In Vitro Regeneration of Stevia rebaudiana Bertoni Using Somaclonal Variation as a Tool for Genetic Diversification
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Callus Initiation and Generation of Somaclone Second Generation from Leaf Explants
2.3. Culture Stabilization
2.4. Analysis of the Morphogenetic Potential of Regenerants
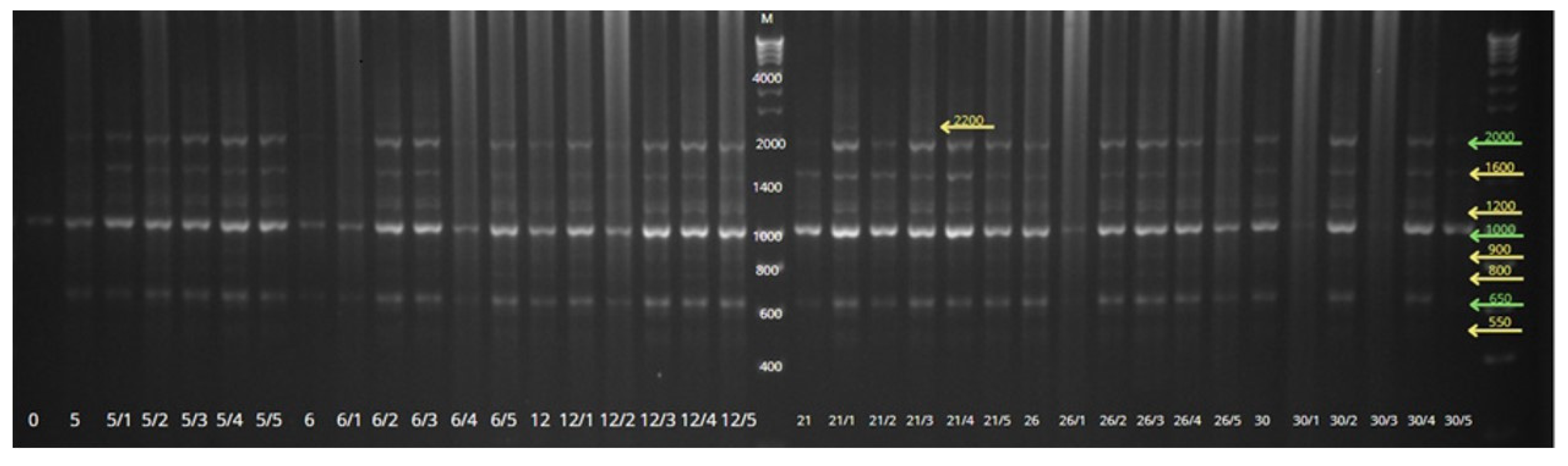
2.5. SCoT Markers—Genotype Analysis
3. Results and Discussion
3.1. Callus Initiation from Leaf Explants and Obtaining Regenerants by Indirect Organogenesis
3.2. Analysis of the Morphogenetic Potential of Regenerants
3.3. SCoT Markers—Genotype Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SGs | steviol glycosides (SGs) |
| MS | Murashige and Skoog |
| BAP | 6-benzylaminopurine |
| NAA | 1-naphthaleneacetic acid |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| Kin | Kinetin |
| SCoT | Start Codon Targeted |
| WUS | WUSCHEL |
References
- Kolanowski, A. Steviol glycosides—Properties and use in foods. Bromat. Chem. Toksykol. 2013, 2, 140–150. [Google Scholar]
- Xu, X.; Yuan, H.; Yu, X.; Huang, S.; Sun, Y.; Zhang, T.; Liu, Q.; Tong, H.; Zhang, Y.; Wang, Y.; et al. The chromosome-level Stevia genome provides insights into steviol glycoside biosynthesis. Hortic. Res. 2021, 8, 129. [Google Scholar] [CrossRef]
- Zhang, H.; An, S.; Hu, J.; Lin, Z.; Liu, X.; Bao, H.; Chen, R. Induction, identification and characterization of polyploidy in Stevia rebaudiana Bertoni. Plant Biotechnol. 2018, 35, 81–86. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- O’Neill, K.; Pirro, S. The complete genome sequence of Stevia rebaudiana, the Sweetleaf. F1000Research 2020, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Ceunen, S.; Geuns, J.M.C. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Wójtowicz, A.T.; Szewczyk, A. The content of phenolic compounds in Stevia rebaudiana (Bertoni) plants derived from melatonin and NaCl treated seeds. Plants 2023, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Luwańska, A.; Perz, A.; Mańkowska, G.; Wielgus, K. Application of in vitro Stevia (Stevia rebaudiana Bertoni) cultures in obtaining steviol glycoside rich material. Herba Pol. 2015, 61, 50–63. [Google Scholar] [CrossRef][Green Version]
- Singh, N.; Verma, S.; Yadav, K. Optimization of the protocols for surface sterilization, regeneration and acclimatization of Stevia rebaudiana Bertoni. J. Agric. Environ. Sci. 2011, 11, 221–227. [Google Scholar]
- Rafiq, M.; Dahot, M.U.; Mangrio, S.M.; Naqvi, H.A.; Qarshi, L.A. In vitro clonal propagation and biochemical analysis of field established Stevia rebaudiana Bertoni. Pak. J. Bot. 2007, 39, 2467–2474. [Google Scholar]
- Górska, K.; Kaszuba, M.; Ligman, S.; Pluskota, W.; Wojciechowicz, J.; Źróbek-Sokolnik, A.; Michalczyk, D.J. (Eds.) Wykłady i Ćwiczenia z Roślinnych Kultur In Vitro [online]. 2010. Available online: https://archiwum.wbp.olsztyn.pl/invitro/index.php?co=wstep (accessed on 17 April 2025).
- Stefaniak, B. Roślinne kultury in vitro. In Komórki Roślinne w Warunkach Stresu. Tom II. Komórki In Vitro; Woźny, A., Przybył, K., Eds.; Wydawnictwo Naukowe UAM: Poznań, Poland, 2007. [Google Scholar]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Mirani, A.A.; Teo, C.H.; Markhand, G.S.; Abul-Soad, A.A.; Harikrishna, J.A. Detection of somaclonal variations in tissue cultured date palm (Phoenix dactylifera L.) using transposable element-based markers. Plant Cell Tissue Organ Cult. 2020, 141, 119–130. [Google Scholar] [CrossRef]
- Pachota, K.A.; Orłowska, R.; Bednarek, P.T. Medium composition affects the tissue culture-induced variation in triticale regenerants. Plant Cell Tissue Organ Cult. 2022, 151, 35–46. [Google Scholar] [CrossRef]
- Dyduch-Siemińska, M.; Wawerska, K.; Gawroński, J. The Potential of Plant Tissue Cultures to Improve the Steviol Glycoside Profile of Stevia (Stevia rebaudiana Bertoni) Regenerants. Int. J. Mol. Sci. 2024, 25, 13584. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Igamberdiev, A.U.; Debnath, S.C. Somaclonal Variation and Clonal Fidelity in Commercial Micropropagation: Challenges and Perspectives. Agronomy 2025, 15, 1489. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef]
- Jadid, N.; Anggraeni, S.; Ramadani, M.R.N.; Arieny, M.; Mas’ud, F. In vitro propagation of Indonesian stevia (Stevia rebaudiana) genotype using axenic nodal segments. BMC Res. Notes 2024, 17, 45. [Google Scholar] [CrossRef]
- Razak, U.; Boon, O.C.; Yu, T.; Lau, L. In vitro micropropagation of Stevia rebaudiana Bertoni in Malaysia. Braz. Arch. Biol. Technol. 2014, 57, 23–28. [Google Scholar] [CrossRef]
- Aguirre-Medina, J.F.; Gálvez-López, A.L.; Aguirre-Cadena, J.F. In vitro organogenesis of Stevia rebaudiana Bert. with different explants and growth regulators. Agro Prod. 2021, 14, 23–29. [Google Scholar] [CrossRef]
- Rokosa, M.T.; Kulpa, D. Micropropagation of Stevia rebaudiana plants. Cienc. Rural 2020, 50, e20181029. [Google Scholar] [CrossRef]
- Ghose, A.K.; Abdullah, S.N.A.; Md Hatta, M.A.; Megat Wahab, P.E. In vitro regeneration of Stevia rebaudiana Bertoni and evaluation of the impacts of growth media nutrients on the biosynthesis of steviol glycosides (SGs). Agronomy 2022, 12, 1957. [Google Scholar] [CrossRef]
- Misal, V.D.; Chavan, A.M. In vitro micropropagation and mass multiplication of Stevia rebaudiana Bertoni. World J. Adv. Res. Rev. 2024, 23, 250–254. [Google Scholar] [CrossRef]
- Dyduch-Siemińska, M. A fast and effective protocol for obtaining genetically diverse stevia (Stevia rebaudiana Bertoni) regenerants through indirect organogenesis. Acta Soc. Bot. Pol. 2021, 76, 47–62. [Google Scholar] [CrossRef]
- Duta-Cornescu, G.; Constantin, N.; Pojoga, D.-M.; Nicuta, D.; Simon-Gruita, A. Somaclonal Variation—Advantage or Disadvantage in Micropropagation of the Medicinal Plants. Int. J. Mol. Sci. 2023, 24, 838. [Google Scholar] [CrossRef]
- Xiong, F.; Zhong, R.; Han, Z.; Jiang, J.; He, L.; Zhuang, W.; Tang, R. Start codon targeted polymorphism for evaluation of functional genetic variation and relationships in cultivated peanut (Arachis hypogaea L.) genotypes. Mol. Biol. Rep. 2011, 38, 3487–3494. [Google Scholar] [CrossRef]
- Chhajer, S.; Jukanti, A.K.; Kalia, R.K. Start codon targeted (SCoT) polymorphism-based genetic relationships and diversity among populations of Tecomella undulata (Sm.) Seem—An endangered timber tree of hot arid regions. Tree Genet. Genomes 2017, 13, 84. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Luo, C.; He, X.H.; Chen, H.; Ou, S.J.; Gao, M.P. Analysis of diversity and relationships among mango cultivars using Start Codon Targeted (SCoT) markers. Biochem. Syst. Ecol. 2010, 38, 1176–1184. [Google Scholar] [CrossRef]
- Qin, G.-X.; Liang, G.-L.; Lei, J.-J. Studies on the genetic relationship of Chinese diploid strawberry species based on SCoT analysis. Acta Hortic. 2014, 1049, 301–304. [Google Scholar] [CrossRef]
- Krishna, S.; Woodrow, C.J.; Staines, H.M.; Haynes, R.K.; Mercereau-Puijalon, O. Re-evaluation of how artemisinins work in light of emerging evidence of in vitro resistance. Trends Mol. Med. 2006, 12, 200–205. [Google Scholar] [CrossRef]
- Rodriguéz-Páez, L.A.; Pineda-Rodriguez, Y.Y.; Pompelli, M.F.; Jimenez-Ramirez, A.M.; Genes-Avilez, O.J.; Jaraba-Navas, J.D.D.; Jarma-Orozco, A.; Combatt-Caballero, E.; Oviedo Zumaqué, L.E.; Suarez-Padron, I.E.; et al. Micropropagation protocols for three elite genotypes of Stevia rebaudiana Bertoni. Horticulturae 2024, 10, 404. [Google Scholar] [CrossRef]
- Abouelela, M.B.; Eid, M.; Ali, F.M.; Owis, A.I. Optimizing a rapid tissue culture method for steviol glycoside production from Stevia rebaudiana to address Egypt’s sugar deficit. Sci. Rep. 2025, 15, 25495. [Google Scholar] [CrossRef]
- Ferreira, M.d.S.; Rocha, A.d.J.; Nascimento, F.d.S.; Oliveira, W.D.d.S.; Soares, J.M.d.S.; Rebouças, T.A.; Morais Lino, L.S.; Haddad, F.; Ferreira, C.F.; Santos-Serejo, J.A.d.; et al. The Role of Somaclonal Variation in Plant Genetic Improvement: A Systematic Review. Agronomy 2023, 13, 730. [Google Scholar] [CrossRef]
- Hesami, M.; Adamek, K.; Pepe, M.; Jones, A.M.P. Effect of explant source on phenotypic changes of in vitro grown Cannabis plantlets over multiple subcultures. Biology 2023, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M.; Armitage, D.W. Epigenetic responses of trees to environmental stress in the context of climate change. Biol. Rev. 2025, 100, 131–148. [Google Scholar] [CrossRef]




| PGR | BAP (mg × dm−3) (Benzylaminopurine) | Kin (mg × dm−3) (Kinetin) | |
|---|---|---|---|
| Medium | |||
| F1 | 0.5 | 0.25 | |
| F2 | 0.5 | - | |
| F3 | 1 | 0.25 | |
| F4 | 1 | - | |
| No. of Somaclone | Number of Explants | Percentage of Explants Producing Callus | Number of Regenerants |
|---|---|---|---|
| 5 | 100 | 100 | 15 |
| 6 | 100 | 98 | 23 |
| 12 | 100 | 100 | 7 |
| 21 | 100 | 95 | 12 |
| 26 | 100 | 97 | 10 |
| 30 | 100 | 100 | 13 |
| No. of Somaclone | F1 Medium | ||
|---|---|---|---|
| Mean Number of Shoots | Mean Shoot Length (cm) | Mean Number of Nodes per Shoot | |
| 5 | 8.00 a * (4.0–12.0) | 3.40 bc (2.5–4.5) | 3.31 a (2.0–4.0) |
| 6 | 3.71 cd (2.0–5.0) | 4.14 ab (0.7–8.0) | 3.06 a (1.0–5.0) |
| 12 | 5.78 b (4.0–7.0) | 2.59 c (2.2–3.0) | 2.93 a (2.0–4.0) |
| 21 | 4.65 bc (3.0–6.0) | 4.17 ab (2.9–5.8) | 2.92 a (2.0–3.0) |
| 26 | 5.29 b (2.0–8.0) | 4.82 a (4.1–5.5) | 2.81 a (2.0–4.0) |
| 30 | 2.50 d (2.0–3.0) | 4.58 a (3.1–6.2) | 3.00 a (2.0–4.0) |
| No. of Somaclone | F2 Medium | ||
|---|---|---|---|
| Mean Number of Shoots | Mean Shoot Length (cm) | Mean Number of Nodes per Shoot | |
| 5 | 4.22 b * (2.0–5.0) | 6.11 ab (3.3–10.2) | 4.75 ab (3.0–5.0) |
| 6 | 6.19 a (2.0–11.0) | 4.61 b (3.7–5.4) | 3.77 b (2.0–5.0) |
| 12 | 3.08 c (1.0–4.0) | 5.04 b (2.5–7.1) | 4.83 ab (3.0–6.0) |
| 21 | 2.82 cd (1.0–4.0) | 5.20 b (2.2–7.1) | 3.81 b (2.0–6.0) |
| 26 | 1.82 d (1.0–2.0) | 7.00 a (4.6–10.3) | 5.29 a (4.0–7.0) |
| 30 | 2.84 cd (2.0–3.0) | 5.98 ab (4.3–7.4) | 4.80 ab (3.0–6.0) |
| No. of Somaclone | F3 Medium | ||
|---|---|---|---|
| Mean Number of Shoots | Mean Shoot Length (cm) | Mean Number of Nodes per Shoot | |
| 5 | 1.00 a * (1.0–1.0) | 1.80 a (1.00–2.50) | 2.00 a (1.0–3.0) |
| 6 | 1.00 a (1.0–1.0) | 1.90 a (1.25–2.75) | 2.50 a (1.0–4.0) |
| 12 | 1.86 a (1.0–3.0) | 2.07 a (1.00–2.50) | 2.71 a (2.0–3.0) |
| 21 | 1.00 a (1.0–1.0) | 1.95 a (1.50–2.50) | 1.90 a (1.0–2.0) |
| 26 | 0.90 b (0.0–1.0) | 1.95 a (1.25–2.50) | 2.90 a (2.0–3.0) |
| 30 | 1.00 a (1.0–1.0) | 1.94 a (1.25–2.25) | 2.70 a (2.0–3.0) |
| No. of Somaclone | F4 Medium | ||
|---|---|---|---|
| Mean Number of Shoots | Mean Shoot Length (cm) | Mean Number of Nodes per Shoot | |
| 5 | 2.13 a * (1.0–3.0) | 2.86 a (2.50–3.00) | 2.87 a (1.0–5.0) |
| 6 | 2.11 a (1.0–3.0) | 2.83 a (2.00–3.50) | 3.11 a (2.0–3.0) |
| 12 | 1.86 a (1.0–3.0) | 4.43 a (2.00–5.83) | 4.43 a (3.0–6.0) |
| 21 | 3.00 a (1.0–3.0) | 4.96 a (1.80–7.10) | 4.14 a (3.0–5.0) |
| 26 | 1.89 a (1.0–2.0) | 3.56 a (2.75–4.33) | 3.11 a (2.0–4.0) |
| 30 | 2.38 a (1.0–3.0) | 4.57 a (3.00–5.33) | 4.00 a (2.0–5.0) |
| No. of Primer | Starter Sequence 5′-3′ | Number of Products | Percentage of Polymorphism % | Size Range (bp) | ||
|---|---|---|---|---|---|---|
| Monomorphic | Polymorphic | Total | ||||
| 2 | CAACAATGGCTACCACCC | 2 | 4 | 6 | 67 | 1000–4000 |
| 4 | CAACAATGGCTACCACCT | 4 | 2 | 6 | 33 | 1400–3800 |
| 21 | ACGACATGGCGACCCACA | 5 | 4 | 9 | 44 | 370–2750 |
| 23 | CACCATGGCTACCACCAG | 2 | 1 | 3 | 33 | 1300–1700 |
| 28 | CCATGGCTACCACCGCCA | 7 | 2 | 9 | 22 | 220–2500 |
| 30 | CCATGGCTACCACCGGCG | 1 | 4 | 5 | 80 | 2250–4000 |
| 33 | CCATGGCTACCACCGCAG | 0 | 3 | 3 | 100 | 750–1000 |
| 46 | ACAATGGCTACCACTGAG | 1 | 2 | 3 | 67 | 900–4000 |
| 75 | CCATGGCTACCACCGGAG | 3 | 6 | 9 | 67 | 550–2200 |
| 83 | ACGACATGGCGACCAGCG | 4 | 5 | 9 | 56 | 500–2500 |
| 90 | CCATGGCTACCACCGGCA | 5 | 3 | 8 | 38 | 500–3000 |
| Total | - | 34 | 36 | 70 | - | 220–4000 |
| Average/ primer | - | 3.1 | 3.3 | 6.4 | 55.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyduch-Siemińska, M.; Gawroński, J. In Vitro Regeneration of Stevia rebaudiana Bertoni Using Somaclonal Variation as a Tool for Genetic Diversification. Genes 2025, 16, 1203. https://doi.org/10.3390/genes16101203
Dyduch-Siemińska M, Gawroński J. In Vitro Regeneration of Stevia rebaudiana Bertoni Using Somaclonal Variation as a Tool for Genetic Diversification. Genes. 2025; 16(10):1203. https://doi.org/10.3390/genes16101203
Chicago/Turabian StyleDyduch-Siemińska, Magdalena, and Jacek Gawroński. 2025. "In Vitro Regeneration of Stevia rebaudiana Bertoni Using Somaclonal Variation as a Tool for Genetic Diversification" Genes 16, no. 10: 1203. https://doi.org/10.3390/genes16101203
APA StyleDyduch-Siemińska, M., & Gawroński, J. (2025). In Vitro Regeneration of Stevia rebaudiana Bertoni Using Somaclonal Variation as a Tool for Genetic Diversification. Genes, 16(10), 1203. https://doi.org/10.3390/genes16101203






