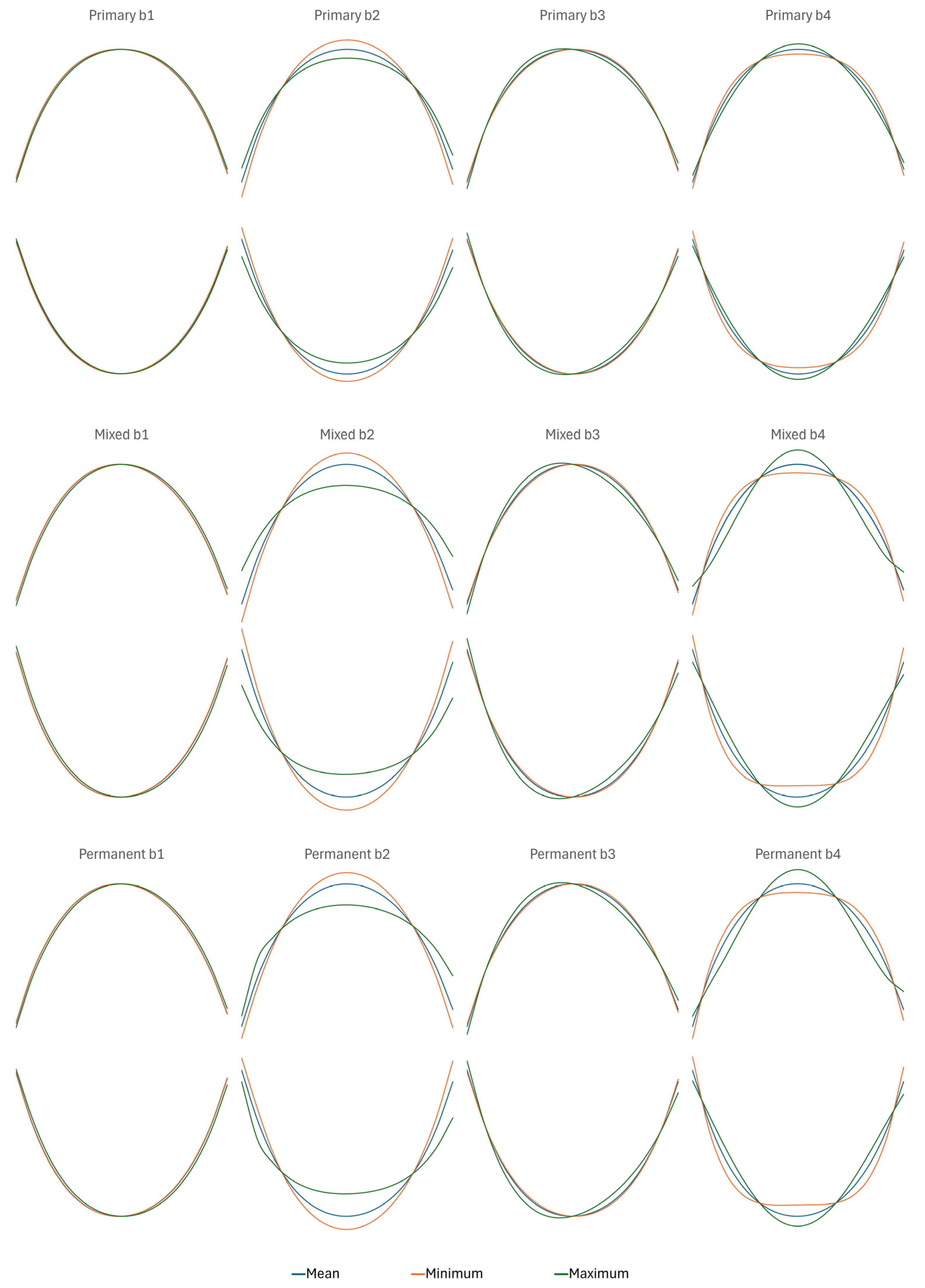
| b1 | Primary | 0.003 (0.002, 0.005) | 0.002 (0.001, 0.005) | 0.003 (0.001, 0.005) | 0.003 (0.001, 0.005) |
| Mixed | 0.001 (0.000, 0.002) | 0.001 (0.000, 0.002) | 0.000 (−0.001, 0.002) | 0.000 (−0.002, 0.001) | |
| Permanent | 0.002 (0.000, 0.005) | 0.004 (0.002, 0.007) | −0.001 (−0.003, 0.002) | −0.006 (−0.008, −0.003) | |
| b2 | Primary | −0.47 (−0.48, −0.47) | −0.48 (−0.48, −0.47) | −0.48 (−0.49, −0.48) | −0.49 (−0.49, −0.48) |
| Mixed | −0.50 (−0.50, −0.49) | −0.50 (−0.51, −0.50) | −0.53 (−0.53, −0.52) | −0.53 (−0.53, −0.52) | |
| Permanent | −0.51 (−0.52, −0.50) | −0.51 (−0.52, −0.50) | −0.53 (−0.53, −0.52) | −0.53 (−0.54, −0.52) | |
| b3 | Primary | −0.007 (−0.010, −0.004) | −0.009 (−0.012, −0.005) | 0.002 (−0.001, 0.006) | 0.001 (−0.002, 0.005) |
| Mixed | −0.005 (−0.008, −0.002) | −0.003 (−0.005, 0.000) | 0.005 (0.003, 0.008) | 0.004 (0.001, 0.007) | |
| Permanent | −0.003 (−0.008, 0.002) | −0.001 (−0.006, 0.005) | −0.003 (−0.008, 0.002) | 0.000 (−0.006, 0.005) | |
| b4 | Primary | −0.05 (−0.05, −0.04) | −0.05 (−0.05, −0.04) | −0.03 (−0.04, −0.03) | −0.04 (−0.04, −0.03) |
| Mixed | −0.04 (−0.04, −0.03) | −0.03 (−0.04, −0.03) | −0.04 (−0.05, −0.04) | −0.05 (−0.05, −0.05 | |
| Permanent | −0.05 (−0.06, −0.04) | −0.04 (−0.05, −0.04) | −0.04 (−0.04, −0.03) | −0.04 (−0.05, −0.03) | |
| Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AB1 | AB2 | AD | b1 | b2 | b3 | b4 | ||||
| Males | Primary | Maxilla | AB1 | 1.00 | 0.72 | 0.59 | 0.12 | 0.20 | −0.08 | −0.54 |
| AB2 | 0.83 | 1.00 | 0.23 | 0.05 | 0.43 | 0.00 | −0.08 | |||
| AD | 0.40 | 0.23 | 1.00 | 0.21 | −0.61 | −0.16 | −0.24 | |||
| b1 | −0.15 | −0.19 | −0.01 | 1.00 | −0.13 | −0.44 | 0.00 | |||
| b2 | 0.46 | 0.64 | −0.50 | −0.20 | 1.00 | 0.07 | 0.05 | |||
| b3 | 0.07 | 0.14 | −0.09 | −0.38 | 0.17 | 1.00 | −0.07 | |||
| b4 | −0.38 | −0.02 | 0.11 | −0.01 | −0.19 | 0.02 | 1.00 | |||
| Mandible | AB1 | 1.00 | 0.59 | 0.59 | −0.04 | −0.18 | −0.05 | −0.44 | ||
| AB2 | 0.74 | 1.00 | 0.18 | −0.09 | 0.24 | −0.08 | 0.14 | |||
| AD | 0.58 | 0.34 | 1.00 | 0.08 | −0.73 | −0.11 | −0.15 | |||
| b1 | 0.11 | 0.00 | 0.21 | 1.00 | −0.04 | −0.54 | 0.04 | |||
| b2 | 0.27 | 0.46 | −0.45 | −0.21 | 1.00 | −0.15 | 0.24 | |||
| b3 | 0.00 | −0.08 | −0.08 | −0.44 | 0.11 | 1.00 | −0.02 | |||
| b4 | −0.24 | 0.12 | −0.08 | −0.11 | 0.32 | −0.17 | 1.00 | |||
| Mixed | Maxilla | AB1 | 1.00 | 0.58 | 0.46 | −0.08 | −0.08 | −0.01 | −0.49 | |
| AB2 | 0.55 | 1.00 | 0.20 | −0.04 | 0.16 | 0.10 | 0.03 | |||
| AD | 0.41 | 0.16 | 1.00 | −0.03 | −0.63 | −0.10 | 0.07 | |||
| b1 | 0.01 | 0.15 | 0.02 | 1.00 | −0.09 | −0.31 | 0.15 | |||
| b2 | 0.00 | 0.21 | −0.64 | 0.02 | 1.00 | −0.17 | −0.01 | |||
| b3 | 0.15 | 0.04 | 0.19 | −0.31 | −0.20 | 1.00 | −0.04 | |||
| b4 | −0.38 | 0.15 | 0.19 | −0.02 | −0.23 | −0.02 | 1.00 | |||
| Mandible | AB1 | 1.00 | 0.42 | 0.51 | 0.12 | 0.00 | −0.16 | −0.33 | ||
| AB2 | 0.53 | 1.00 | 0.28 | 0.06 | 0.29 | −0.08 | 0.24 | |||
| AD | 0.47 | 0.35 | 1.00 | 0.14 | −0.53 | −0.20 | 0.03 | |||
| b1 | −0.15 | −0.05 | −0.21 | 1.00 | −0.03 | −0.23 | −0.02 | |||
| b2 | −0.07 | 0.24 | −0.53 | 0.02 | 1.00 | 0.17 | 0.05 | |||
| b3 | 0.07 | −0.03 | 0.00 | −0.58 | −0.08 | 1.00 | 0.01 | |||
| b4 | −0.33 | 0.20 | 0.07 | 0.05 | 0.03 | 0.08 | 1.00 | |||
| Permanent | Maxilla | AB1 | 1.00 | 0.63 | 0.41 | 0.30 | 0.28 | −0.22 | −0.14 | |
| AB2 | 0.23 | 1.00 | 0.06 | 0.47 | 0.69 | −0.42 | −0.01 | |||
| AD | 0.37 | 0.35 | 1.00 | 0.18 | −0.54 | −0.24 | −0.17 | |||
| b1 | 0.17 | 0.13 | −0.04 | 1.00 | 0.25 | −0.86 | 0.11 | |||
| b2 | 0.02 | 0.33 | −0.70 | 0.17 | 1.00 | −0.28 | −0.16 | |||
| b3 | −0.34 | −0.07 | −0.04 | −0.75 | −0.06 | 1.00 | 0.04 | |||
| b4 | −0.19 | 0.69 | 0.01 | 0.34 | 0.28 | −0.11 | 1.00 | |||
| Mandible | AB1 | 1.00 | 0.63 | 0.33 | 0.33 | 0.23 | −0.12 | −0.37 | ||
| AB2 | 0.28 | 1.00 | −0.06 | 0.51 | 0.65 | −0.26 | 0.08 | |||
| AD | 0.59 | 0.19 | 1.00 | 0.29 | −0.75 | 0.10 | 0.06 | |||
| b1 | −0.13 | −0.27 | −0.17 | 1.00 | 0.10 | −0.68 | −0.03 | |||
| b2 | −0.07 | 0.46 | −0.60 | −0.07 | 1.00 | −0.26 | −0.15 | |||
| b3 | 0.29 | 0.22 | 0.22 | −0.79 | 0.01 | 1.00 | 0.18 | |||
| b4 | −0.50 | 0.32 | −0.21 | 0.17 | 0.20 | −0.29 | 1.00 | |||
| Maxilla | Mandible | ||||||
|---|---|---|---|---|---|---|---|
| Primary | Mixed | Permanent | Primary | Mixed | Permanent | ||
| AB1 | Novel | 0.71 | 0.17 | 0.41 | 0.79 | 0.27 | 0.46 |
| Transmitted | 0.00 | 0.46 | 0.01 | 0.00 | 0.52 | 0.39 | |
| Total | 0.71 | 0.63 | 0.42 | 0.79 | 0.79 | 0.85 | |
| AB2 | Novel | 0.82 | 0.21 | 0.00 | 0.83 | 0.24 | 0.19 |
| Transmitted | 0.00 | 0.55 | 0.84 | 0.00 | 0.58 | 0.48 | |
| Total | 0.82 | 0.76 | 0.84 | 0.83 | 0.82 | 0.67 | |
| AD | Novel | 0.74 | 0.23 | 0.56 | 0.83 | 0.22 | 0.27 |
| Transmitted | 0.00 | 0.56 | 0.23 | 0.00 | 0.59 | 0.36 | |
| Total | 0.74 | 0.79 | 0.79 | 0.83 | 0.81 | 0.63 | |
| b1 | Novel | - | - | - | - | - | - |
| Transmitted | - | - | - | - | - | - | |
| Total | - | - | - | - | - | - | |
| b2 | Novel | 0.70 | 0.35 | 0.53 | 0.53 | 0.00 | 0.00 |
| Transmitted | 0.00 | 0.32 | 0.00 | 0.00 | 0.62 | 0.36 | |
| Total | 0.70 | 0.67 | 0.53 | 0.53 | 0.62 | 0.36 | |
| b3 | Novel | - | - | - | - | - | - |
| Transmitted | - | - | - | - | - | - | |
| Total | - | - | - | - | - | - | |
| b4 | Novel | 0.79 | 0.42 | 0.79 | 0.79 | 0.42 | 0.49 |
| Transmitted | 0.00 | 0.35 | 0.00 | 0.00 | 0.35 | 0.35 | |
| Total | 0.79 | 0.77 | 0.79 | 0.79 | 0.77 | 0.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, T.; Mahmood, Z.; Giri, J.; Townsend, G.; Brook, A.H. Fluctuating Genetic Influences at Three Different Stages of Development of Dental Arches: A Complex System. Genes 2025, 16, 189. https://doi.org/10.3390/genes16020189
Hughes T, Mahmood Z, Giri J, Townsend G, Brook AH. Fluctuating Genetic Influences at Three Different Stages of Development of Dental Arches: A Complex System. Genes. 2025; 16(2):189. https://doi.org/10.3390/genes16020189
Chicago/Turabian StyleHughes, Toby, Zuliani Mahmood, Jamal Giri, Grant Townsend, and Alan Henry Brook. 2025. "Fluctuating Genetic Influences at Three Different Stages of Development of Dental Arches: A Complex System" Genes 16, no. 2: 189. https://doi.org/10.3390/genes16020189
APA StyleHughes, T., Mahmood, Z., Giri, J., Townsend, G., & Brook, A. H. (2025). Fluctuating Genetic Influences at Three Different Stages of Development of Dental Arches: A Complex System. Genes, 16(2), 189. https://doi.org/10.3390/genes16020189






