Transcriptional Regulation Analysis Provides Insight into the Function of GSK3β Gene in Diannan Small-Ear Pig Spermatogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Short-Read RNA-Seq and Long-Read Iso-Seq
2.2. Transcript Amplification and Sequence Determination
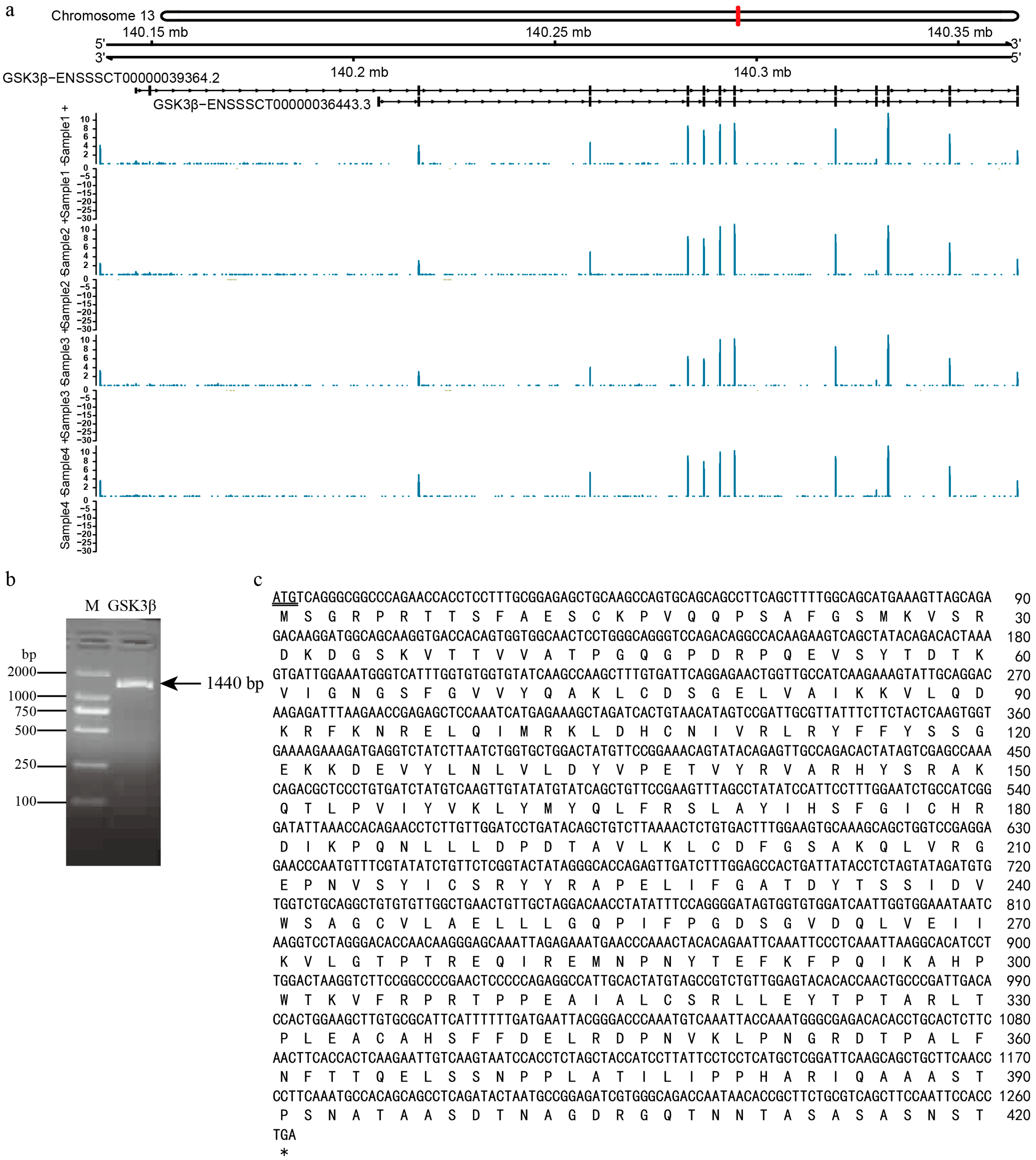
2.3. Characteristics Analysis of Transcript ENSSSCT00000039364
2.4. Protein–Protein Interaction Analysis of GSK3β
2.5. Regulatory Network Analysis of GSK3β
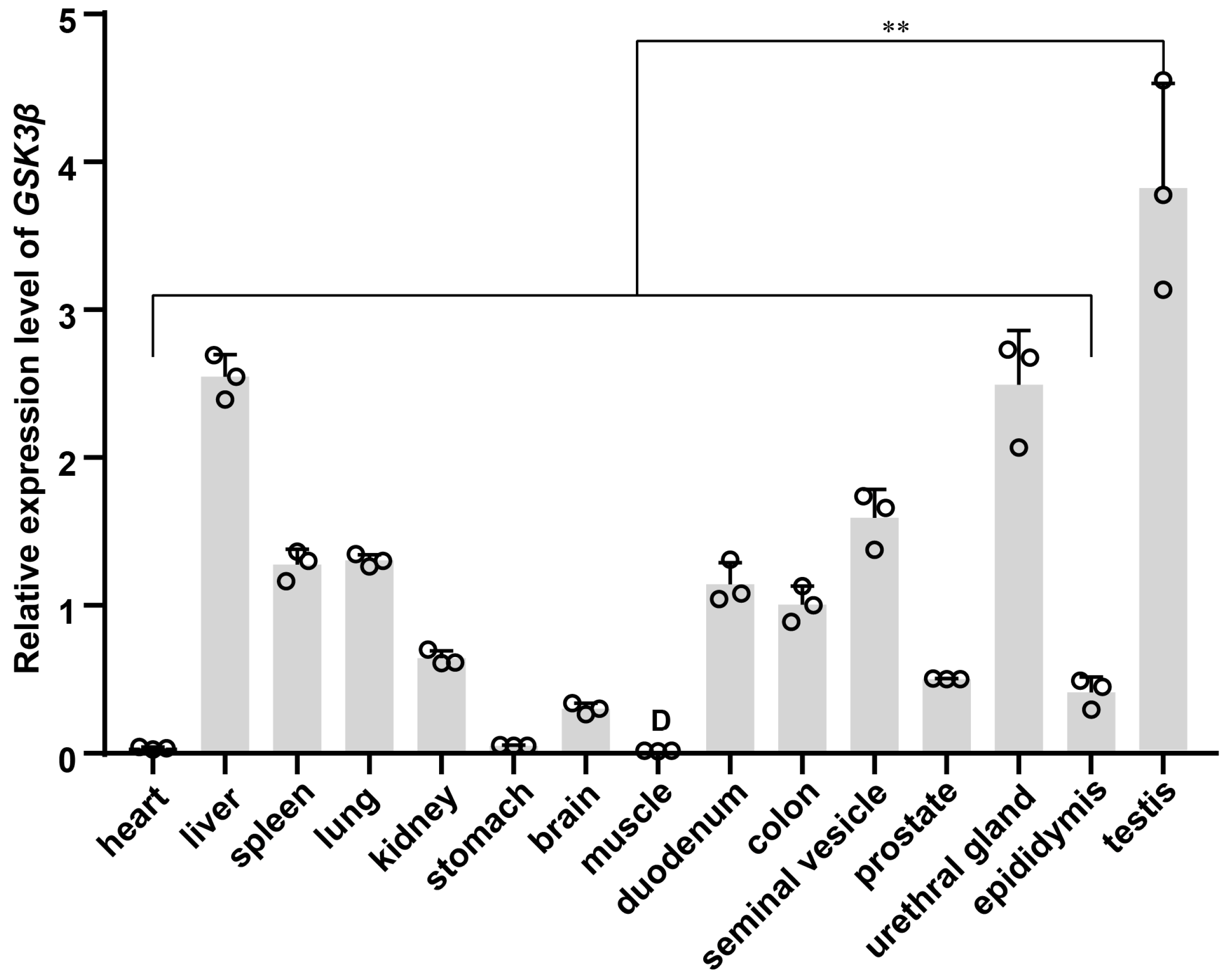
2.6. Multi-Tissue Expression Analysis of the GSK3β Gene
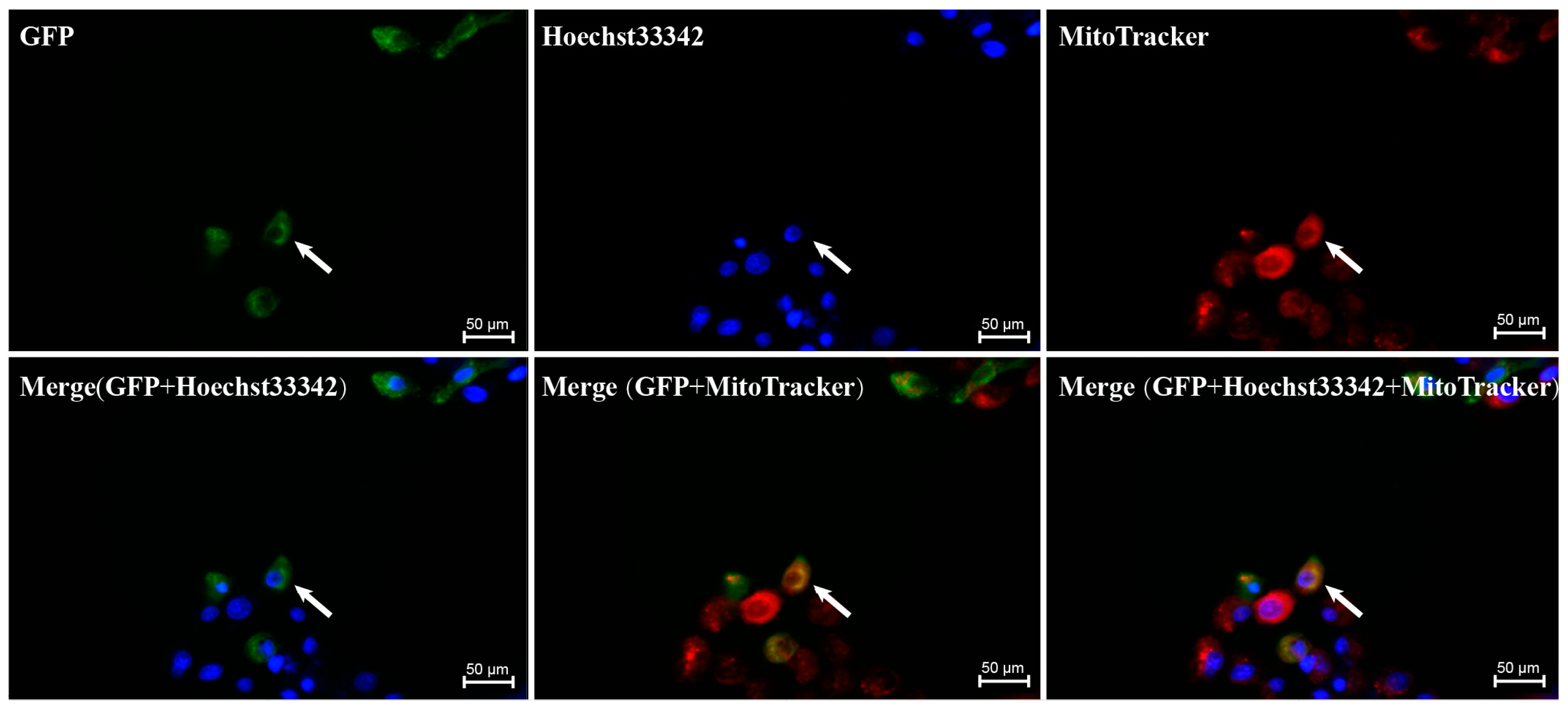
2.7. Subcellular Localization Detection of GSK3β
3. Results
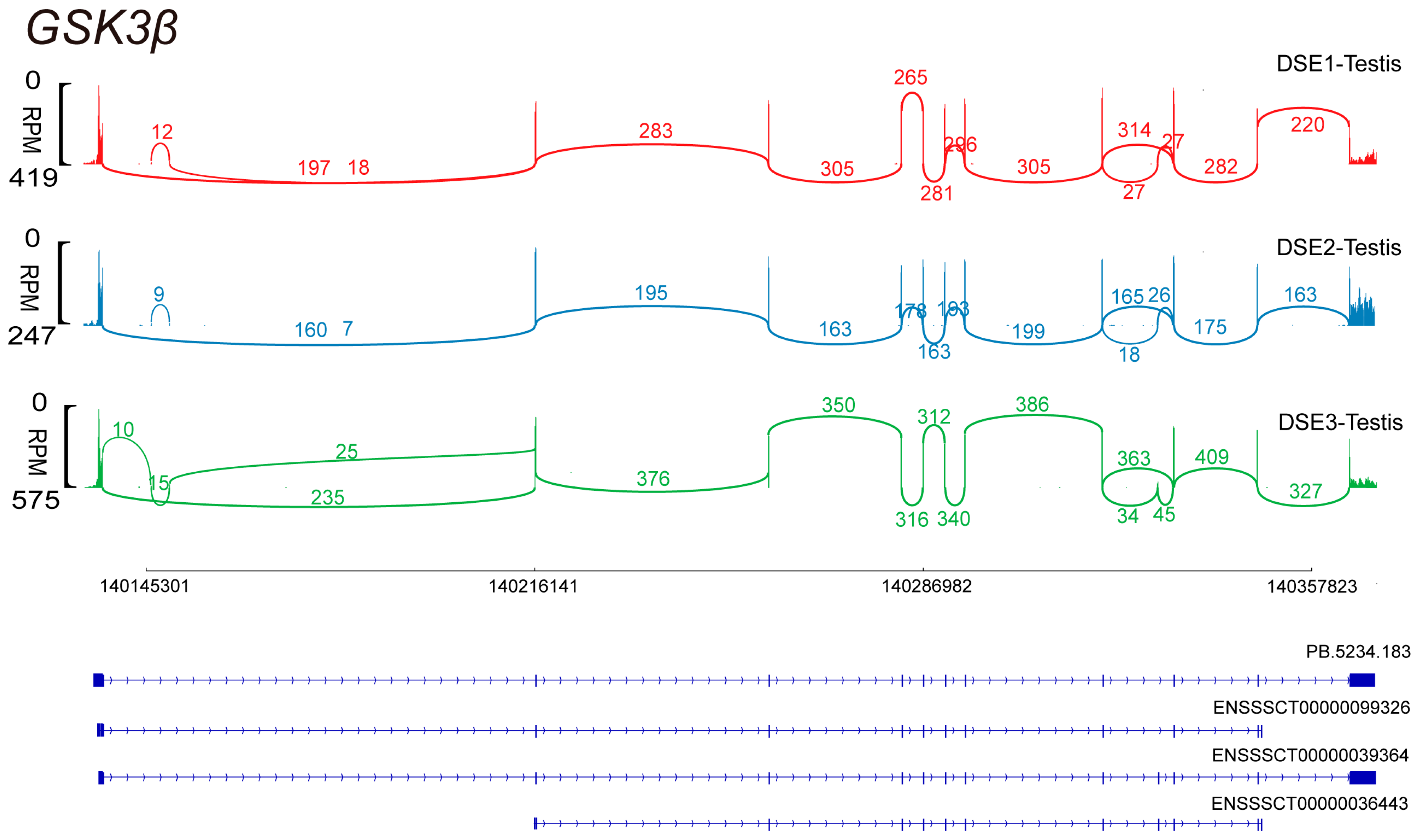
3.1. Alternative Splicing of GSK3β
3.2. GSK3β Gene Expression Characteristics
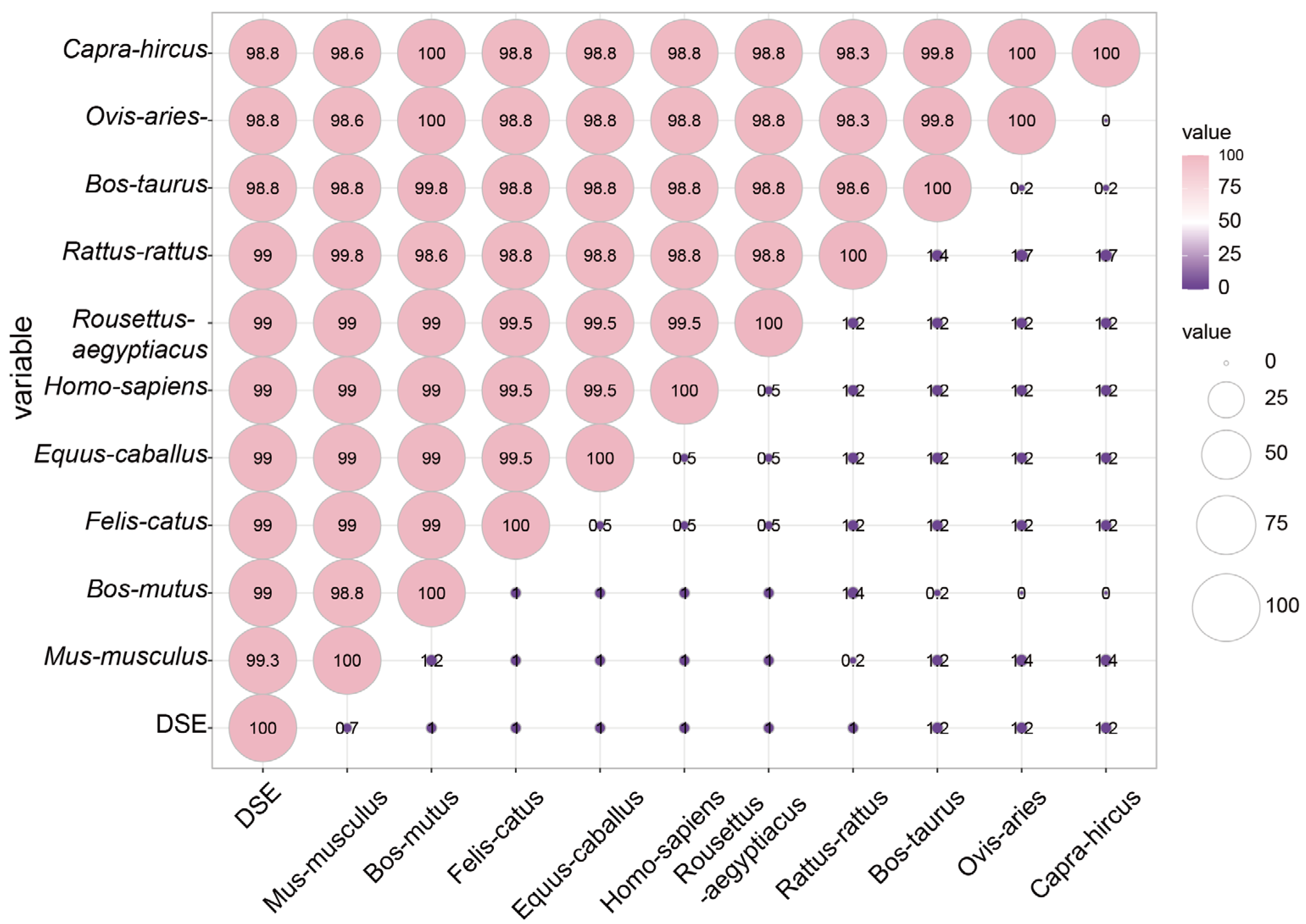
3.3. Homology Analysis of GSK3β Proteins across Species
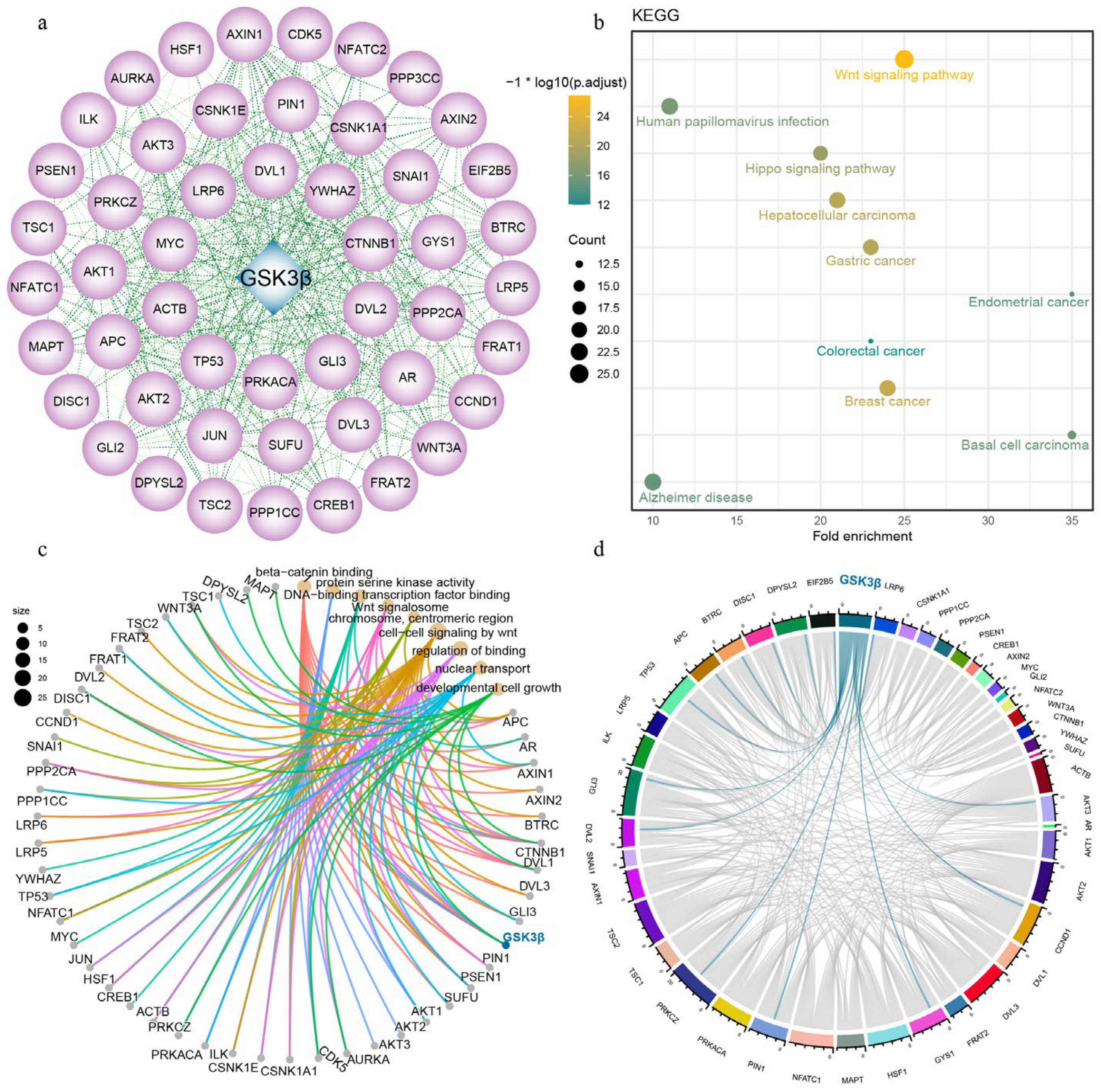
3.4. Protein–Protein Interaction
3.5. ceRNA Regulatory Network of GSK3β
3.6. Expression Pattern of GSK3β across Multi-Tissue
3.7. Subcellular Localization Results of GSK3β
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, S.L.; Ni, F.D.; Yang, W.X. The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene 2019, 706, 201–210. [Google Scholar] [CrossRef]
- Somanath, P.R.; Jack, S.L.; Vijayaraghavan, S. Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. J. Androl. 2004, 25, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Wang, L.; Zheng, Y.; Hoque, S.M.; Lv, Y.; Zeng, W. Glycogen Synthase Kinase-3 Regulates Sperm Motility and Acrosome Reaction via Affecting Energy Metabolism in Goats. Front. Physiol. 2019, 10, 968. [Google Scholar] [CrossRef]
- Dudiki, T.; Kadunganattil, S.; Ferrara, J.K.; Kline, D.W.; Vijayaraghavan, S. Changes in carboxy methylation and tyrosine phosphorylation of protein phosphatase PP2A are associated with epididymal sperm maturation and motility. PLoS ONE 2015, 10, e0141961. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Goswami, S.; Dudiki, T.; Popkie, A.P.; Phiel, C.J.; Kline, D.; Vijayaraghavan, S. Targeted disruption of glycogen synthase kinase 3A (GSK3A) in mice affects sperm motility resulting in male infertility. Biol. Reprod. 2015, 92, 65. [Google Scholar] [CrossRef]
- Bhat, R.V.; Budd, S.L. GSK3beta signalling: Casting a wide net in Alzheimer’s disease. Neurosignals 2002, 11, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.J.; Olanrewaju, A.J.; Arowosegbe, M.; Omotuyi, O.I. Molecular docking based screening analysis of GSK3B. Bioinformation 2019, 15, 201–208. [Google Scholar] [CrossRef]
- Serenó, L.; Coma, M.; Rodríguez, M.; Sánchez-Ferrer, P.; Sánchez, M.B.; Gich, I.; Agulló, J.M.; Pérez, M.; Avila, J.; Guardia-Laguarta, C.; et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 2009, 35, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Rockenstein, E.; Torrance, M.; Adame, A.; Mante, M.; Bar-On, P.; Rose, J.B.; Crews, L.; Masliah, E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 2007, 27, 1981–1991. [Google Scholar] [CrossRef]
- Magdesian, M.H.; Carvalho, M.M.; Mendes, F.A.; Saraiva, L.M.; Juliano, M.A.; Juliano, L.; Garcia-Abreu, J.; Ferreira, S.T. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J. Biol. Chem. 2008, 283, 9359–9368. [Google Scholar] [CrossRef]
- Vanden Dries, V.; Stygelbout, V.; Pierrot, N.; Yilmaz, Z.; Suain, V.; De Decker, R.; Buée, L.; Octave, J.N.; Brion, J.P.; Leroy, K. Amyloid precursor protein reduction enhances the formation of neurofibrillary tangles in a mutant tau transgenic mouse model. Neurobiol. Aging 2017, 55, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Jope, R.S.; Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- Wang, Q.M.; Park, I.K.; Fiol, C.J.; Roach, P.J.; DePaoli-Roach, A.A. Isoform differences in substrate recognition by glycogen synthase kinases 3 alpha and 3 beta in the phosphorylation of phosphatase inhibitor 2. Biochemistry 1994, 33, 143–147. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, K.; Woodgett, J.R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert Opin. Ther. Targets 2008, 12, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.; Dias, T.; Cavaco, J.; Oliveira, P.F. Testicular metabolic reprogramming in neonatal streptozotocin-induced type 2 diabetic rats impairs glycolytic flux and promotes glycogen synthesis. J. Diabetes Res. 2015, 2015, 973142. [Google Scholar] [CrossRef]
- Acebron, S.P.; Karaulanov, E.; Berger, B.S.; Huang, Y.L.; Niehrs, C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol. Cell. 2014, 54, 663–674. [Google Scholar] [CrossRef]
- Koch, S.; Acebron, S.P.; Herbst, J.; Hatiboglu, G.; Niehrs, C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell 2015, 163, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Suzuki, A.; Kamo, Y.; Uchida, C.; Tanemura, K.; Hara, K.; Uchida, T. Prolyl isomerase Pin1 is required sperm production by promoting mitosis progression of spermatogonial stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 388–393. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D.; Jiang, Z.; Luo, Y.; Norris, C.; Zhang, M.; Tian, X.; Tang, Y. Akt3 is responsible for the survival and proliferation of embryonic stem cells. Biol. Open 2017, 6, 850–861. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, M.; Guo, J.; Liu, Z.; Zhou, R.; Guo, F.; Li, K.; Mu, Y. The effects of flavonoid apigenin on male reproductive health: Inhibition of spermatogonial proliferation through downregulation of Prmt7/Akt3 pathway. Int. J. Mol. Sci. 2021, 22, 12209. [Google Scholar] [CrossRef]
- Dachtler, J.; Elliott, C.; Rodgers, R.J.; Baillie, G.S.; Clapcote, S.J. Missense mutation in DISC1 C-terminal coiled-coil has GSK3β signaling and sex-dependent behavioral effects in mice. Sci. Rep. 2016, 6, 18748. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhu, L.; Li, Z.; Zuo, P.; Wang, P.; Feng, J.; Mi, Y.; Zhang, C.; Xu, Y.; et al. ASPM promotes hepatocellular carcinoma progression by activating Wnt/β-catenin signaling through antagonizing autophagy-mediated Dvl2 degradation. FEBS Open Bio 2021, 11, 2784–2799. [Google Scholar] [CrossRef] [PubMed]
- Wilczynska, A.; Bushell, M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A.; Lowe, S.W. The microcosmos of cancer. Nature 2012, 482, 347–355. [Google Scholar] [CrossRef]
- Gorospe, M.; Abdelmohsen, K. MicroRegulators come of age in senescence. Trends Genet. 2011, 27, 233–241. [Google Scholar] [CrossRef]
- Feng, H.; Liu, T.; Yousuf, S.; Zhang, X.; Huang, W.; Li, A.; Xie, L.; Miao, X. Identification of potential miRNA-mRNA regulatory network and the key miRNAs in intramuscular and subcutaneous adipose. Front. Vet. Sci. 2022, 9, 976603. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.A.; Stunz, L.L.; Hostager, B.S. TRAF3 as a Multifaceted Regulator of B Lymphocyte Survival and Activation. Front. Immunol. 2018, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Liu, D.; Su, S.; Wang, L.; Zhao, Z.; Ma, Y.; Li, Q.; Jia, C.; Xu, J.; Zhou, Y.; et al. Comparison of splenocyte microRNA expression profiles of pigs during acute and chronic toxoplasmosis. BMC Genom. 2019, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Malcher, A.; Rozwadowska, N.; Stokowy, T.; Kolanowski, T.; Jedrzejczak, P.; Zietkowiak, W.; Kurpisz, M. Potential biomarkers of nonobstructive azoospermia identified in microarray gene expression analysis. Fertil. Steril. 2013, 100, 1686–1694. [Google Scholar] [CrossRef]
- Yang, H.M.; Liu, G.; Nie, Z.Y.; Nie, D.S.; Deng, Y.; Lu, G.X. Molecular cloning of a novel rat gene Tsarg1, a member of the DnaJ/HSP40 protein family. DNA Seq. 2005, 16, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Muyllaert, D.; Kremer, A.; Jaworski, T.; Borghgraef, P.; Devijver, H.; Croes, S.; Dewachter, I.; Van Leuven, F. Glycogen synthase kinase-3beta, or a link between amyloid and tau pathology? Genes Brain Behav. 2008, 7, 57–66. [Google Scholar] [CrossRef]
- Gómez de Barreda, E.; Pérez, M.; Gómez Ramos, P.; de Cristobal, J.; Martín-Maestro, P.; Morán, A.; Dawson, H.N.; Vitek, M.P.; Lucas, J.J.; Hernández, F.; et al. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol. Dis. 2010, 37, 622–629. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhao, G.; Yang, F.; Li, C.; Lin, W.; Dai, H.; Zhai, L.; Xi, X.; Yuan, Q.; Huo, J. Transcriptional Regulation Analysis Provides Insight into the Function of GSK3β Gene in Diannan Small-Ear Pig Spermatogenesis. Genes 2024, 15, 655. https://doi.org/10.3390/genes15060655
Zhang X, Zhao G, Yang F, Li C, Lin W, Dai H, Zhai L, Xi X, Yuan Q, Huo J. Transcriptional Regulation Analysis Provides Insight into the Function of GSK3β Gene in Diannan Small-Ear Pig Spermatogenesis. Genes. 2024; 15(6):655. https://doi.org/10.3390/genes15060655
Chicago/Turabian StyleZhang, Xia, Guiying Zhao, Fuhua Yang, Changyao Li, Wan Lin, Hongmei Dai, Lan Zhai, Xuemin Xi, Qingting Yuan, and Jinlong Huo. 2024. "Transcriptional Regulation Analysis Provides Insight into the Function of GSK3β Gene in Diannan Small-Ear Pig Spermatogenesis" Genes 15, no. 6: 655. https://doi.org/10.3390/genes15060655
APA StyleZhang, X., Zhao, G., Yang, F., Li, C., Lin, W., Dai, H., Zhai, L., Xi, X., Yuan, Q., & Huo, J. (2024). Transcriptional Regulation Analysis Provides Insight into the Function of GSK3β Gene in Diannan Small-Ear Pig Spermatogenesis. Genes, 15(6), 655. https://doi.org/10.3390/genes15060655





