Genome-Wide Analysis of the HECT-Type E3 Ubiquitin Ligase Gene Family in Nicotiana benthamiana: Evidence Implicating NbHECT6 and NbHECT13 in the Response to Tomato Yellow Leaf Curl Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the HECT Genes in N. benthamiana
2.2. Phylogenetic Tree Construction, Exon–Intron Structure, and Promoter Analyses
2.3. Chromosomal Localization and Collinearity Analyses
2.4. Tissue-Specific Expression Pattern Analysis
2.5. Plant Materials and TYLCV Inoculation
2.6. RNA Extraction and Quantitative PCR (qPCR) Analysis
2.7. VIGS Experiment and Silencing Efficiency Assay
2.8. Genomic DNA Extraction and Viral Accumulation Determination
2.9. Statistical Analysis
3. Results
3.1. Identification and Physicochemical Properties of the HECT Gene Family in N. benthamiana
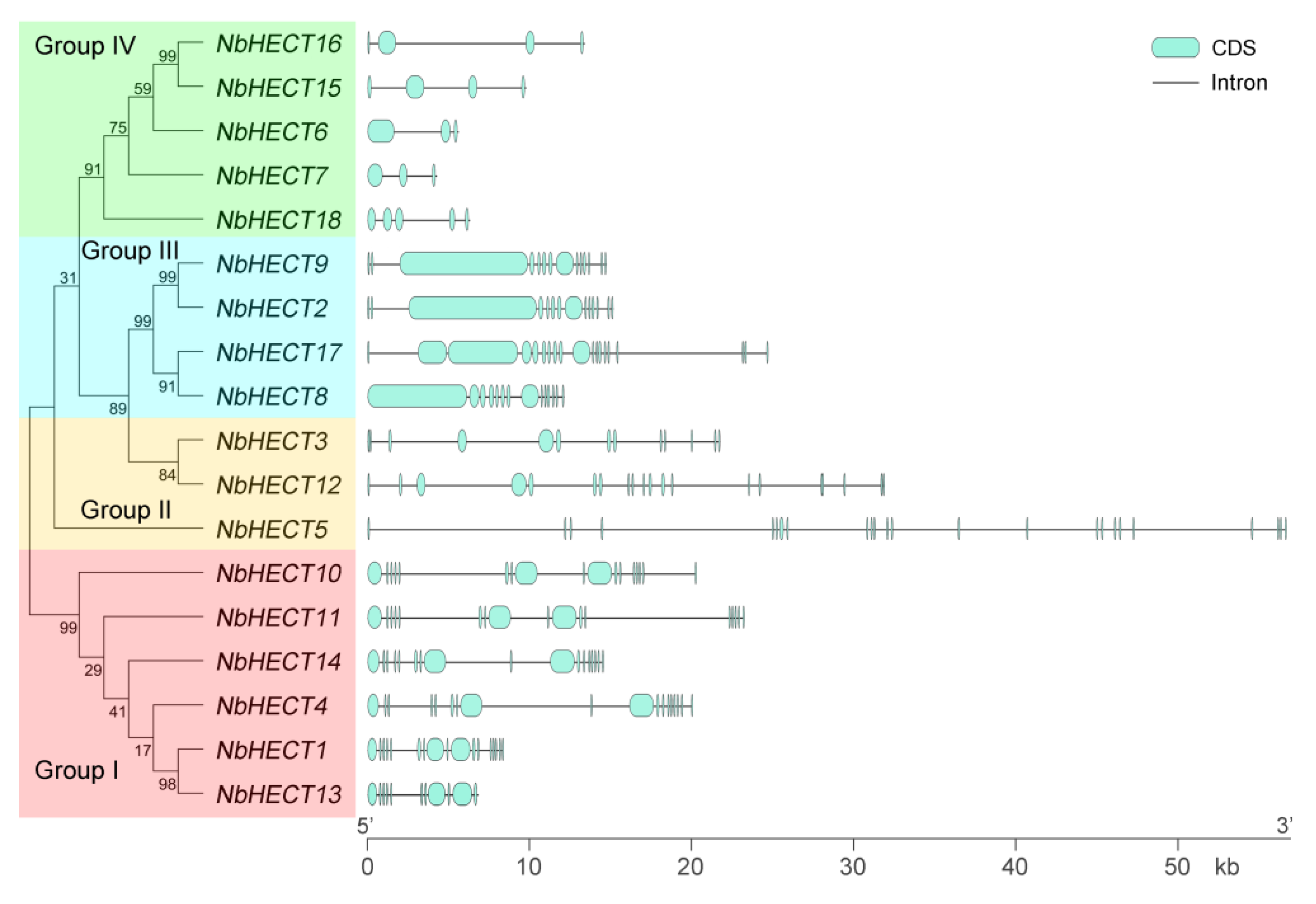
3.2. Phylogenetic Analysis of the NbHECT Gene Family
3.3. Gene Structure Analysis of the NbHECT Gene Family
3.4. Chromosomal Location and Synteny Analyses of the NbHECT Genes
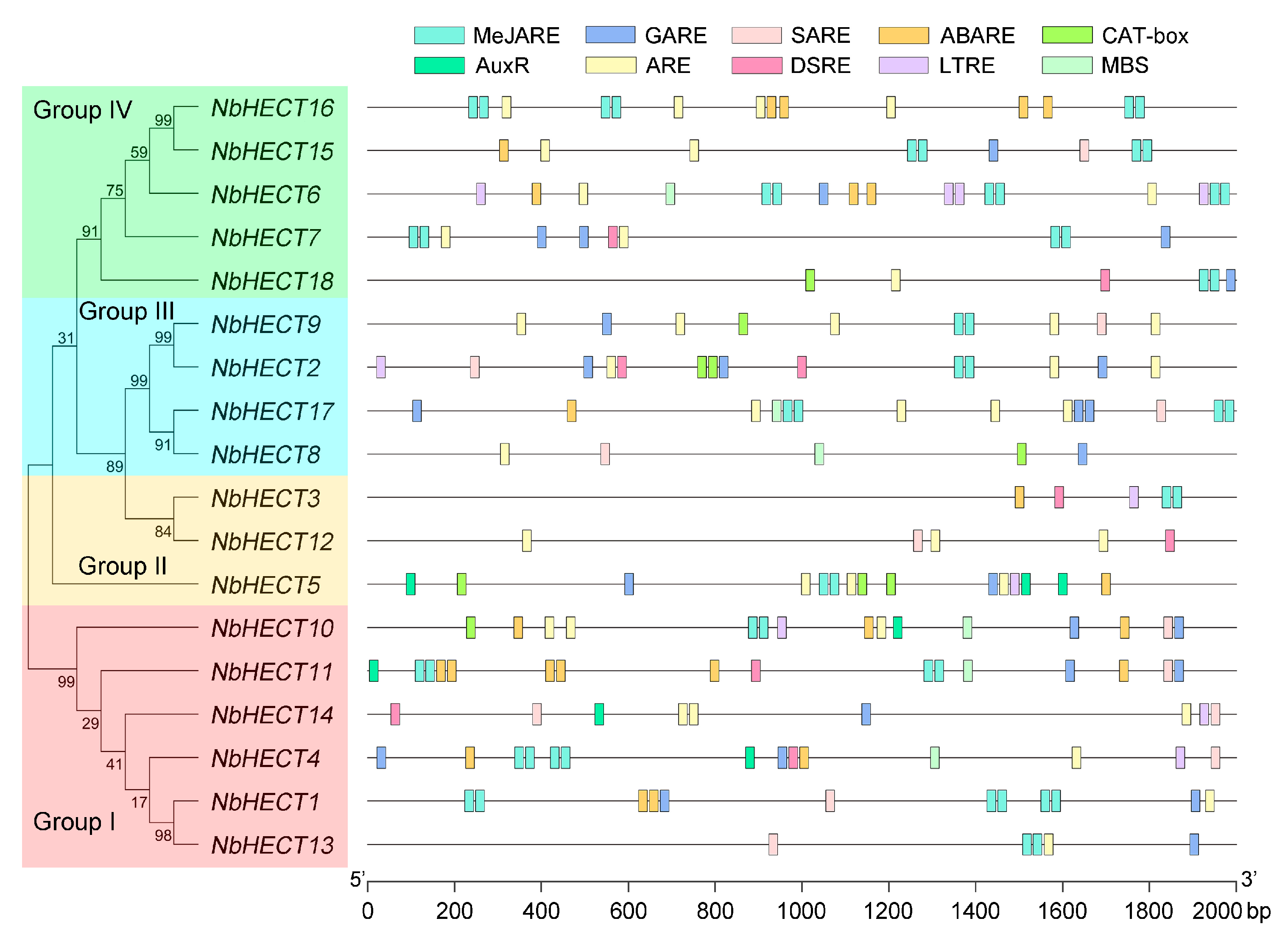
3.5. Promoter Analysis of the NbHECT Genes
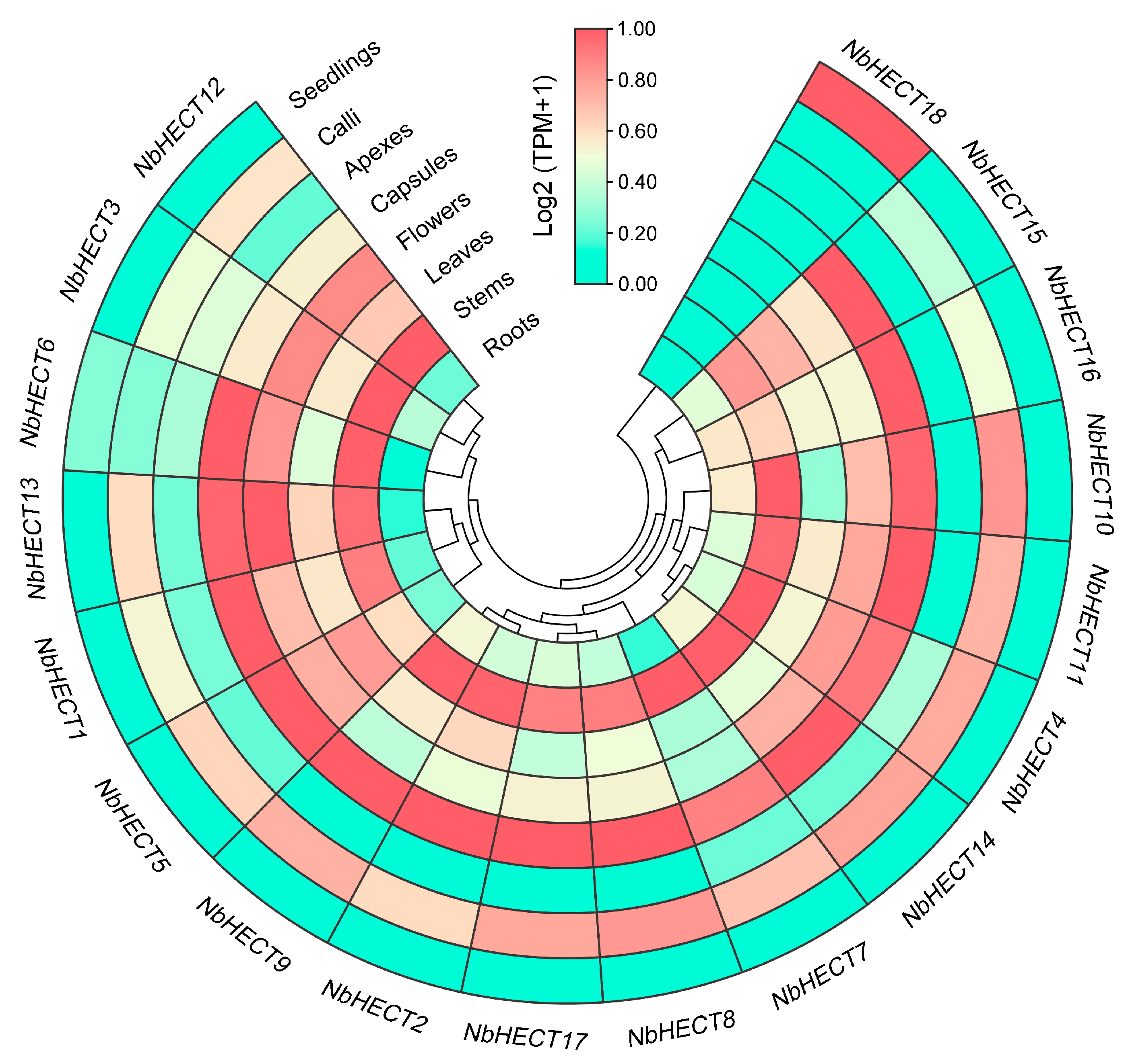
3.6. Tissue-Specific Expression Patterns of the NbHECT Genes
3.7. Expression Profiles of NbHECT Genes in Response to TYLCV Infection
3.8. Disruption of the Expression of NbHECTs Increases Host Susceptibility to TYLCV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niklas, K.J.; Kutschera, U. The evolution of the land plant life cycle. New Phytol. 2010, 185, 27–41. [Google Scholar] [CrossRef]
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering protein function: From classification to complexes. Essays Biochem. 2022, 66, 255–285. [Google Scholar] [CrossRef]
- Romero, P.A.; Arnold, F.H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 2009, 10, 866–876. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Bartel, B.; Citovsky, V. Focus on ubiquitin in plant biology. Plant Physiol. 2012, 160, 1. [Google Scholar] [CrossRef]
- Sharma, B.; Joshi, D.; Yadav, P.K.; Gupta, A.K.; Bhatt, T.K. Role of ubiquitin-mediated degradation system in plant biology. Front. Plant Sci. 2016, 7, 806. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Q.; Xue, H.W. The ubiquitin-proteasome system in plant responses to environments. Plant Cell Environ. 2019, 42, 2931–2944. [Google Scholar] [CrossRef]
- Lobaina, D.P.; Tarazi, R.; Castorino, T.; Vaslin, M.F.S. The ubiquitin-proteasome system (UPS) and viral infection in plants. Plants 2022, 11, 2476. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Shabek, N. Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Isono, E.; Li, J.; Pulido, P.; Siao, W.; Spoel, S.H.; Wang, Z.; Zhuang, X.; Trujillo, M. Protein degrons and degradation: Exploring substrate recognition and pathway selection in plants. Plant Cell 2024, 36, 3074–3098. [Google Scholar] [CrossRef]
- Mattern, M.; Sutherland, J.; Kadimisetty, K.; Barrio, R.; Rodriguez, M.S. Using ubiquitin binders to decipher the ubiquitin code. Trends Biochem. Sci. 2019, 44, 599–615. [Google Scholar] [CrossRef]
- Agrata, R.; Komander, D. Ubiquitin-A structural perspective. Mol. Cell 2025, 85, 323–346. [Google Scholar] [CrossRef]
- Chen, L.; Hellmann, H. Plant E3 ligases: Flexible enzymes in a sessile world. Mol. Plant 2013, 6, 1388–1404. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Saxena, H.; Negi, H. Genome-wide analysis of HECT E3 ubiquitin ligase gene family in Solanum lycopersicum. Sci. Rep. 2021, 11, 15891. [Google Scholar] [CrossRef]
- Buetow, L.; Huang, D.T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2016, 17, 626–642. [Google Scholar] [CrossRef]
- Toma-Fukai, S.; Shimizu, T. Structural diversity of ubiquitin E3 Ligase. Molecules 2021, 26, 6682. [Google Scholar] [CrossRef]
- Rotin, D.; Kumar, S. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2009, 10, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Maspero, E.; Mari, S.; Valentini, E.; Musacchio, A.; Fish, A.; Pasqualato, S.; Polo, S. Structure of the HECT:ubiquitin complex and its role in ubiquitin chain elongation. EMBO Rep. 2011, 12, 342–349. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Miao, Y. New aspects of HECT-E3 ligases in cell senescence and cell death of plants. Plants 2019, 8, 483. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Laterrot, H. Epidemiology and Control of Tomato Yellow Leaf Curl Virus. In Plant Virus Epidemiology; Plumb, R.T., Thresh, J.M., Eds.; Blackwell: Oxford, UK, 1983; pp. 315–321. [Google Scholar]
- Prasad, A.; Sharma, N.; Hari-Gowthem, G.; Muthamilarasan, M.; Prasad, M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant Sci. 2020, 25, 897–911. [Google Scholar] [CrossRef]
- Gong, P.; Tan, H.; Zhao, S.; Li, H.; Liu, H.; Ma, Y.; Zhang, X.; Rong, J.; Fu, X.; Lozano-Durán, R.; et al. Geminiviruses encode additional small proteins with specific subcellular localizations and virulence function. Nat. Commun. 2021, 12, 4278. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qiao, R.; Yang, X.; Gong, P.; Zhou, X. Occurrence, distribution, and management of tomato yellow leaf curl virus in China. Phytopathol. Res. 2022, 4, 28. [Google Scholar] [CrossRef]
- Cao, X.; Huang, M.; Wang, S.; Li, T.; Huang, Y. Tomato yellow leaf curl virus: Characteristics, influence, and regulation mechanism. Plant Physiol. Biochem. 2024, 213, 108812. [Google Scholar] [CrossRef]
- Zhong, X.; Li, J.; Yang, L.; Wu, X.; Xu, H.; Hu, T.; Wang, Y.; Wang, Y.; Wang, Z. Genome-wide identification and expression analysis of wall-associated kinase (WAK) and WAK-like kinase gene family in response to tomato yellow leaf curl virus infection in Nicotiana benthamiana. BMC Plant Biol. 2023, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Glick, E.; Zrachya, A.; Levy, Y.; Mett, A.; Gidoni, D.; Belausov, E.; Citovsky, V.; Gafni, Y. Interaction with host SGS3 is required for suppression of RNA silencing by tomato yellow leaf curl virus V2 protein. Proc. Natl. Acad. Sci. USA 2008, 105, 157–161. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, S.; Barton, E.; Fan, Y.; Ji, Y.; Wang, X.; Zhou, Y. Tomato yellow leaf curl virus V2 protein plays a critical role in the nuclear export of V1 protein and viral systemic infection. Front. Microbiol. 2020, 11, 1243. [Google Scholar] [CrossRef]
- Wang, L.; Tan, H.; Medina-Puche, L.; Wu, M.; Garnelo Gomez, B.; Gao, M.; Shi, C.; Jimenez-Gongora, T.; Fan, P.; Ding, X.; et al. Combinatorial interactions between viral proteins expand the potential functional landscape of the tomato yellow leaf curl virus proteome. PLoS Pathog. 2022, 18, e1010909. [Google Scholar] [CrossRef]
- Rosas-Diaz, T.; Cana-Quijada, P.; Wu, M.; Hui, D.; Fernandez-Barbero, G.; Macho, A.P.; Solano, R.; Castillo, A.G.; Wang, X.W.; Lozano-Duran, R.; et al. The transcriptional regulator JAZ8 interacts with the C2 protein from geminiviruses and limits the geminiviral infection in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 1826–1840. [Google Scholar] [CrossRef]
- Hanley-Bowdoin, L.; Bejarano, E.R.; Robertson, D.; Mansoor, S. Geminiviruses: Masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 2013, 11, 777–788. [Google Scholar] [CrossRef]
- Wu, M.; Wei, H.; Tan, H.; Pan, S.; Liu, Q.; Bejarano, E.R.; Lozano-Durán, R. Plant DNA polymerases α and δ mediate replication of geminiviruses. Nat. Commun. 2021, 12, 2780. [Google Scholar] [CrossRef]
- Ghosh, D.; Chakraborty, S. Selective REcruitmeNt of plant DNA polymerases by geminivirus. Trends Genet. 2022, 38, 211–213. [Google Scholar] [CrossRef]
- Gong, P.; Zhao, S.; Liu, H.; Chang, Z.; Li, F.; Zhou, X. Tomato yellow leaf curl virus V3 protein traffics along microfilaments to plasmodesmata to promote virus cell-to-cell movement. Sci. China Life Sci. 2022, 65, 1046–1049. [Google Scholar] [CrossRef]
- Corrales-Gutierrez, M.; Medina-Puche, L.; Yu, Y.; Wang, L.; Ding, X.; Luna, A.P.; Bejarano, E.R.; Castillo, A.G.; Lozano-Duran, R. The C4 protein from the geminivirus Tomato yellow leaf curl virus confers drought tolerance in Arabidopsis through an ABA-independent mechanism. Plant Biotechnol. J. 2020, 18, 1121–1123. [Google Scholar] [CrossRef]
- Padmanabhan, C.; Zheng, Y.; Shamimuzzaman, M.; Wilson, J.R.; Gilliard, A.; Fei, Z.; Ling, K.S. The tomato yellow leaf curl virus C4 protein alters the expression of plant developmental genes correlating to leaf upward cupping phenotype in tomato. PLoS ONE 2022, 17, e0257936. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, P.; Ren, Y.; Liu, H.; Li, H.; Li, F.; Zhou, X. The novel C5 protein from tomato yellow leaf curl virus is a virulence factor and suppressor of gene silencing. Stress Biol. 2022, 2, 19. [Google Scholar] [CrossRef]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The rise and rise of Nicotiana benthamiana: A Plant for all reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef]
- Downes, B.P.; Stupar, R.M.; Gingerich, D.J.; Vierstra, R.D. The HECT ubiquitin-protein ligase (UPL) family in Arabidopsis: UPL3 has a specific role in trichome development. Plant J. 2003, 35, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hua, K.; Xu, R.; Zeng, D.; Wang, R.; Dong, G.; Zhang, G.; Lu, X.; Fang, N.; Wang, D.; et al. The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell 2021, 33, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Qin, A.; Deng, W.; Chen, R.; Yu, H.; Wang, Y.; Song, J.; Zeng, L. Genome-wide identification and transcriptome profiling expression analysis of the U-box E3 ubiquitin ligase gene family related to abiotic stress in maize (Zea mays L.). BMC Genom. 2024, 25, 132. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, C.; Rahman, S.U.; Wang, Y.; Wang, A.; Tao, S. Genome-wide identification and evolution of HECT genes in soybean. Int. J. Mol. Sci. 2015, 16, 8517–8535. [Google Scholar] [CrossRef]
- Meng, X.; Yang, T.; Liu, J.; Zhao, M.; Wang, J. Genome-wide identification and evolution of HECT genes in wheat. PeerJ 2020, 8, e10457. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Bombarely, A.; Rosli, H.G.; Vrebalov, J.; Moffett, P.; Mueller, L.A.; Martin, G.B. A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol. Plant Microbe Interact. 2012, 25, 1523–1530. [Google Scholar] [CrossRef]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef] [PubMed]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 296–1297. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Wang, X.; Sun, Y.; Joseph, P.V.; Paterson, A.H. Detection of colinear blocks and synteny and evolutionary analyses based on utilization of MCScanX. Nat. Protoc. 2024, 19, 2206–2229. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, M.; Chen, S.; Sun, J.; Wang, J.; Meng, D.; Li, J.; Zhang, L.; Guo, L. The complete genome assembly of Nicotiana benthamiana reveals the genetic and epigenetic landscape of centromeres. Nat. Plants 2024, 10, 1928–1943. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Tung, J.; Zhang, X.; Liu, D.; Deng, Y.; Tian, Z.; Chen, H.; Wang, T.; Yin, W.; et al. High-quality assembled and annotated genomes of Nicotiana tabacum and Nicotiana benthamiana reveal chromosome evolution and changes in defense arsenals. Mol. Plant 2024, 17, 423–437. [Google Scholar] [CrossRef]
- Bally, J.; Nakasugi, K.; Jia, F.; Jung, H.; Ho, S.Y.; Wong, M.; Paul, C.M.; Naim, F.; Wood, C.C.; Crowhurst, R.N.; et al. The extremophile Nicotiana benthamiana has traded viral defence for early vigour. Nat. Plants 2015, 1, 15165. [Google Scholar] [CrossRef]
- Ranawaka, B.; An, J.; Lorenc, M.T.; Jung, H.; Sulli, M.; Aprea, G.; Roden, S.; Llaca, V.; Hayashi, S.; Asadyar, L.; et al. A multi-omic Nicotiana benthamiana resource for fundamental research and biotechnology. Nat. Plants 2023, 9, 1558–1571. [Google Scholar] [CrossRef]
- Nakasugi, K.; Crowhurst, R.N.; Bally, J.; Wood, C.C.; Hellens, R.P.; Waterhouse, P.M. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS ONE 2013, 8, e59534. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, H.; Zhou, X. Molecular characterization and pathogenicity of tomato yellow leaf curl virus in China. Virus Genes 2009, 39, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Souvan, J.M.; Bally, J.; de Felippes, F.F.; Waterhouse, P.M. Exploring the source of TYLCV resistance in Nicotiana benthamiana. Front. Plant Sci. 2024, 15, 1404160. [Google Scholar] [CrossRef]
- Wu, M.; Ding, X.; Fu, X.; Lozano-Duran, R. Transcriptional reprogramming caused by the geminivirus Tomato yellow leaf curl virus in local or systemic infections in Nicotiana benthamiana. BMC Genom. 2019, 20, 542. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Z.Q.; Liu, X.; Zhao, L.; Zhou, X.; Xie, Y. Identification and analysis of potential genes regulated by an alphasatellite (TYLCCNA) that contribute to host resistance against tomato yellow leaf curl China virus and its betasatellite (TYLCCNV/TYLCCNB) infection in Nicotiana benthamiana. Viruses 2019, 11, 442. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, L.; Li, J.; Tang, Z.; Wu, C.; Zhang, L.; Zhou, X.; Wang, Y.; Wang, Z. Integrated next-generation sequencing and comparative transcriptomic analysis of leaves provides novel insights into the ethylene pathway of Chrysanthemum morifolium in response to a Chinese isolate of chrysanthemum virus B. Virol. J. 2022, 19, 182. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, Z.Q.; Xiao, R.; Cao, L.; Wang, Y.; Xie, Y.; Zhou, X. Mimic phosphorylation of a βC1 protein encoded by TYLCCNB impairs its functions as a viral suppressor of RNA silencing and a symptom determinant. J. Virol. 2017, 91, e00300-17. [Google Scholar] [CrossRef] [PubMed]
- Dinesh-Kumar, S.P.; Anandalakshmi, R.; Marathe, R.; Schiff, M.; Liu, Y. Virus-induced gene silencing. Methods Mol. Biol. 2003, 236, 287–294. [Google Scholar] [PubMed]
- Zhong, X.; Wang, Z.Q.; Xiao, R.; Wang, Y.; Xie, Y.; Zhou, X. iTRAQ analysis of the tobacco leaf proteome reveals that RNA-directed DNA methylation (RdDM) has important roles in defense against geminivirus-betasatellite infection. J. Proteom. 2017, 152, 88–101. [Google Scholar] [CrossRef]
- Cheng, R.; Mei, R.; Yan, R.; Chen, H.; Miao, D.; Cai, L.; Fan, J.; Li, G.; Xu, R.; Lu, W.; et al. A new distinct geminivirus causes soybean stay-green disease. Mol. Plant 2022, 15, 927–930. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.; Shen, D.; Wang, J.; Ling, X.; Hu, Z.; Chen, T.; Hu, J.; Huang, J.; Yu, W.; et al. Tomato yellow leaf curl virus intergenic siRNAs target a host long noncoding RNA to modulate disease symptoms. PLoS Pathog. 2019, 15, e1007534. [Google Scholar] [CrossRef]
- Deanna, R.; Acosta, M.C.; Scaldaferro, M.; Chiarini, F. Chromosome Evolution in the Family Solanaceae. Front. Plant Sci. 2022, 12, 787590. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Yang, J.; Yao, S.; Zhao, K.; Wang, D.; Qin, Q.; Bian, Z.; Li, Y.; Lan, Y.; et al. Jasmonate signaling enhances RNA silencing and antiviral defense in rice. Cell Host Microbe 2020, 28, 89–103.e8. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Li, Y.; Wang, Z.; Wu, Z.; Zhang, Q.; Jia, H.; Dong, X.; Qi, L.; Shi, J.; et al. Gibberellin positively regulates tomato resistance to tomato yellow leaf curl virus (TYLCV). Plants 2024, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xing, S.; Cui, H.; Chen, X.; Wang, X. Genome-wide identification and characterization of the apple (Malus domestica) HECT ubiquitin-protein ligase family and expression analysis of their responsiveness to abiotic stresses. Mol. Genet. Genom. 2016, 291, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Cui, D.L.; Batool, K.; Yang, Y.Q.; Lu, Y.H. Comprehensive genomic survey, characterization and expression analysis of the hect gene family in Brassica rapa L. and Brassica oleracea L. Genes 2019, 10, 400. [Google Scholar] [PubMed]
- Xie, X.; Hu, S.; Liu, L.; Pan, H.; Huang, H.; Cao, X.; Qiao, G.; Han, X.; Qiu, W.; Lu, Z.; et al. Genome-wide analysis of HECT E3 ligases members in Phyllostachys edulis provides insights into the role of PeHECT1 in plant abiotic stress response. Int. J. Mol. Sci. 2024, 25, 11896. [Google Scholar] [CrossRef]
- Al-Saharin, R.; Hellmann, H.; Mooney, S. Plant E3 ligases and their role in abiotic stress response. Cells 2022, 11, 890. [Google Scholar] [CrossRef]
- Wang, S.; Lv, X.; Zhang, J.; Chen, D.; Chen, S.; Fan, G.; Ma, C.; Wang, Y. Roles of E3 ubiquitin ligases in plant responses to abiotic stresses. Int. J. Mol. Sci. 2022, 23, 2308. [Google Scholar] [CrossRef]
- Barajas, D.; Li, Z.; Nagy, P.D. The Nedd4-type Rsp5p ubiquitin ligase inhibits tombusvirus replication by regulating degradation of the p92 replication protein and decreasing the activity of the tombusvirus replicase. J. Virol. 2009, 83, 11751–11764. [Google Scholar] [CrossRef]
- OuYang, X.; Wang, L.; Luo, X.; Li, C.; An, X.; Yao, L.; Huang, W.; Zhang, Z.; Zhang, S.; Liu, Y.; et al. Pepper vein yellow virus P0 protein triggers NbHERC3, NbBax, and NbCRR mediated hypersensitive response. J. Basic Microbiol. 2024, 64, e2400023. [Google Scholar] [CrossRef]
- Lozano-Durán, R.; Rosas-Díaz, T.; Gusmaroli, G.; Luna, A.P.; Taconnat, L.; Deng, X.W.; Bejarano, E.R. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 2011, 23, 1014–1032. [Google Scholar] [CrossRef]
- Lozano-Duran, R.; Bejarano, E.R. Geminivirus C2 protein might be the key player for geminiviral co-option of SCF-mediated ubiquitination. Plant Signal. Behav. 2011, 6, 999–1001. [Google Scholar] [CrossRef]
- Shen, Q.; Hu, T.; Bao, M.; Cao, L.; Zhang, H.; Song, F.; Xie, Q.; Zhou, X. Tobacco RING E3 ligase NtRFP1 mediates ubiquitination and proteasomal degradation of a geminivirus-encoded βC1. Mol. Plant 2016, 9, 911–925. [Google Scholar] [CrossRef]
- Chen, I.H.; Chang, J.E.; Wu, C.Y.; Huang, Y.P.; Hsu, Y.H.; Tsai, C.H. An E3 ubiquitin ligase from Nicotiana benthamiana targets the replicase of Bamboo mosaic virus and restricts its replication. Mol. Plant Pathol. 2019, 20, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, L.; Huang, X.; Geng, Y.; Li, F.; Qin, Q.; Wang, R.; Ji, S.; Zhao, S.; Xie, Q.I.; et al. OsRFPH2-10, a ring-H2 finger E3 ubiquitin ligase, is involved in rice antiviral defense in the early stages of rice dwarf virus infection. Mol. Plant 2014, 7, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Qin, X.Y.; Guo, H.F.; Zheng, C.; Zhang, Z.Y.; Chen, Q.; Wang, X.B.; Han, C.G.; Wang, Y. The E3 ligase HRD1 enhances plant antiviral immunity by targeting viral movement proteins. Cell Rep. 2025, 44, 115449. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, J.; Sun, X.; Li, J.; Cao, X.; Yao, S.; Han, Y.; Chen, C.; Du, L.; Li, S.; et al. Perception of viral infections and initiation of antiviral defence in rice. Nature 2025, 641, 173–181. [Google Scholar] [CrossRef]
- De Silva, A.; Kim, K.; Weiland, J.; Hwang, J.; Chung, J.; Pereira, H.S.; Patel, T.R.; Teyra, J.; Patel, A.; Mira, M.M.; et al. Suppressing Tymovirus replication in plants using a variant of ubiquitin. PLoS Pathog. 2025, 21, e1012899. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Gene ID a | Gene Position | Strand | Size | |||||
|---|---|---|---|---|---|---|---|---|---|
| Genomic DNA (bp) | Number of Exons | CDS b (bp) | Protein (aa) | MW c (kDa) | pI d | ||||
| NbHECT1 | Niben261Chr01g0009001 | Niben261Chr01:939808–948152 | forward | 8345 | 17 | 4767 | 1588 | 176.63 | 5.52 |
| NbHECT2 | Niben261Chr01g0481001 | Niben261Chr01:48077477–48092623 | forward | 15,147 | 14 | 10,248 | 3415 | 373.11 | 5.03 |
| NbHECT3 | Niben261Chr01g1345011 | Niben261Chr01:134516798–134538509 | forward | 21,712 | 13 | 3354 | 1117 | 126.60 | 8.56 |
| NbHECT4 | Niben261Chr02g1146005 | Niben261Chr02:114614528–114634495 | forward | 19,968 | 18 | 5508 | 1835 | 198.99 | 5.84 |
| NbHECT5 | Niben261Chr04g0871004 | Niben261Chr04:87053772–87110416 | forward | 56,645 | 24 | 2649 | 882 | 100.56 | 5.51 |
| NbHECT6 | Niben261Chr04g1019003 | Niben261Chr04:101911370–101916954 | forward | 5585 | 3 | 2577 | 858 | 98.89 | 6.68 |
| NbHECT7 | Niben261Chr06g0028002 | Niben261Chr06:2825647–2829881 | reverse | 4235 | 3 | 1767 | 588 | 67.63 | 8.08 |
| NbHECT8 | Niben261Chr06g1000013 | Niben261Chr06:100017780–100029875 | forward | 12,096 | 14 | 10,179 | 3392 | 374.42 | 4.97 |
| NbHECT9 | Niben261Chr06g1026001 | Niben261Chr06:102542575–102557284 | forward | 14,710 | 14 | 10,248 | 3415 | 373.07 | 5.00 |
| NbHECT10 | Niben261Chr09g1363007 | Niben261Chr09:136341123–136361305 | forward | 20,183 | 17 | 5577 | 1858 | 199.45 | 5.69 |
| NbHECT11 | Niben261Chr10g0448001 | Niben261Chr10:44821858–44845061 | reverse | 23,204 | 17 | 5634 | 1877 | 201.67 | 5.62 |
| NbHECT12 | Niben261Chr12g0194001 | Niben261Chr12:19384125–19415980 | forward | 31,856 | 20 | 4194 | 1397 | 157.50 | 8.27 |
| NbHECT13 | Niben261Chr12g0254002 | Niben261Chr12:25391951–25398716 | reverse | 6766 | 11 | 4104 | 1367 | 151.86 | 5.54 |
| NbHECT14 | Niben261Chr13g0020011 | Niben261Chr13:2046409–2060925 | reverse | 14,517 | 17 | 5520 | 1839 | 198.30 | 5.77 |
| NbHECT15 | Niben261Chr14g0693017 | Niben261Chr14:69254130–69263876 | reverse | 9747 | 4 | 2286 | 761 | 86.53 | 5.94 |
| NbHECT16 | Niben261Chr15g0756024 | Niben261Chr15:75656777–75670163 | reverse | 13,387 | 4 | 2133 | 710 | 81.42 | 5.62 |
| NbHECT17 | Niben261Chr19g0507029 | Niben261Chr19:50718936–50743619 | reverse | 24,684 | 19 | 10,440 | 3479 | 383.67 | 5.05 |
| NbHECT18 | Niben261Chr19g0656003 | Niben261Chr19:65653994–65660273 | forward | 6280 | 5 | 2280 | 759 | 85.83 | 7.48 |
| Gene Pairs | Ka a | Ks b | Ka/Ks | Duplicate Type | Purifying Selection c | Duplication Date (MY d) | |
|---|---|---|---|---|---|---|---|
| NbHECT2 | NbHECT9 | 0.02 | 0.05 | 0.31 | Segmental | Yes | 1.75 |
| NbHECT3 | NbHECT12 | 0.03 | 0.08 | 0.38 | Segmental | Yes | 2.59 |
| NbHECT4 | NbHECT10 | 0.11 | 0.57 | 0.20 | Segmental | Yes | 18.91 |
| NbHECT4 | NbHECT11 | 0.11 | 0.55 | 0.20 | Segmental | Yes | 18.23 |
| NbHECT4 | NbHECT14 | 0.03 | 0.06 | 0.41 | Segmental | Yes | 2.16 |
| NbHECT7 | NbHECT15 | 0.20 | 0.63 | 0.32 | Segmental | Yes | 20.99 |
| NbHECT7 | NbHECT16 | 0.22 | 0.64 | 0.34 | Segmental | Yes | 21.38 |
| NbHECT10 | NbHECT11 | 0.01 | 0.07 | 0.20 | Segmental | Yes | 2.33 |
| NbHECT11 | NbHECT14 | 0.10 | 0.55 | 0.19 | Segmental | Yes | 18.45 |
| NbHECT15 | NbHECT16 | 0.01 | 0.09 | 0.11 | Segmental | Yes | 3.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Yu, S.; Ye, F.; Zhang, Y.; Wu, X.; Shi, M.; Zhao, G.; Shen, Y.; Lu, Z.; Yu, Z.; et al. Genome-Wide Analysis of the HECT-Type E3 Ubiquitin Ligase Gene Family in Nicotiana benthamiana: Evidence Implicating NbHECT6 and NbHECT13 in the Response to Tomato Yellow Leaf Curl Virus Infection. Genes 2025, 16, 1150. https://doi.org/10.3390/genes16101150
Shen J, Yu S, Ye F, Zhang Y, Wu X, Shi M, Zhao G, Shen Y, Lu Z, Yu Z, et al. Genome-Wide Analysis of the HECT-Type E3 Ubiquitin Ligase Gene Family in Nicotiana benthamiana: Evidence Implicating NbHECT6 and NbHECT13 in the Response to Tomato Yellow Leaf Curl Virus Infection. Genes. 2025; 16(10):1150. https://doi.org/10.3390/genes16101150
Chicago/Turabian StyleShen, Jin, Shasha Yu, Fang Ye, Yiming Zhang, Xue Wu, Mengxuan Shi, Gen Zhao, Yang Shen, Zhoufo Lu, Zaihang Yu, and et al. 2025. "Genome-Wide Analysis of the HECT-Type E3 Ubiquitin Ligase Gene Family in Nicotiana benthamiana: Evidence Implicating NbHECT6 and NbHECT13 in the Response to Tomato Yellow Leaf Curl Virus Infection" Genes 16, no. 10: 1150. https://doi.org/10.3390/genes16101150
APA StyleShen, J., Yu, S., Ye, F., Zhang, Y., Wu, X., Shi, M., Zhao, G., Shen, Y., Lu, Z., Yu, Z., Li, X., Zhong, X., & Wang, Z. (2025). Genome-Wide Analysis of the HECT-Type E3 Ubiquitin Ligase Gene Family in Nicotiana benthamiana: Evidence Implicating NbHECT6 and NbHECT13 in the Response to Tomato Yellow Leaf Curl Virus Infection. Genes, 16(10), 1150. https://doi.org/10.3390/genes16101150








