Genetic Etiology of Developmental and Epileptic Encephalopathy in a Turkish Cohort: A Single-Center Study with Targeted Gene Panel and Whole Exome Sequencing
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Targeted Gene Panel and Whole Exome Sequencing
2.3. Variant Evaluation
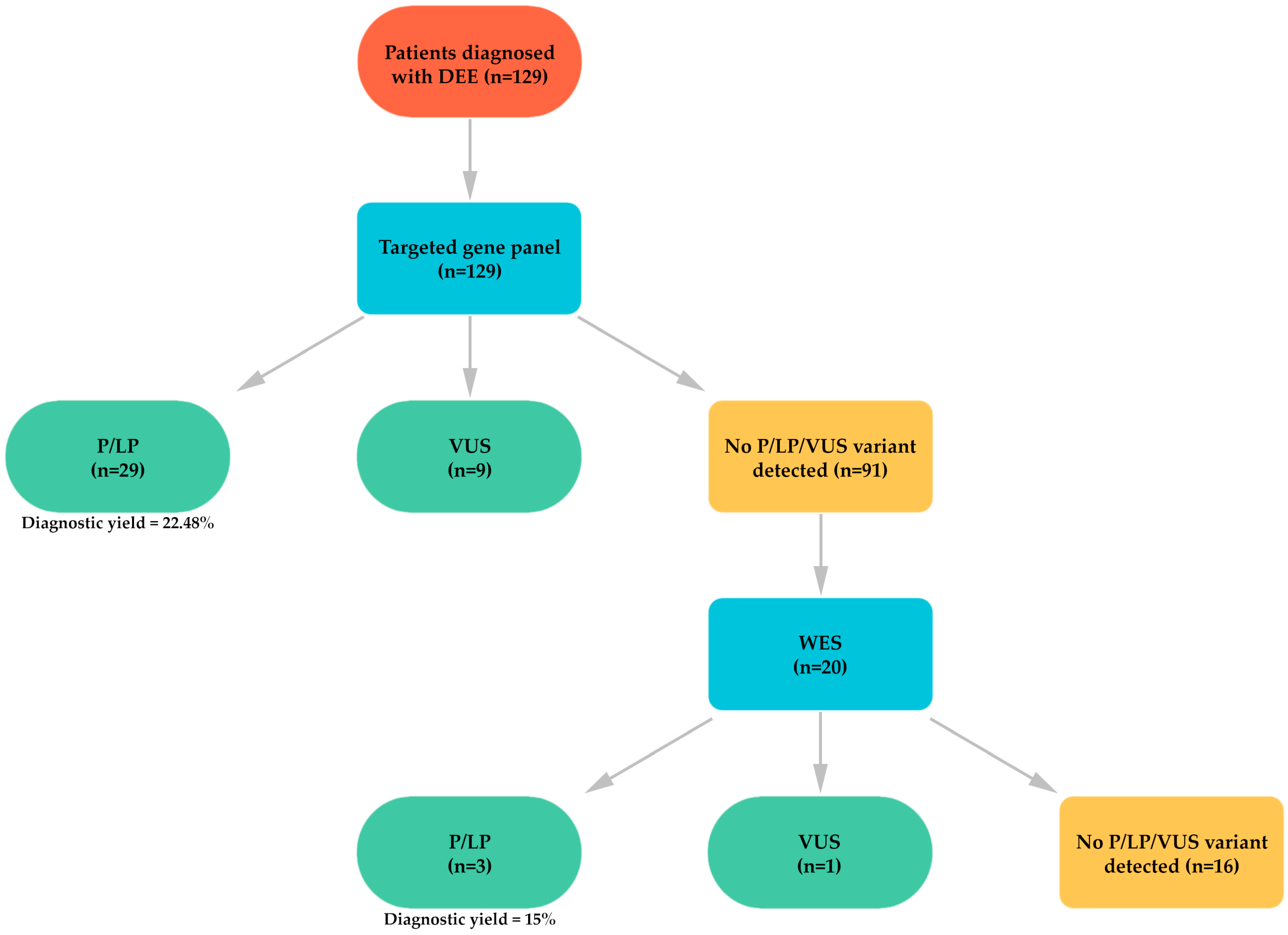
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Essajee, F.; Urban, M.; Smit, L.; Wilmshurst, J.M.; Solomons, R.; van Toorn, R.; Moosa, S. Utility of genetic testing in children with developmental and epileptic encephalopathy (DEE) at a tertiary hospital in South Africa: A prospective study. Seizure 2022, 101, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Chen, C.; Yang, L.; Zhang, C.; Deng, X.; Yang, L.; Mao, L.; Liu, F.; Wang, G.; Duan, H.; et al. The genetic spectrum features of 2261 Chinese children with epilepsy and intellectual disability. BMC Med. 2025, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Triono, A.; Herini, E.S.; Mooiindie, K.H.; Iskandar, K.; Gunadi. Association between phenotypes and genotype of developmental and epileptic encephalopathy in next-generation sequencing methods in infants: A scoping review. Med. J. Malays. 2025, 80, 521–530. [Google Scholar]
- Nguyen, Y.T.M.; Vu, B.Q.; Nguyen, D.K.; Quach, N.V.; Bui, L.T.; Hong, J.; Bui, C.B. Genotype-driven therapeutics in DEE and metabolic epilepsy: Navigating treatment efficacy and drug resistance. Sci Rep. 2024, 14, 21606. [Google Scholar] [CrossRef]
- Gallagher, D.; Pérez-Palma, E.; Bruenger, T.; Ghanty, I.; Brilstra, E.; Ceulemans, B.; Chemaly, N.; de Lange, I.; Depienne, C.; Guerrini, R.; et al. Genotype-phenotype associations in 1018 individuals with SCN1A-related epilepsies. Epilepsia 2024, 65, 1046–1059. [Google Scholar] [CrossRef]
- Frost, L.D.; Bicknell, B.; Lalor, K. Multi-Phenotype-Genotype Correlations in a Dravet Syndrome Case with Compounded SCN1A and SCN2A Mutations. In Proceedings of the AFMR Southeastern Regional Meeting, Birmingham, AL, USA, 15–16 May 2025. [Google Scholar]
- Jang, S.S.; Kim, S.Y.; Kim, H.; Hwang, H.; Chae, J.H.; Kim, K.J.; Kim, J.I.; Lim, B.C. Diagnostic Yield of Epilepsy Panel Testing in Patients With Seizure Onset Within the First Year of Life. Front. Neurol. 2019, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, M.; Jallouli, O.; Ben Aissa, A.; Souissi, A.; Kamoun, F.; Fakhfakh, F.; Masmoudi, S.; Ben Ayed, I.; Charfi Triki, C. Customized targeted massively parallel sequencing enables the identification of novel pathogenic variants in Tunisian patients with developmental and epileptic encephalopathy. Epilepsia Open 2024, 9, 1697–1709. [Google Scholar] [CrossRef]
- Ream, M.A.; Mikati, M.A. Clinical utility of genetic testing in pediatric drug-resistant epilepsy: A pilot study. Epilepsy Behav. 2014, 37, 241–248. [Google Scholar] [CrossRef]
- Møller, R.S.; Larsen, L.H.; Johannesen, K.M.; Talvik, I.; Talvik, T.; Vaher, U.; Miranda, M.J.; Farooq, M.; Nielsen, J.E.; Svendsen, L.L.; et al. Gene Panel Testing in Epileptic Encephalopathies and Familial Epilepsies. Mol. Syndromol. 2016, 7, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.M.; da Silva, C.; Alexander, J.J.; Hegde, M.; Escayg, A. Diagnostic Yield from 339 Epilepsy Patients Screened on a Clinical Gene Panel. Pediatr. Neurol. 2017, 77, 61–66. [Google Scholar] [CrossRef]
- Rim, J.H.; Kim, S.H.; Hwang, I.S.; Kwon, S.S.; Kim, J.; Kim, H.W.; Cho, M.J.; Ko, A.; Youn, S.E.; Kim, J.; et al. Efficient strategy for the molecular diagnosis of intractable early-onset epilepsy using targeted gene sequencing. BMC Med. Genom. 2018, 11, 6. [Google Scholar] [CrossRef]
- Atli, E.I.; Atli, E.; Yalcintepe, S.; Demir, S.; Kalkan, R.; Eker, D.; Gurkan, H. Customised targeted massively parallel sequencing enables more precise diagnosis of patients with epilepsy. Intern. Med. J. 2022, 52, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Hoelz, H.; Herdl, C.; Gerstl, L.; Tacke, M.; Vill, K.; von Stuelpnagel, C.; Rost, I.; Hoertnagel, K.; Abicht, A.; Hollizeck, S.; et al. Impact on Clinical Decision Making of Next-Generation Sequencing in Pediatric Epilepsy in a Tertiary Epilepsy Referral Center. Clin. EEG Neurosci. 2020, 51, 61–69. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Ki, C.S.; Lee, J. Determining the best candidates for next-generation sequencing-based gene panel for evaluation of early-onset epilepsy. Mol. Genet. Genom. Med. 2020, 8, e1376. [Google Scholar] [CrossRef]
- Murthy, M.C.; Banerjee, B.; Shetty, M.; Mariappan, M.; Sekhsaria, A. A retrospective study of the yield of next-generation sequencing in the diagnosis of developmental and epileptic encephalopathies and epileptic encephalopathies in 0–12 years aged children, at a single tertiary care hospital in South India. Epileptic Disord. 2024, 26, 609–625. [Google Scholar] [CrossRef]
- Sadleir, L.G.; Mountier, E.I.; Gill, D.; Davis, S.; Joshi, C.; De Vile, C.; Kurian, M.A.; Study, D.D.D.; Mandelstam, S.; Wirrell, E.; et al. Not all SCN1A epileptic encephalopathies are Dravet syndrome: Early pro-found Thr226Met phenotype. Neurology 2017, 89, 1035–1042. [Google Scholar] [CrossRef]
- Usluer, S.; Salar, S.; Arslan, M.; Yiş, U.; Kara, B.; Tektürk, P.; Baykan, B.; Meral, C.; Türkdoğan, D.; Bebek, N.; et al. SCN1A gene sequencing in 46 Turkish epilepsy patients disclosed 12 novel mutations. Seizure 2016, 39, 34–43. [Google Scholar] [CrossRef]
- Wallace, R.H.; Hodgson, B.L.; Grinton, B.E.; Gardiner, R.M.; Robinson, R.; Rodriguez-Casero, V.; Sadleir, L.; Morgan, J.; Harkin, L.A.; Dibbens, L.M.; et al. Sodium channel alpha1-subunit mutations in severe myoclonic epilepsy of infancy and infantile spasms. Neurology 2003, 61, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kawawaki, H.; Horino, A.; Thuji, H.; Nukui, M.; Kuki, I.; Okazaki, S.; Tomiwa, K. A case of West syndrome with a deletion at chromosome 2q24.3-q31.3. No Hattatsu 2017, 49, 131–135. [Google Scholar]
- Pavone, P.; Polizzi, A.; Marino, S.D.; Corsello, G.; Falsaperla, R.; Marino, S.; Ruggieri, M. West syndrome: A comprehensive review. Neurol. Sci. 2020, 41, 3547–3562. [Google Scholar] [CrossRef] [PubMed]
- Krey, I.; Krois-Neudenberger, J.; Hentschel, J.; Syrbe, S.; Polster, T.; Hanker, B.; Fiedler, B.; Kurlemann, G.; Lemke, J.R. Genotype-phenotype correlation on 45 individuals with West syndrome. Eur. J. Paediatr. Neurol. 2020, 25, 134–138. [Google Scholar] [CrossRef]
- Wong, V.C.; Fung, C.W.; Kwong, A.K. SCN2A mutation in a Chinese boy with infantile spasm—Response to Modified Atkins Diet. Brain Dev. 2015, 37, 729–732. [Google Scholar] [CrossRef]
- Yüksel, M.F.; Doğulu, N.; Yıldırım, M.; Köse, E.; Bektaş, Ö.; Eminoğlu, F.T.; Teber, S. Metabolic etiologies in children with infantile epileptic spasm syndrome: Experience at a tertiary pediatric neurology center. Brain Dev. 2024, 46, 213–218. [Google Scholar] [CrossRef]
- Banne, E.; Abudiab, B.; Abu-Swai, S.; Repudi, S.R.; Steinberg, D.J.; Shatleh, D.; Alshammery, S.; Lisowski, L.; Gold, W.; Carlen, P.L.; et al. Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview. Cells 2021, 10, 824. [Google Scholar] [CrossRef]
- Jia, J.L.; Chen, S.; Sivarajah, V.; Stephens, D.; Cortez, M.A. Latitudinal differences on the global epidemiology of infantile spasms: Systematic review and meta-analysis. Orphanet J. Rare Dis. 2018, 13, 216. [Google Scholar] [CrossRef]
- Bahi-Buisson, N.; Villeneuve, N.; Caietta, E.; Jacquette, A.; Maurey, H.; Matthijs, G.; Van-Esch, H.; Delahaye, A.; Moncla, A.; Milh, M.; et al. Recurrent mutations in the CDKL5 gene: Genotype-phenotype relationships. Am. J. Med. Genet. Part A 2012, 158A, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.M.; Tian, F.Y.; Shen, Y.W.; Yang, C.Y.; Yuan, H.; Li, P.; Gao, Z.B. Functional characterization and in vitro pharmacological rescue of KCNQ2 pore mutations associated with epileptic encephalopathy. Acta Pharmacol. Sin. 2023, 44, 1589–1599. [Google Scholar] [CrossRef]
- Berg, A.T.; Thompson, C.H.; Myers, L.S.; Anderson, E.; Evans, L.; Kaiser, A.J.E.; Paltell, K.; Nili, A.N.; DeKeyser, J.L.; Abramova, T.V.; et al. Expanded clinical phenotype spectrum correlates with variant function in SCN2A-related disorders. Brain 2024, 147, 2761–2774. [Google Scholar] [CrossRef]
- Takeguchi, R.; Haginoya, K.; Uchiyama, Y.; Fujita, A.; Nagura, M.; Takeshita, E.; Inui, T.; Okubo, Y.; Sato, R.; Miyabayashi, T.; et al. Two Japanese cases of epileptic encephalopathy associated with an FGF12 mutation. Brain Dev. 2018, 40, 728–732. [Google Scholar] [CrossRef]
- Xian, J.; Parthasarathy, S.; Ruggiero, S.M.; Balagura, G.; Fitch, E.; Helbig, K.; Gan, J.; Ganesan, S.; Kaufman, M.C.; Ellis, C.A.; et al. Assessing the landscape of STXBP1-related disorders in 534 individuals. Brain. 2022, 145, 1668–1683. [Google Scholar] [CrossRef]
- Oliver, K.L.; Trivisano, M.; Mandelstam, S.A.; De Dominicis, A.; Francis, D.I.; Green, T.E.; Muir, A.M.; Chowdhary, A.; Hertzberg, C.; Goldhahn, K.; et al. WWOX developmental and epileptic encephalopathy: Understanding the epileptology and the mortality risk. Epilepsia 2023, 64, 1351–1367. [Google Scholar] [CrossRef]
- Kacker, S.; Phitsanuwong, C.; Oetomo, A.; Nordli, D.R., Jr. Late infantile epileptic encephalopathy: A distinct developmental and epileptic encephalopathy syndrome. Epileptic Disord. 2024, 26, 98–108. [Google Scholar] [CrossRef]
- Poduri, A. HCN1 Gain-Of-Function Mutations—A New Cause of Epileptic Encephalopathy. Epilepsy Curr. 2014, 14, 348–349. [Google Scholar] [CrossRef]
- Desprairies, C.; Valence, S.; Maurey, H.; Helal, S.I.; Weckhuysen, S.; Soliman, H.; Mefford, H.C.; Spentchian, M.; Héron, D.; Leguern, E.; et al. Three novel patients with epileptic encephalopathy due to biallelic mutations in the PLCB1 gene. Clin. Genet. 2020, 97, 477–482. [Google Scholar] [CrossRef]
- Johannesen, K.M.; Liu, Y.; Koko, M.; Gjerulfsen, C.E.; Sonnenberg, L.; Schubert, J.; Fenger, C.D.; Eltokhi, A.; Rannap, M.; Koch, N.A.; et al. Genotype-phenotype correlations in SCN8A-related disorders reveal prognostic and therapeutic implications. Brain 2022, 145, 2991–3009. [Google Scholar] [CrossRef]
- Hack, J.B.; Horning, K.; Juroske Short, D.M.; Schreiber, J.M.; Watkins, J.C.; Hammer, M.F. Distinguishing Loss-of-Function and Gain-of-Function SCN8A Variants Using a Random Forest Classification Model Trained on Clinical Features. Neurol. Genet. 2023, 9, e200060. [Google Scholar] [CrossRef] [PubMed]
- Happ, H.C.; Carvill, G.L. A 2020 View on the Genetics of Developmental and Epileptic Encephalopathies. Epilepsy Curr. 2020, 20, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Seri, S.; Verdecchia, M.; Bombardieri, R. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001, 23, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, X.; Tang, Z.; Qiu, X.; Guo, Z.; Huang, D.; Xiong, H.; Guo, Q. The Correlation Between Tuberous Sclerosis Complex Genotype and Renal Angiomyolipoma Phenotype. Front. Genet. 2020, 11, 575750. [Google Scholar] [CrossRef] [PubMed]
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Fuchs, Z.; Gosnell, E.S.; Gupta, N.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Vecchio, D.; Macchiaiolo, M.; Gonfiantini, M.V.; Panfili, F.M.; Petrizzelli, F.; Liorni, N.; Cortellessa, F.; Sinibaldi, L.; Rana, I.; Agolini, E.; et al. Widening the infantile hypotonia with psychomotor retardation and characteristic Facies-1 Syndrome’s clinical and molecular spectrum through NALCN in-silico structural analysis. Front. Genet. 2024, 15, 1477940. [Google Scholar] [CrossRef]
- Karimi, A.H.; Karimi, M.R.; Farnia, P.; Parvini, F.; Foroutan, M. A Homozygous Truncating Mutation in NALCN Causing IHPRF1: Detailed Clinical Manifestations and a Review of Literature. Appl. Clin. Genet. 2020, 13, 151–157. [Google Scholar] [CrossRef]
- Takenouchi, T.; Inaba, M.; Uehara, T.; Takahashi, T.; Kosaki, K.; Mizuno, S. Biallelic mutations in NALCN: Expanding the genotypic and phenotypic spectra of IHPRF1. Am. J. Med. Genet. A 2018, 176, 431–437. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Liu, X.; Zhao, Y.; Zhang, J. A novel CTBP1 variant in a Chinese pediatric patient with a phenotype distinct from hypotonia, ataxia, developmental delay, and tooth enamel defect syndrome. Front. Genet. 2024, 15, 1344682. [Google Scholar] [CrossRef]
- Jafari Khamirani, H.; Zoghi, S.; Saber Sichani, A.; Dianatpour, M.; Mohammadi, S.; Mohammad Bagher Tabei, S.; Alireza Dastgheib, S. Exome sequencing identified a de novo frameshift pathogenic variant of CTBP1 in an extremely rare case of HADDTS. J. Genet. 2021, 100, 68. [Google Scholar] [CrossRef]
- Helbig, I.; Heinzen, E.L.; Mefford, H.C. Primer Part 1-The building blocks of epilepsy genetics. Epilepsia 2016, 57, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Mercimek-Mahmutoglu, S.; Patel, J.; Cordeiro, D.; Hewson, S.; Callen, D.; Donner, E.J.; Hahn, C.D.; Kannu, P.; Kobayashi, J.; Minassian, B.A.; et al. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia 2015, 56, 707–716. [Google Scholar] [CrossRef]
- Vetri, L.; Calì, F.; Saccone, S.; Vinci, M.; Chiavetta, N.V.; Carotenuto, M.; Roccella, M.; Costanza, C.; Elia, M. Whole Exome Sequencing as a First-Line Molecular Genetic Test in Developmental and Epileptic Encephalopathies. Int. J. Mol. Sci. 2024, 25, 1146. [Google Scholar] [CrossRef] [PubMed]
- Oliwa, A.; Hendson, G.; Longman, C.; Synnes, A.; Seath, K.; Barnicoat, A.; Hall, J.G.; Patel, M.S. Lethal respiratory course and additional features expand the phenotypic spectrum of PIEZO2-related distal arthrogryposis type 5. Am. J. Med. Genet. A 2023, 191, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Seidahmed, M.Z.; Maddirevula, S.; Miqdad, A.M.; Al Faifi, A.; Al Samadi, A.; Alkuraya, F.S. Confirming the involvement of PIEZO2 in the etiology of Marden-Walker syndrome. Am. J. Med. Genet. A 2021, 185, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Fornari Caprara, A.L.; Fighera, M.R.; Tamiozzo, R.L. A 24-year-Old Male with Marden-Walker Syndrome and Epilepsy: Case Report. Neurol. India 2023, 71, 767–771. [Google Scholar] [CrossRef] [PubMed]
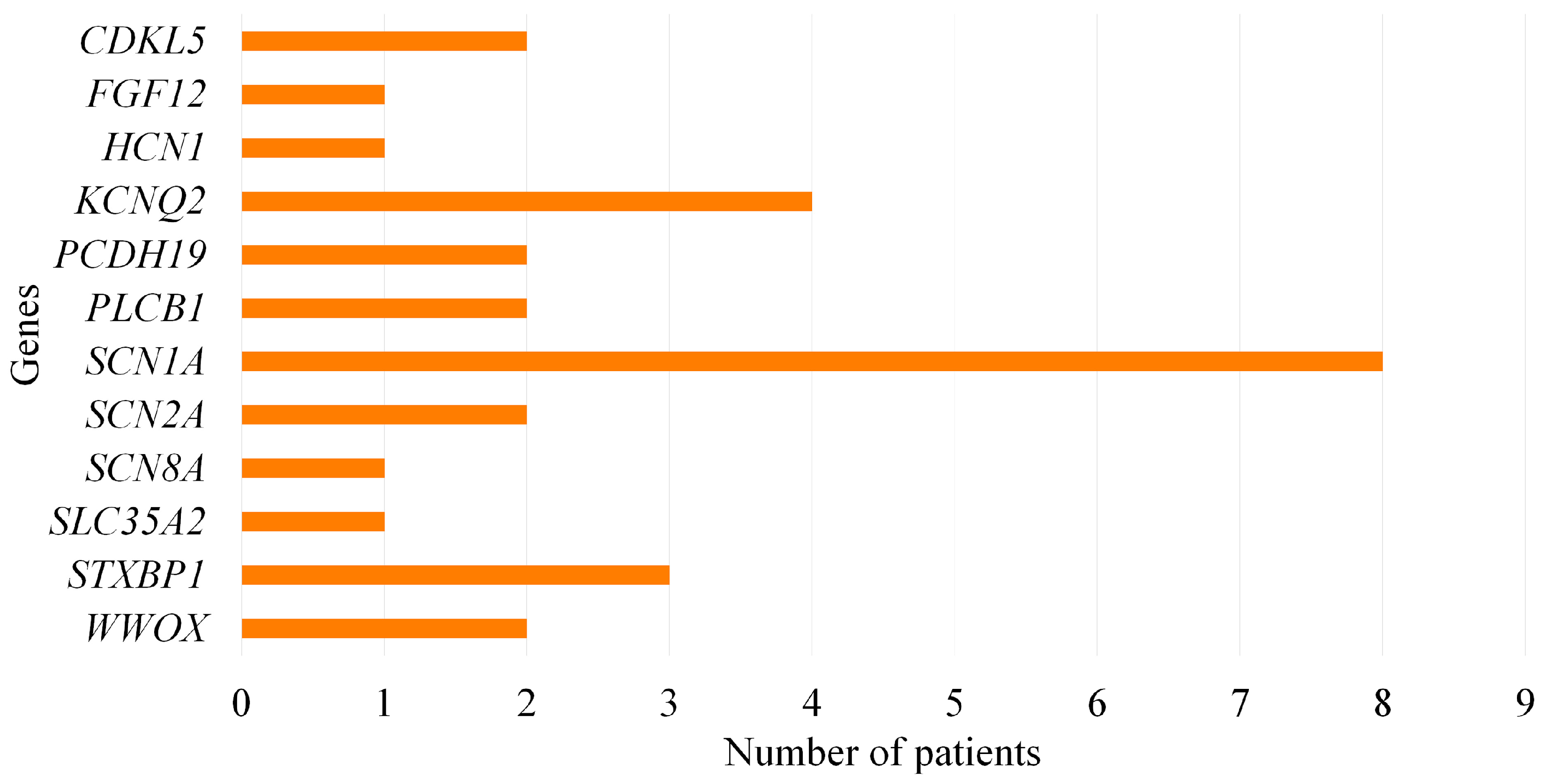
| Gene | Variant | Reported ACMG Classification | Case ID | Sex/Age at the Study (Years/Months) * | Clinical Features/Epileptic Syndrome | Novel/ Previously Reported Variant | Pattern of Inheritance (De Novo/Maternal/ Paternal) | Consanguinity |
|---|---|---|---|---|---|---|---|---|
| CDKL5 | Heterozygous Frameshift chrX:18616648 NM_001323289.2 c.892_911delinsG p.Q298VfsTer46 | P (PVS1, PS2, PM2) | 3 | F/10 | Epileptiform anomaly on a disorganized background in EEG/EIDEE | Novel | de novo | N/A |
| Heterozygous Missense chrX:18622278 NM_001323289.2 c.1234A>C p.K412Q | LP (PS2, PM2) | 4 | M/5 months | Seizures/EIDEE | Novel | de novo | N/A | |
| FGF12 | Heterozygous Missense chr3:192053223 NM_004113.6 c.341G>A p.R114H | P (PS2, PS3, PM2, PP3, PP5) | 6 | F/6 | Thin corpus callosum and decreased white matter volume in MRI, focal epileptiform anomaly on severely disorganized background in EEG/EIDEE | ClinVar 2584544 | de novo | No |
| HCN1 | Heterozygous Missense chr5:45695831 NM_021072.4 c.365A>G p.K122R | LP (PS2, PM2, PP2) | 12 | F/10 months | Myoclonic seizures, focal epileptiform anomaly in EEG/LIDEE | Novel | de novo | No |
| KCNQ2 | Heterozygous Missense chr20:62071057 NM_172107.4 c.821C>T p.T274M | P (PS2, PS3, PM1, PM2, PM5, PP2, PP3) | 15 | F/1 | Seizures/EIDEE | ClinVar 167208 | de novo | N/A |
| Heterozygous Missense chr20:62070961 NM_172107.4 c.917C>T p.A306V | P (PM1, PM2, PM5, PP2, PP3, PP5) | 16 | M/3 months | Disorganized background in EEG/EIDEE | ClinVar 219235 | N/A | N/A | |
| Heterozygous Missense chr20:62076118 NM_172107.4 c.584C>T p.S195F | P (PM1, PM2, PM5, PP2, PP3, PP5) | 17 | M/2 months | Focal epileptiform anomaly on a disorganized background in EEG/EIDEE | ClinVar 590184 | N/A | N/A | |
| Heterozygous Missense chr20:62076083 NM_172107.4 c.619C>T p.R207W | P (PS2, PS3, PM1, PM2, PM5, PP2, PP3) | 18 | M/5 | Autism, focal epileptiform anomaly in EEG/EIDEE | ClinVar 7386 | de novo | N/A | |
| PCDH19 | Heterozygous Frameshift chrX:99662504 NM_001184880.2 c.1091dup p.Y366Lfs*10 | P (PVS1, PS4, PM2) | 21 | F/16 | Intellectual disability, scoliosis, dermatological symptoms/LIDEE | ClinVar 206353 | N/A | N/A |
| Heterozygous Frameshift chrX:99662504 NM_001184880.2 c.1091dup p.Y366Lfs*10 | P (PVS1, PS4, PM2) | 22 | F/17 | Menstrual irregularity, dermatological symptoms/LIDEE | ClinVar 206353 | N/A | N/A | |
| PLCB1 | Homozygous Missense chr20:8713941 NM_015192.4 c.1945A>G p.R649G | LP (PM2, PP1, PP2, PP3, PP4) | 23 | F/6 | Multifocal epileptiform anomaly in EEG, malignant migrating focal seizures/LIDEE | SNP rs1219807693 | Carrier parents | No |
| Homozygous Missense chr20:8713941 NM_015192.4 c.1945A>G p.R649G | LP (PM2, PP1, PP2, PP3, PP4) | 24 | M/3 | Multifocal epileptiform anomaly in EEG, malignant migrating focal seizures/LIDEE | SNP rs1219807693 | Carrier parents | No | |
| SCN1A | Heterozygous Frameshift chr2:166905410 NM_001165963.4 c.1013delA p.N338fs | P (PVS1, PS2, PM2) | 25 | M/7 months | Thin corpus callosum in MRI, focal epileptiform anomaly in EEG/WS | Novel | de novo | N/A |
| Heterozygous Missense chr2:166908424 NM_001165963.4 c.769T>C p.C257R | P (PS2, PS4, PM1, PM2, PP2, PP3) | 26 | F/4 | Multifocal generalized epileptiform anomaly in EEG/DS | ClinVar 189934 | de novo | No | |
| Heterozygous Splice region chr2:166895930 NM_001165963.4 c.2589+3A>T | P (PS2, PS3, PM2, PP3) | 27 | F/9 months | Focal epileptiform anomaly in EEG/DS | ClinVar 189938 | de novo | N/A | |
| Heterozygous Missense chr2:166854586 NM_001165963.4 c.4438G>T p.G1480C | P (PS2, PM1, PM2, PM5, PP2, PP3) | 28 | F/3 | Focal epileptiform anomaly in EEG/DS | ClinVar 206830 | de novo | N/A | |
| Heterozygous Stop gained chr2:166898897 NM_001165963.4 c.2048C>A p.S683Ter | P (PVS1, PS2, PS4, PM2) | 29 | M/10 | Seizures/DS | ClinVar 2431708 | de novo | Yes | |
| Heterozygous Nonsense chr2:166859193 NM_001165963.4 c.4040G>A p.W1347Ter | P (PVS1, PS2, PS4, PM2) | 30 | F/10 months | Focal epileptiform anomaly on a disorganized background in EEG/DS | ClinVar 1458263 | de novo | Yes | |
| Heterozygous Missense chr2:166901554 NM_001165963.4 c.1661A>T p.Q554L | P (PS2, PM2, PM5, PP2, PP3) | 31 | F/6 | Intellectual disability/DS | Novel | de novo | No | |
| Heterozygous Missense chr2:166859091 NM_001165963.4 c.4175A>G p.N139S | P (PS2, PM1, PM2, PP2, PP3) | 32 | F/16 | Seizures/DS | Novel | de novo | N/A | |
| SCN2A | Heterozygous Missense chr2:166223837 NM_001040142.2 c.3631G>A p.E1211K | P (PS2, PM2, PP2, PP3, PP5) | 33 | M/8 | Severe neuromotor delay, behavioral disorder, multifocal epileptiform anomaly on a disorganized background in EEG/WS | ClinVar 29886 | de novo | N/A |
| Heterozygous Missense chr2:166201183 NM_001040142.2 c.2681T>G p.I894S | P (PS2, PM1, PM2, PP2, PP3) | 34 | M/4 months | Seizures/EIDEE | Novel | de novo | N/A | |
| SCN8A | Heterozygous Missense chr12:52200516 NM_001330260.2 c.5246A>G p.Y1749C | LP (PM2, PP2, PP3) | 36 | M/16 | Psychomotor delay, ischemia sign in MRI/LIDEE | Novel | N/A | No |
| SLC35A2 | Heterozygous Missense chrX:48762497 NM_005660.3 c.773C>T p.S258F | LP (PS2, PM2) | 40 | M/6 | Generalized epileptiform anomaly in EEG/WS | Novel | de novo | No |
| STXBP1 | Heterozygous Nonsense chr9:130425598 NM_001032221.6 c.547delC p.Leu183Ter | LP (PVS1, PM2) | 44 | M/1 | Hearing loss, autism-like behavior, focal epileptiform anomaly in EEG/EIDEE | Novel | N/A | No |
| Heterozygous Splice acceptor chr9:130428443 NM_001032221.6 c.664-2A>G | P (PVS1, PM2, PP3) | 45 | F/1 | Multifocal generalized epileptiform anomaly in EEG/EIDEE | Novel | N/A | No | |
| Heterozygous Missense chr9:130444839 NM_001032221.6 c.1702G>A p.G568R | P (PS1, PS2, PM1, PM2, PP2, PP3) | 47 | F/16 | Focal epileptiform anomaly on a disorganized background in EEG/EIDEE | Novel | de novo | N/A | |
| WWOX | Homozygous Missense chr16:78458877 NM_016373.4 c.716T>G p.L239R | P (PM2, PM3, PP3, PP5) | 49 | M/3 months | Multifocal epileptiform anomaly in EEG/WS | ClinVar 871669 | Carrier parents | Yes |
| Homozygous Missense chr16:78458877 NM_016373.4 c.716T>G p.L239R | P (PM2, PM3, PP3, PP5) | 50 | F/11 months | Hypsarrhythmia and multifocal epileptiform anomaly on a disorganized background in EEG/EIDEE | ClinVar 871669 | Carrier parents | Yes |
| Genes | Variant | Reported ACMG Classification | Case ID | Sex/Age at the Study (Years/Month) | Clinical Features/Epileptic Syndrome | Novel/ Previously Reported Variant | Pattern of Inheritance (De Novo/Maternal/ Paternal) | Consanguinity |
|---|---|---|---|---|---|---|---|---|
| CACNA1A | Heterozygous Missense chr19:13409974 NM_001127222.2 c.2473G>A p.V825M | VUS (PM2, PP2) | 1 | M/5 months | Learning disability, generalized epileptiform anomaly on a disorganized background in EEG/WS | ClinVar 1485168 | Paternal | Yes |
| FGF12 | Heterozygous Splice region chr3:191888452 NM_004113.6 c.415-9_415-7delTTCinsCTT | VUS (PM2) | 5 | M/2 | Microcephaly, thin corpus callosum, and decreased white matter volume in MRI, focal epileptiform anomaly in EEG/WS | Novel | Paternal | No |
| GABRA1 | Heterozygous Missense chr5:161277883 NM_001127644.2 c.67G>A p.G23R | VUS (PM2, PP2) | 7 | M/2 | Seizures/DS | ClinVar 2923865 | Paternal | N/A |
| HNRNPU | Heterozygous Missense chr1:245021533 NM_031844.3 c.1274G>C p.G425A | VUS (PM2, PP3) | 13 | M/6 months | Microcephaly, lobar holoprosencephaly, dysgenesis of corpus callosum, and decreased white matter volume in MRI, generalized focal epileptiform anomaly in EEG/WS | ClinVar 696822 | Maternal | No |
| KCNB1 | Heterozygous Missense chr20:48099013 NM_004975.4 c.5C>A p.P2Q | VUS (PM2, PP2, BP6) | 14 | M/1 | Developmental delay, thin corpus callosum in MRI/WS | ClinVar 1918447 | N/A | No |
| MDH2 | Homozygous Missense chr7:75692842 NM_005918.4 c.565C>A p.P189A | VUS (PM2) | 19 | M/9 | Intellectual disability/EIDEE | SNP rs782733818 | Carrier parents | Yes |
| NECAP1 | Homozygous Initiator codon chr12:8234886 NM_015509.4 c.2T>A p.M1? | VUS (PM2) | 20 | M/3 | Thin corpus callosum in MRI/LIDEE | Novel | Carrier parents | Yes |
| SPTAN1 | Heterozygous Missense chr9:131343295 NM_001130438.3 c.1418A>T p.Y473F | VUS (PM2, PP2) | 42 | M/2 | Autism/DS | Novel | Maternal | N/A |
| Heterozygous Missense chr9:131341992 NM_001130438.3 c.1298A>G p.Y433C | VUS (PM2) | 43 | M/9 months | Seizures/WS | ClinVar 1716606 | Paternal | N/A |
| Genes | Variant | Reported ACMG Classification | Case ID | Sex/Age at the Study (Years) | Clinical Features/Epileptic Syndrome | OMIM Phenotype (MIM#) | Pattern of Inheritance (De Novo/Maternal/ Paternal) | Consanguinity |
|---|---|---|---|---|---|---|---|---|
| PIEZO2 | Heterozygous Missense chr18:10696484 NM_001378183.1 c.6542C>T p.P2181L | VUS (PM2, PP2) | 51 | F/1 | Microcephaly, respiratory distress, thin corpus callosum, enlarged lateral ventricles, polymicrogyria, dystonia, frequent spike, polyspike, and slow-wave discharges in the left central and right temporal regions, accompanying bicycling movements of both feet in EEG/EIDEE | Marden–Walker Syndrome (248700), AD | N/A | N/A |
| TSC1 | Heterozygous Missense chr9:135787758 NM_000368.5 c.824A>G p.Y275C | LP (PS2, PM2, PP3) | 52 | M/1 | Bilateral renal echogenicity, nephrolithiasis, hypsarrhythmia in EEG/WS | Tuberous sclerosis-1 (191100), AD | de novo | No |
| NALCN | Homozygous Splice donor chr13:101728223 NM_052867.4 c.3954+1G>A | LP (PVS1, PM2) | 53 | F/9 | Cerebral palsy, respiratory distress, feeding difficulties, moderate cerebral dysfunction accompanied by mild-to-moderate active multifocal epileptic abnormalities in EEG, scoliosis, laryngomalacia, atelectasis, secundum atrial septal defect, asymmetric septal hypertrophy/WS | Hypotonia, infantile, with psychomotor retardation and characteristic facies-1 (615419), AR | Carrier parents | No |
| CTBP1 | Heterozygous Missense chr4:1207317 NM_001012614.2 c.970T>G p.S324A | LP (PS2, PM2, PP2) | 59 | M/1 | Hypotonia, infantile spasms, feeding difficulties, tooth enamel disease, myoclonus, pectus excavatum, pachygyria, thin corpus callosum, hypoplastic inferior vermis/WS | Hypotonia, ataxia, developmental delay, and tooth enamel defect syndrome (617915), AD | de novo | Yes |
| Study | Number of Patients | Number of Genes | Yield (%) | Frequently Affected Genes |
|---|---|---|---|---|
| Ream et al. (2014) [11] | 25 | 40–53 | 46.2 | PCDH19, SCN1A, SLC2A1, SPTAN1, SLC9A6, EFHC |
| Møller et al. (2016) [12] | 216 | 46 | 23 | SCN1A, CDKL5, GABRA1, GABRB3, KCNQ2, SCN2A, SCN8A, SLC2A1, STXBP1 |
| Butler et al. (2017) [13] | 339 | 110 | 18 | TSC2, SCN1A, KCNQ2, CDKL5, SCN2A, SCN8A, SCN1B, STXBP1, TPP1, PCDH19, CACNA1A, GABRA1, GRIN2A, SLC2A1 |
| Rim et al. (2018) [14] | 74 | 172 | 37.8 | SXTBP1, CDKL5, KCNQ2, SCN1A, SYNGAP1, GNAO1, KCNT1 |
| Atli et al. (2020) [15] | 80 | 110 | 36.25 | TSC2, TSC1, KCNQ2, AMT, CACNA1H, KCNT1, SCN1A, GRIN2A, CNTNAP2, GLDC, MECP2, ASAH1 |
| Hoelz et al. (2020) [16] | 91 | 5–434 | 18 | SCN1A, TSC1, SCN8A, SYNGAP1, CPT2, KCNB1, PCDH19, KCNQ2, CHD2, CACNA1A, STXBP1 |
| Lee et al. (2020) [17] | 116 | 40 | 34.5 | SCN1A, PRRT2, ARX, SCN2A, KCNQ2, PCDH19, STXBP1, DEPDC5, SCN8A |
| Essajee et al. (2022) [3] | 41 | 308 | 43.9 | SCN1A, KANSL1, KCNQ2, CDKL5, IQSEC2, MC1A, STXBP1 |
| Ben Said et al. (2024) [10] | 40 | 116 | 30 | SCN1A, CHD2, CDKL5, SZT2, KCNT1, GNAO1, PCDH19, GRIN2A, MECP2, SYNGAP1 |
| Present study (2024) | 129 | 55 | 22.48 | SCN1A, KCNQ2, STXBP1, CDKL5, PCDH19, PLCB1, SCN2A, WWOX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunnetci-Akkoyunlu, D.; Kara, B.; Ozer, T.; Deniz, A.; Sakarya-Gunes, A.; Isik, E.B.; Dogruoglu, B.; Ilkay, Z.; Yilmaz, M.; Sahin, S.; et al. Genetic Etiology of Developmental and Epileptic Encephalopathy in a Turkish Cohort: A Single-Center Study with Targeted Gene Panel and Whole Exome Sequencing. Genes 2025, 16, 1152. https://doi.org/10.3390/genes16101152
Sunnetci-Akkoyunlu D, Kara B, Ozer T, Deniz A, Sakarya-Gunes A, Isik EB, Dogruoglu B, Ilkay Z, Yilmaz M, Sahin S, et al. Genetic Etiology of Developmental and Epileptic Encephalopathy in a Turkish Cohort: A Single-Center Study with Targeted Gene Panel and Whole Exome Sequencing. Genes. 2025; 16(10):1152. https://doi.org/10.3390/genes16101152
Chicago/Turabian StyleSunnetci-Akkoyunlu, Deniz, Bulent Kara, Tolgahan Ozer, Adnan Deniz, Ayfer Sakarya-Gunes, Elif Busra Isik, Buket Dogruoglu, Zeynep Ilkay, Mehtap Yilmaz, Sumeyye Sahin, and et al. 2025. "Genetic Etiology of Developmental and Epileptic Encephalopathy in a Turkish Cohort: A Single-Center Study with Targeted Gene Panel and Whole Exome Sequencing" Genes 16, no. 10: 1152. https://doi.org/10.3390/genes16101152
APA StyleSunnetci-Akkoyunlu, D., Kara, B., Ozer, T., Deniz, A., Sakarya-Gunes, A., Isik, E. B., Dogruoglu, B., Ilkay, Z., Yilmaz, M., Sahin, S., Eren-Keskin, S., Cine, N., & Savli, H. (2025). Genetic Etiology of Developmental and Epileptic Encephalopathy in a Turkish Cohort: A Single-Center Study with Targeted Gene Panel and Whole Exome Sequencing. Genes, 16(10), 1152. https://doi.org/10.3390/genes16101152







