Abstract
MicroRNAs (miRNAs) are small non-coding conserved molecules with lengths varying between 18-25nt. Plants miRNAs are very stable, and probably they might have been transferred across kingdoms via food intake. Such miRNAs are also called exogenous miRNAs, which regulate the gene expression in host organisms. The miRNAs present in the cluster bean, a drought tolerant legume crop having high commercial value, might have also played a regulatory role for the genes involved in nutrients synthesis or disease pathways in animals including humans due to dietary intake of plant parts of cluster beans. However, the predictive role of miRNAs of cluster beans for gene–disease association across kingdoms such as cattle and humans are not yet fully explored. Thus, the aim of the present study is to (i) find out the cluster bean miRNAs (cb-miRs) functionally similar to miRNAs of cattle and humans and predict their target genes’ involvement in the occurrence of complex diseases, and (ii) identify the role of cb-miRs that are functionally non-similar to the miRNAs of cattle and humans and predict their targeted genes’ association with complex diseases in host systems. Here, we predicted a total of 33 and 15 functionally similar cb-miRs (fs-cb-miRs) to human and cattle miRNAs, respectively. Further, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed the participation of targeted genes of fs-cb-miRs in 24 and 12 different pathways in humans and cattle, respectively. Few targeted genes in humans like LCP2, GABRA6, and MYH14 were predicted to be associated with disease pathways of Yesinia infection (hsa05135), neuroactive ligand-receptor interaction (hsa04080), and pathogenic Escherichia coli infection (hsa05130), respectively. However, targeted genes of fs-cb-miRs in humans like KLHL20, TNS1, and PAPD4 are associated with Alzheimer’s, malignant tumor of the breast, and hepatitis C virus infection disease, respectively. Similarly, in cattle, targeted genes like ATG2B and DHRS11 of fs-cb-miRs participate in the pathways of Huntington disease and steroid biosynthesis, respectively. Additionally, the targeted genes like SURF4 and EDME2 of fs-cb-miRs are associated with mastitis and bovine osteoporosis, respectively. We also found a few cb-miRs that do not have functional similarity with human and cattle miRNAs but are found to target the genes in the host organisms and as well being associated with human and cattle diseases. Interestingly, a few genes such as NRM, PTPRE and SUZ12 were observed to be associated with Rheumatoid Arthritis, Asthma and Endometrial Stromal Sarcoma diseases, respectively, in humans and genes like SCNN1B associated with renal disease in cattle.
1. Introduction
In the ecosystem, quite often organisms interact with each other directly or indirectly. The prokaryotic cells communicate through quorum sensing whereas eukaryotic cells transmit signals through hormones and cytokines [1]. In living organisms, quite often microRNAs (miRNAs) regulate multiple cell activities such as stress tolerance, plant defense mechanisms, growth and development [2]. In recent years, it has been reported that miRNAs might have been transmitted from one species to another [3] and might have targeted the genes not only at the endogenous level but also at the exogenous level [4,5]. Moreover, the cross-kingdom mobility of miRNAs has been studied between bacteria and animals [6], plants and insects [7], and plants and fungi [8]. Also, numerous exogenous plant miRNAs exhibit perfect complementarity to human genes as well as to the genes of other mammals [9]. The experimental detection of plant miRNAs in human plasma, serum, urine, saliva and other body fluids have been reported in the recent past [10,11]. The RT-PCR technique reveals the stability of plant miRNAs in the human sera [12,13] and such stability is achieved by the methylation at 2′-hydroxyl group of sugar at 3′ end of miRNA [14]. The presence of plant miRNAs in humans has been reported when plant products in the form of diet travelled through the gastrointestinal tract [15]. It was reported earlier that plant miRNA “MIR168a” targets the low-density lipoprotein receptor (LDL) adaptor protein 1 (LDLRAP1) of humans’ liver cells that led to the uptake of low-density lipoprotein receptor from blood [3]. Additionally, it was showed that human miRNAs might have translocated and regulated the genes responsible for growth of Plasmodium falciparum [16]. The studies on virus–host interaction showed that viruses used host miRNA biosynthesis machinery for the expression of their own miRNAs [17]. In addition, the oilve miRNAs, viz., oeu-sR20, oeu-sR27 and oeu-sR34 showed functional homology with human miRNA “hsa-miR34” that regulates the expression of genes in human tumor cells [15]. Recently, it was found that miRNAs of Avacado (Persea americana) regulate the function of human genes like FLT1 (Fms Related Tyrosine Kinase 1) and SOCS3 (Suppressor of Cytokine Signalling 3) [10]. The miR160 and miR2673 of Brassica oleracea reported to regulate the expression of human lung-cancer-related genes [18].
The cluster bean, also known as guar, is a drought-tolerant crop of the legume family. It is grown in India for vegetable, green manure and seed production. It is also recognized as a medicinal plant and possesses a high quantity of phytochemicals [19,20]. It is commonly used to cure various diseases like ulcer, secretion, hyperglycemia, and cathartic [21]. The edible parts of this crop are consumed by humans and cattle as food and fodder. Through such intake, probably the cluster bean plant-derived miRNAs might have been translocated to humans and cattle. Subsequently, these miRNAs might have played different roles in the regulation of gene–disease association in animals. Thus, in the present study, we tried to identify the cluster bean miRNAs (cb-miRs) that are functionally similar and functionally non-similar to miRNAs of humans and cattle. Further, we predicted the target genes of the identified functionally similar cb-miRs (fs-cb-miRs) and functionally non-similar cb-miRs (fns-cb-miRs), pathways involving the predicted genes, and the gene–disease associations. Thus, the present findings may supplement the existing knowledge on the role of cb-miRs in regulating genes associated with human and cattle diseases. Additionally, the findings may help promote the use of cluster bean plant parts as dietary supplement for humans and as fodder for animals. The findings may act as a remedial outbreak against various diseases as well as in advising therapeutic strategies for animal diseases.
2. Material and Methods
2.1. Data Source
A total of 171 and 21 cluster bean miRNAs were collected from [22] and [23], respectively, after removing the redundancy. The prefixes Ct and Cte of cluster bean miRNAs, used earlier [22,23], were retained as such in their ids. For functional-similarity study of cb-miRs, a total of 1052 miRNAs of cattle and 2781 miRNAs of human were collected from miRbase [24]. The mRNA sequences of cattle were downloaded from the National Center for Biotechnology Information (NCBI), whereas human mRNAs available in the database of psRNATarget server [25] were considered in the study. The miRNAs and mRNAs of cattle and humans were used to carry out the cross-kingdom analysis.
2.2. Cross-Kingdom miRNA Similarity
The cross-kingdom mechanisms involve cell communication as well as intra-species and inter-species interactions through miRNAs. Here, a total of 192 cb-miRs (=171 + 21) were considered to perform cross-kingdom analysis in human and cattle. The MirCompare tool [26] was used to predict the similarity between cb-miRs and human miRNAs, cattle miRNAs with suitable parameters (r-value 0.55, similarity ≥ 60% and seed-region threshold value 5). The cb-miRs with the said remarkable and high similarity were considered as s-cb-miRs. Further, the secondary structures of pre-miRNAs of cluster beans, humans and cattle were predicted by RNA module of Vienna Package to filter out those s-miRNAs satisfying the structural properties from the miRNAs identified by MirCompare, i.e., those s-miRNAs (mature miRNAs of length 21 nt) localized on the secondary structure of pre-miRNAs. While s-cb-miRs that have sequence homology < 60% with miRNAs of cattle and human were considered as non-similar cb-miRs (ns-cb-miRs). Thus, the total miRNAs of cluster bean were bifurcated into s-cb-miRs and ns-cb-miRs. The s-cb-miRs may have functional and likely evolutionary importance while the ns-cb-miRs may have functional importance alone. Therefore, both types of cb-miRs were studied for the regulation of genes and their associated diseases in humans and cattle. The workflow meant for cross-kingdom analysis of miRNAs is given in Figure 1.
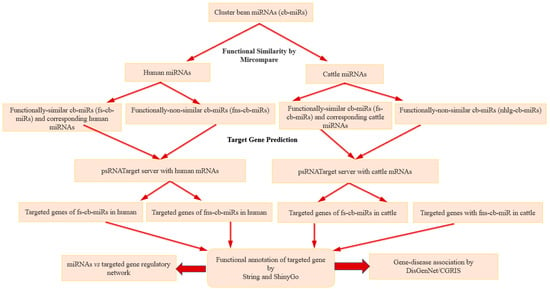
Figure 1.
Workflow of cross-kingdom gene regulation through miRNAs of cluster beans in humans and cattle.
2.3. Identification of Functionally Similar cbmiRs (fs-cb-miRs) and Prediction of Potential Target Genes in Humans and Cattle
The s-cb-miRs and their corresponding paired miRNAs in humans and cattle were considered for target gene prediction and, thus, submitted in the psRNATarget server [25]. Subsequently, only those genes that are commonly targeted by the referred pairs (s-cb-miRs and corresponding paired miRNA in the host system) are considered and referred as “targeted genes” for further down-stream analysis on annotation, pathways and gene–disease association whereas the s-cb-miRs in the referred pairs are referred as functionally similar cb-miRs (fs-cb-miRs), as they along with their paired miRNAs target the same gene in the host system (human, cattle), i.e., functionally similar while targeting the host gene. Additionally, the ns-cb-miRs targeting genes in host systems are referred to as functionally non-similar cb-miRs (fns-cb-miRs). To identify the probable targeted genes, the parameters (i) maximum expectation value = 3, (ii) length of complementary score = 20, (iii) maximum energy to unpair target site = 25, and (iv) translation inhibition = 9 nt–11 nt range were set in the psRNATarget Server [23,27,28] (Figure 1). As the mRNAs of cattle are not available in the psRNATarget server, the mRNAs collected from NCBI were uploaded in said server for identifying the genes targeted by s-cb-miRs, following the approach outlined above for humans. However, the targeted genes of fns-cb-miRs were identified from the psRNATarget server by submitting the fns-cb-miRs alone as input for humans and cattle separately.
2.4. Functional Annotation and Pathway Analysis of cb-miRs’ Targeted Genes
The human and cattle targeted genes of both fs-cb-miRs (33) and fns-cb-miRs (159) were submitted to ShinyGO v.0.77 [29] and String v.11.5 [30] for functional annotation and KEGG pathway [31] analysis, respectively. The gene ontology (GO) analysis was performed through AgriGO v2.0 tool [32]. Subsequently, the WEGO tool [33] was used for the representation of GO terms in different classes: biological process, cellular component and molecular function. Further, the significant GO terms were filtered out on the basis of p-value (<0.05) and FDR (<0.05). The pathway analysis of human and cattle genes targeted by cb-miRs was carried by KEGG mapper [34]. Finally, the gene regulatory network analyses involving the identified cb-miRs and their target genes of humans and cattle were performed by Cytoscape v3.3.0 [35].
2.5. Disease Association with cb-miRs’ Target Genes
The targeted genes that were significantly annotated in human were mapped against the DisGeNET database [36] for the analysis of their disease association. The evidence levels of gene–disease association, developed by the NIH-funded Clinical Genome Resource (ClinGen), were qualitatively classified into (i) definitive, (ii) strong, (iii) moderate, (iv) limited, (v) conflicting evidence and (vi) no reported evidence categories [37]. It was reported earlier that the predicted cross-kingdom targeted genes of human have plausible association with several diseases [38]. In case of cattle, the identified targeted genes were searched in literature and CGRIS (http://bioinformatics.iasri.res.in/cgris; accessed on 29 June 2023) for gene–disease association.
3. Results
3.1. Identification of fs-cb-miRs and fns-cb-miRs to Human and Cattle miRNAs
With input of 192 cb-miRs and 2042 human miRNAs, the similarity hits were found from the MirCompare tool. Similarly, while providing 192 cb-miRs and 206 cattle miRNAs as input to the MirCompare tool. The functional similarities between cluster bean miRNAs and human/cattle miRNAs are given in Figure 2 under different ranges of % similarity. Subsequently, cut off values of parameters r < 0.55, similarity ≥ 60% and seed region = 5 were kept in the MirCompare tool. This has resulted in 33 as fs-cb-miRs and 159 as fns-cb-miRs with human miRNAs, while 15 unique cb-miRs resulted as fs-cb-miRs and 177 as fns-cb-miRs with cattle miRNAs (Figure 3). The predicted secondary structures of both fs-cb-miRs and their corresponding human miRNAs were checked for the location of mature miRNAs. As an example, the secondary structures of Ct-mir-3130 and hsa-mir-1910 are given in Figure 4. The human miRNAs: hsa-miR-6754-3p and hsa-miR-6804-5p were found to have similarity with the cb-miRs: Cte-miR824-3p and Cte-miR6183, respectively. The percentage similarity in the former case was 73%, whereas in the latter case it was 71%. The cattle miRNAs bta-miR-7865 and bta-miR-2338 were found to have similarity with the cb-miRs Ct-miR-3061 and Ct-miR-3033 of cluster beans, respectively. The observed similarities in the former and latter cases were 74% and 69%, respectively.
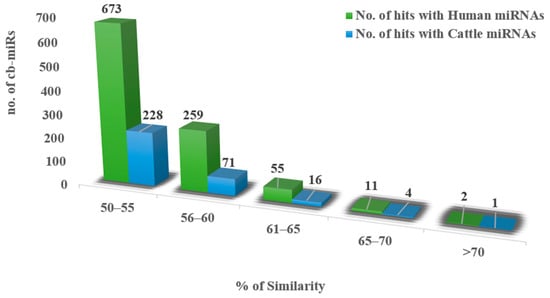
Figure 2.
X-axis represents different ranges of % similarities whereas the y-axis represents the number of cb-miRs having similarities with human miRNAs (green color) and cattle miRNAs (blue color).
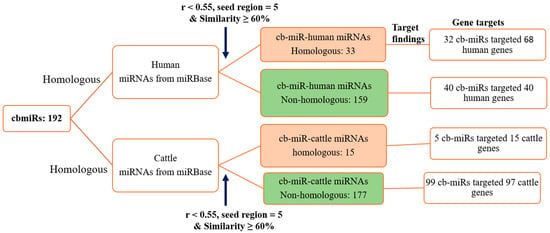
Figure 3.
Functional similarity of cluster bean miRNAs (cb-miRs) with human and cattle miRNAs. The observed functionally similar (fs) and functionally non-similar (fns) cb-miRs targeting genes in humans and cattle are indicated in orange color and green color boxes, respectively.
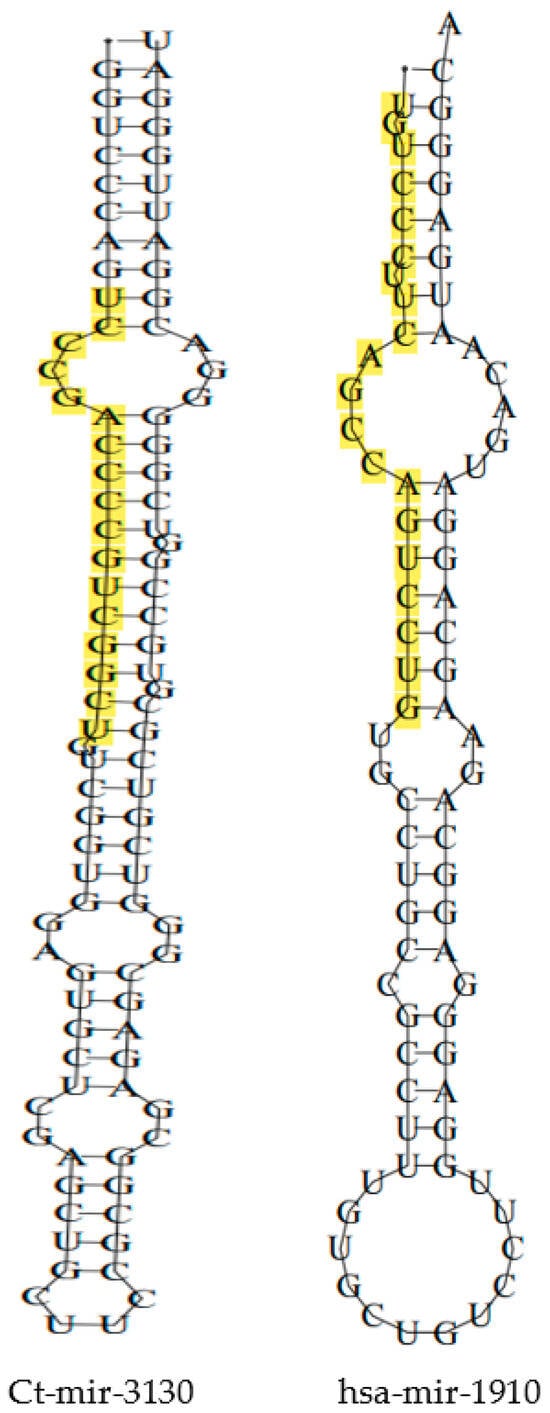
Figure 4.
Two-dimensional structures of fs-cb-miR and its corresponding human miRNA with the location of mature miRNA being highlighted.
3.2. Prediction of Target Genes of cb-miRs in Human and Cattle
The identified 33 (~17%) and 15 (~7.8%) fs-cb-miRs along with their corresponding paired miRNAs in humans and cattle, respectively, were subjected to the target gene prediction using the psRNATarget server. Subsequently, a total of 32 and 5 fs-cb-miRs have uniquely targeted 68 and 15 genes in human and cattle, respectively (Figure 3). Similarly, we found 40 targeted genes for 40 fns-cb-miRs in case of human and 97 unique targeted genes for 99 fns-cb-miRs in cattle (Figure 3). The perfect and near perfect complementary matches of cb-miRs to their target mRNAs show the probability of post-transcriptional gene expression by mechanisms such as translation inhibition and cleavage of mRNA [39].
3.3. Functional Annotation and Pathway Analysis of Human and Cattle Genes Targeted by cb-miRs
A total of 68 and 15 unique genes in humans and cattle, respectively, were identified as targets for fs-cb-miRs from the psRNAtarget server. Subsequently, the ShinyGO v.0.77 and String v.11.5 were used to annotate and identify the involvement of targeted genes in various pathways. The annotation terms were filtered out with p value < 0.05 resulting in 846 terms in humans and 653 terms in cattle. Among the GO annotation terms of human, 16.31% (138), 17.38% (147) and 66.31% (561) were found to be involved in cellular component, molecular function and biological process, respectively, for targeted genes of fs-cb-miRs whereas these annotation terms in cattle were 9.34% (61), 12.71% (83) and 77.95% (509) for targeted genes of fs-cb-miRs (Figure 5). A total of 24 and 11 KEGG pathways were identified for targeted genes of fs-cb-miRs in human (Table 1) and cattle (Table 2), respectively. It was also observed in the tables that few genes like HMGCS2, LCP2, PPP2R5C and GABRA6 in humans and genes like ATG2B in cattle have participated in more than five pathways. On the other hand, 40 fns-cb-miRs have targeted 40 human genes and 99 fns-cb-miRs have targeted 97 distinct cattle genes. Among the GO annotation terms of targeted genes of humans for fns-cb-miRs, 13.61% (230), 17.40% (294) and 68.99% (1166) were found to be involved in the cellular component, molecular function and biological processes whereas in the case of cattle the percentage of GO terms were 15.14% (157), 14.08% (146) and 70.78% (734) for targeted genes of fns-cb-miRs (Figure 6). Also, 46 and 44 KEGG pathways were identified for targeted genes of fns-cb-miRs in human (Table 3) and cattle (Table 4), respectively.
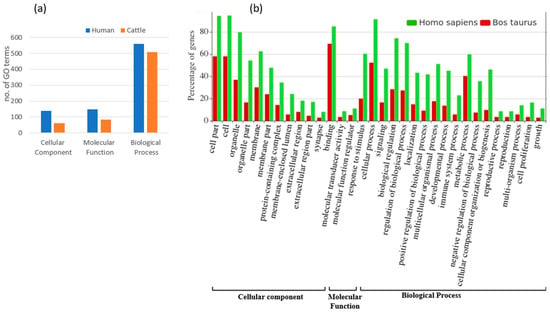
Figure 5.
(a) Number of GO terms involved in cellular component, molecular function and biological process for targeted genes of fs-cb-miRs in human (blue) and cattle (red). (b) Functional annotation and GO terms of targeted genes of fs-cb-miRs in cattle (red) and human (green).

Table 1.
List of human genes targeted by fs-cb-miRs and their involvement in KEGG pathways.

Table 2.
List of cattle genes targeted by fs-cb-miRs and their involvement in KEGG pathways.
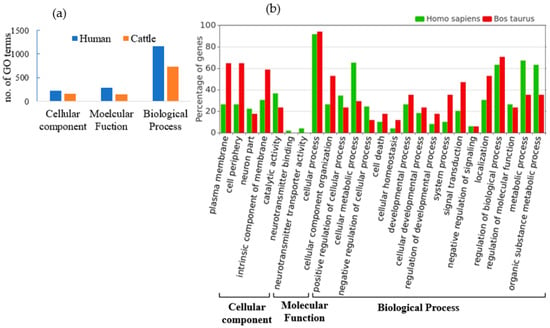
Figure 6.
(a) Number of GO terms involved in cellular component, molecular function and biological process for human (blue) and cattle (orange) for targeted genes of fns-cb-miRs. (b) Functional annotation and GO terms of targeted genes of fns-cb-miRs in cattle (red) and human (green).

Table 3.
List of human genes targeted by fns-cb-miRs and their involvement in KEGG pathways.

Table 4.
List of cattle genes targeted by fns-cb-miRs and involvement in KEGG pathways.
3.4. Gene Regulatory Network Analysis
The regulatory network between fs-cb-miRs and their targeted genes in humans and cattle were visualized by cytoscape and depicted in Figure 7. The integrative analyses of cb-miRs and their target genes responsible for diseases in cattle and human provide useful information to understand complex biological systems and processes involved in miRNA–mRNA–disease associations. A total of eight and five cb-miRs (ellipse shape) showed their likely integration with the gene networks in humans and cattle, respectively. The targeted genes in the network are shown in rectangular boxes (Figure 7a,b). The length of edges in the network shows the strength of unpaired energy (UPE) that in turn depicts the interaction between miRNA and mRNA. The fs-cb-miRs like Ct-miR-3037, Ct-miR-3169, Cte-miR824-3p, Ct-miR-3135, Cte-miR8741, and Cte-miR7780-3p were found to target more than four genes. The targeted genes of fs-cb-miRs, viz., DAZAP2, KLH20, TNS1, BBIP1, HMGCS2, PAPD4, FAM212B and KIAA1549 are having disease associations. Similarly, in the case of cattle, fs-cb-miRs like Cte-miR5084, Cte-miR531, Ct-miR-3061, Ct-miR-3069 and Ct-miR-3135 have targeted the genes having several gene associations. The genes having disease associations are highlighted in yellow color. Further, a regulatory network was developed for targeted genes of fns-cb-miRs and it was observed that few genes are participating in the gene regulatory network in the case of humans (Figure 8) while no regulatory network was formed in the case of cattle.
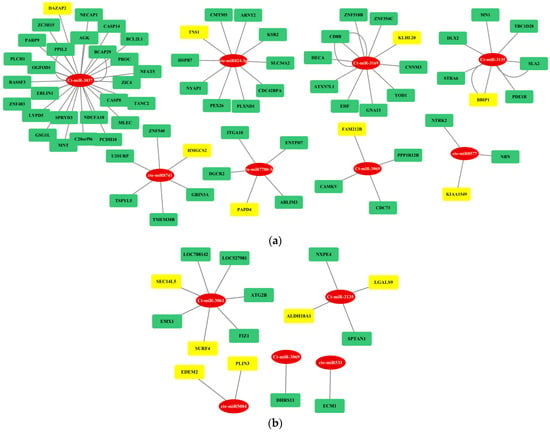
Figure 7.
Gene regulatory network analysis of fs-cb-miRs (red color) and their cross-kingdom targeted genes (yellow or green colors) in (a) humans and (b) cattle. Here, miRNA and mRNA are represented in eclipse and rectangular shapes, respectively. Genes in yellow color show their association with diseases.
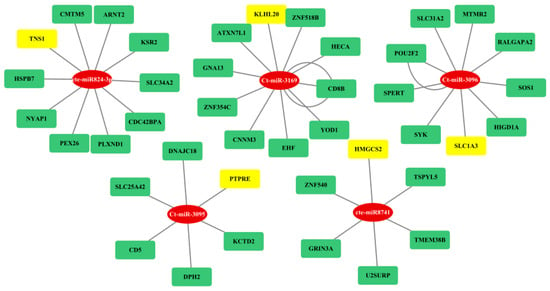
Figure 8.
Gene regulatory network analysis of fns-cb-miRs (in red color) and their targeted genes (yellow or green colors) in human. Here, miRNA and mRNA are represented in rectangular shapes (miRNAs in red color and mRNAs in both green and yellow colors). Rectangular boxes in yellow color show the genes associated with diseases.
3.5. Association of Target Genes of fs-cb-miRs and fns-cb-miRs with Human and Cattle Diseases
The targeted genes of fs-cb-miRs in human were found to be associated with multiple diseases given in DisGeNET database, which is one of the largest available collections of genes and variants involved in human diseases. A total of eight out forty targeted genes were found to be associated with diseases based on first four evidence levels of DisGeNET. Most of the gene–disease associations were categorized into “strong” (50%) evidence level and others into “definitive” (3%), “moderate” (8%) and “limited” (39%). The fs-cb-miRs, targeted genes and their associated diseases, disease association type, and references are given in Table 5. Further, it was observed that the fs-cb-miR-targeted gene KLHL20 was involved in Alzheimer’s disease (Figure 7a) while another gene, PAPD4, was found as a biomarker for diseases Hepatitis C virus infection. Additionally, the variations in the targeted genes: TNS1, FAM212B and KIAA1549 have led to the diseases such as malignant tumors of the breast, Crohn’s disease and Pilocytic astrocytoma, respectively (Table 5). In contrast, the fns-cb-miRs and their targeted gene-association information along with references are given in Table 6. Here, the targeted genes like DNTT, PTPRE, CNRIP1, CLEC4G, LMTK2, PHKA1, and KLHL were found associated with diseases like Schrizophrenia, Asthma, Colorectal Carcinoma, Liver Carcinoma, Glycogen Storage Disease, Chronic Lymphocytic Leukemia, Alcoholic Intoxication and Alzheimer diseases with a disease association type as a biomarker (Table 6).

Table 5.
List of fs-cb-miRs targeted gene–disease association in human and their involvement in various disease.

Table 6.
List of fns-cb-miRs targeted gene–disease association in human and their involvement in various disease.
In the case of cattle, the literature was searched for analyzing the gene–disease association. The fs-cb-miR targeted gene–disease association information and corresponding references are given in Table 7. A total of 11 out of 15 targeted genes, PLIN3, EDEM2, ECM1, SURF4, SEC14L5, DHRS11, LGALS9, ALDH18A1, SPTAN1, NXPE4 and FIZ1 of fs-cb-miRs, were found associated with the diseases like bovine respiratory disease, mastitis resistance, and Hyperprolinemia type II, etc. (Table 7). On the other hand, the fns-cb-miRs and their targeted gene–disease association information are given in Table 8 the genes like CFLAR, TRPA1, RB1 and SCNN1B were found involved in malignant glioma, respiratory disease, sporadic retinoblastoma and renal disease, respectively, in cattle (Table 8).

Table 7.
List of fs-cb-miR targeted genes in cattle, disease associations and references.

Table 8.
List of fns-cb-miRs and their targeted genes of cattle and involvement in different disease.
4. Discussion
The cross-kingdom role of edible plant miRNAs, also known as food-derived miRNAs, play an important role in inter-species regulation [3,38,80,81,82,83]. These studies further demonstrated that the plant miRNAs act in a similar manner to human miRNAs after entering the gastrointestinal (GI) tract [82]. The stability of plant-derived miRNAs has been studied under high temperature and chemical degradation processes as well as in the gastrointestinal tract of humans and animal serum [84,85]. The small-molecule carriers such as exosomes, microvesicles and high-density lipoprotein are responsible for the stability of exogenous miRNAs as they protect from degradation [5,86,87]. The miRNA-mir2911 of Chinese herb honeysuckle (Lonicera japonica) is highly stable and helps in protecting against influenza virus (Zhou et al., 2015) and novel Coronavirus SARS-CoV-2 [88]. Similarly, “miR471” and “miR519” from lettuce targeted the Hepatitis B virus (HBV) [81]. Recently, it was reported that plant miRNA “miR159” helps in reducing the proliferation of breast cancer cells [39]. Moreover, the plant derived miRNAs, which regulate multiple gene expression in cross-kingdom species, may lead to a new approach to cast light on the nutritional and functional value of plants [18]. It was suggested that exogenous miRNAs (plant miRNAs) have targeted the genes in the human genome [6,89]. Similarly, the miRNAs of Moringa oleifera, Ocimum basilicum and Medicago truncatula target the genes in human [26,90,91]. Likewise, the role of plant-derived miRNAs has been studied in mammals including humans and mice in the recent past. To be specific, the understanding of plant-derived miRNAs in mammals encompassing antiviral, antitumor, anti-inflammatory, anti-apoptotic, immune-modulating, and regulatory impacts on intestinal function were studied recently [82]. It has also been studied that the MiR171 variant from the Arabidopsis and tomato targeted the mTOR pathway of the HEK293 cell of humans [92]. Interestingly, the human miRNAs “hsa-miR-21-5p” and “hsa-miR-24-3p” targeted the gene cyclin-dependent kinase inhibitor Sol1 of Candida albicans and inhibit its cell growth [93]. These findings support the cross-kingdom analysis. However, to our limited knowledge, the role of plant-derived-miRNAs in cattle has been reported for the first time by us.
The present study is carried out to (i) identify functionally similar-cb-miRs (fs-cb-miRs), i.e., cbmiRs having sequence similarities with human and cattle miRNAs as well as satisfying structural properties of miRNAs and target the same gene as that targeted by their corresponding similarity pairs in host organisms (human, cattle), (ii) identify functionally-non-similar-miRNAs (fns-cb-miRs) that regulate genes in the host (human, cattle) organisms, and (iii) the involvement of targeted genes in regulatory networks, pathways and disease association. The fs-cb-miRs were studied for functional and probable evolutionary importance in the host systems like humans and cattle. Whereas fns-cb-miRs were studied for their functional role in humans and cattle. The targeted genes of both fs-cb-miRs and fns-cb-miRs in host systems have also been studied through regulatory network and pathway analysis. In addition, the disease associations of the targeted genes have been studied.
Our results revealed high similarity to the extent of more than 70% between human miRNA “hsa-mir-6754-3p” and cb-miR “Cte-mir824-3p” as well as between “hsa-mir-6804-5p” and “Cte-mir6183”. The hsa-mir-6754-3p and hsa-mir-6804-5p target the genes TNS1 and FNDC3A, respectively. These targeted genes code for the corresponding transcription factors that are responsible for the regulation of gene expression [42]. Similar findings have been reported that 84 miRNAs of wheat have targeted the 787 human genes [94]. Our findings on fs-cb-miRs fall in line with the results reported earlier [95] about the similarity between the human miRNA “hsa-mir341” and olive plant miRNAs:oeu-sR20, oeu-sR27, oeu-sR34.
In the case of cattle, the fs-cb-miR “Ct-mir-3061” has 74% similarity with the cattle miRNA “bta-miR-7895” and was observed to interact with the FIZ1 gene responsible for regulating cellular processes as well as being associated with various biological functions [74]. Whereas the fs-cb-miR “Ct-miR-3033” has 69% similarity with the cattle miRNA “bta-mir-2338” and was found interacting with a GAIP-interacting protein C-terminus (GIPC3) gene that has a potential role in cell growth, differentiation, and survival [75].
The gene regulatory networks play an important role in various vital processes of life including cell differentiation, metabolism, cell cycle and signal transduction [95]. The fs-cb-miR “Cte-miR824-3p”, which is functionally similar to the human miRNAs hsa-miR-4279, hsa-miR-6754-3p, hsa-miR-6845-3p, hsa-miR-6887-3p, hsa-miR-877-3p and hsa-miR-6894-5p, was found to interact with the human genes TNS1, SLC34A2, KSR2, NYAP1, HSPB7, CMTM5, PLXND1, ARNT2, and CDC42BPA involved in regulation of phosphate homeostasis [96], cell growth and differentiation [97], neuronal development and synaptic plasticity [98], maintaining cardiac structure and function [99], tumor suppression [100], angiogenesis during development [101], neuronal development [102], and cytoskeletal organization [103] and cancer [104], respectively. Similar findings on the regulation of genes by miRNAs have also been reported in Bacopa monnieri [105] and wheat [94].
The KEGG pathway analysis of the targeted genes of fs-cb-miRs in both humans and cattle revealed that the targeted genes were involved in multiple pathways (Table 1). For example, the targeted gene LCP2 is involved in eight pathways including important pathways like, Rap1 signaling pathway, osteoclast differentiation, Yersinia infection, platelet activation, T-cell receptor signaling pathway, and Fc epsilon RI signaling pathway [51]. Whereas in the case of cattle, only two genes ALDH18A1 and ATG2B were involved in more than one pathway [31].
Further, we investigated the gene–disease associations in human and cattle by using cb-miRs targeted genes through DisGeNET database [36] and literature, respectively. Here, 17 targeted genes of fs-cb-miRs show their involvement in 16 different diseases in human. Few targeted genes of fs-cb-miRs like TNS1 (Cte-miR824-3p), UBE2K (Cte-miR168), FNDC3A (Cte-miR6183) and PAPD4 (Cte-miR7780-3p) were involved in malignant tumors of the breast, Alzheimer’s disease, Angelman syndrome and Hepatitis C virus infection, respectively. The gene–disease associations (Table 5) were reported earlier [42,45]. These findings are in line with earlier reported findings such as the miRNAs “pku-miR167a-5p” and “pku-miR167b-3p” from Picrorhiza kurroa having targeted the genes:“PPP3R2” and “MYOZ3”, respectively, involved in astheno-zoospermia and muscular dystrophy in humans [106]. In the case of cattle, it was observed that six cb-miRs have targeted twelve genes. The targeted genes, PLIN3, EDEM2, SURF4, and LGALS9, with mutations/variations have led to metabolic diseases, bovine osteoporosis, mastitis resistance and bovine respiratory disease, respectively. These mentioned gene–disease associations were reported earlier in the literature [64,65,67,70]. Interestingly, lncRNA “LOC788142” was targeted by the fs-cb-miRs and found expressed in various tissues in cattle, including muscle, liver, and adipose tissue [107].
Our findings also revealed that fns-cb-miRs target the human genes HMGCS2, PPP2R5C, LCP2, and GABRA6 involved in more than four pathways such as signaling pathways, metabolism regulation pathways and a few disease related pathways. The signaling pathways are calcium, Glucagon, insulin, AMPK, and PI3k-Aktin. Further, the gene regulatory network was developed between the targeted genes and fns-cb-miRs. The gene–disease association study revealed that 22 targeted genes were associated with 17 different diseases. Here, mostly genes are working as biomarkers in gene–disease-associations. Three genes, namely, CLEC4G, LCP2, ST8SIA1 are acting as biomarkers in liver carcinoma [51]. Similarly, gene DNTT and UHMk1 are predicted as biomarkers in Schizophrenia. The targeted genes of fns-cb-mirs of cattle were also studied and we found that nine targeted genes are involved in forty-four pathways. These pathways were mainly related to signaling, but few disease-related pathways were also found. Furthermore, four fns-cb-miRs and their respective targeted genes were associated with several diseases in cattle. Here, dysfunction in the fns-cb-miR’s targeted gene SCNN1B lead to renal disease in cattle [79]. Thus, the above findings may pave the way for insights into the role of plant-derived miRNAs in animal cells. However, wet-lab validation of findings is required for a deeper understanding and confirmation of the role of plant-derived miRNAs across the animal kingdom.
5. Conclusions
The plant-derived miRNAs are likely to have an expected functional similarity with the miRNAs of cross-kingdom species like humans and cattle due to the use of plant parts as their dietary intake. In the present study, we found fs-cb-miRs and fns-cb-miRs having functional similarity and functional non-similarity with the miRNAs of humans and cattle through an in silico approach. These fs-cb-miRs were found to target various genes like LCP2, GABRA6, and MYH14 in humans and ATG2B and DHRS11 in cattle. Mostly the fs-cb-miRs’ targeted genes of humans were involved in the signaling pathways like PPAR, Rap1, T cell receptor, Fc epsilon RI, and Retrograde endocannabinoid. The gene–disease-association in humans showed that targeted genes of fs-cb-miRs like KLHL20, BBIP1, and PAPD4 have acted as biomarkers for the diseases such as Alzheimer’s disease, Bardet-Biedl syndrome 18, and Hepatitis C virus infection, respectively. In cattle, the targeted genes of fs-cb-miRs are involved in Apoptosis, Necroptosis, Chagas disease and Hepatitis C. Further, the gene–disease-association showed that mutation/variation in the genes like EDEM2, ECM1, SURF4, and DHRS11 caused bovine osteoporosis, bovine hereditary angioneurotic edema, mastitis resistance and bovine viral diarrhea in cattle. In contrast, fns-cb-miRs’ targeted genes in humans and cattle also showed their involvement in various pathways and diseases. Genes like NRM, SLC6A6, and PTPRE are involved in Rheumatoid Arthritis, Myocardial Ischemia and Asthma diseases in humans. In the case of cattle, genes like TRPA1, RB1 and SCNN1B are predicted to be involved in respiratory diseases, retinoblastoma and renal diseases, respectively. Thus, our findings reflect the translation of plant/food derived miRNAs in cross kingdom species and their functional significance in the gene–disease associations. In the future, these plant-derived miRNAs may become an alternative to current trend of using synthetic-miRNA-based drugs, which are time-consuming and expensive compared to the natural miRNA supplements.
Author Contributions
Conceptualization, S.S. and A.R.R.; data curation, T.K.S., J.P., S.V. and A.K.; formal analysis, S.S., T.K.S., J.P., S.V., A.K. and K.G.; investigation, S.S. and A.R.R.; methodology, S.S. and A.R.R.; writing—original draft, S.S.; writing—review and editing, A.R.R., T.K.S. and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ICAR-Consortia Research Platform on Genomics grant number F.No. CRP-Genomics/IX/2023-24 of Indian Council of Agricultural Research (ICAR). All the authors acknowledge the funding support provided by ICAR-Consortia Research Platform on Genomics. The funding body played no role in design or conclusion of this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available either in literature or in openly available repositories (public domain databases). The miRNAs of cluster bean are available in literature [22,23]. The miRNAs of cattle and human are available in miRbase ([24]; https://mirbase.org/download/hairpin.fa, accessed on 29 June 2023). The mRNA sequences of cattle are available in National Center for Biotechnology Information (NCBI) at https://www.ncbi.nlm.nih.gov/, accessed on 29 June 2023, whereas human mRNAs are available in the database of psRNATarget server ([25]; https://www.zhaolab.org/psRNATarget/, accessed on 29 June 2023].
Acknowledgments
All authors would like to thank the Coordinator, ICAR-Consortia Research Platform on Genomics and Director, ICAR-Indian Agricultural Statistics Research Institute, New Delhi for providing support and computational facilities for conducting the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zen, K.; Zhang, J.; Zhang, C.Y.; Chen, X. New roles for microRNAs in cross-species communication. RNA Biol. 2013, 10, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Silva, M.R.; das Neves, R.F.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; de Souza, W.; et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.D.; Wu, J.; Ren, J.; Meng, L.; Wu, Q.; Dong, H.; Wu, J.; Kao, T.Y.; et al. Escherichia coli noncoding RNAs can affect gene expression and physiology of Caenorhabditis elegans. Nat. Commun. 2012, 3, 1073. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Ivashuta, S.I.; Petrick, J.S.; Heisel, S.E.; Zhang, Y.; Guo, L.; Reynolds, T.L.; Rice, J.F.; Allen, E.; Roberts, J.K. Endogenous small RNAs in grain: Semi-quantification and sequence homology to human and animal genes. Food Chem. Toxicol. 2009, 47, 353–360. [Google Scholar] [CrossRef]
- Bhatt, D.H.; Jha, N.; KJSr Pandya, H.A. In silico exploration of miRNA from EST data of avocado and predicting its cross-kingdom effects on human. Pharma. Innov. J. 2017, 6, 543–548. [Google Scholar]
- Köberle, V.; Pleli, T.; Schmithals, C.; Augusto Alonso, E.A.; Haupenthal, J.; Bönig, H.; Peveling-Oberhag, J.; Biondi, R.M.; Zeuzem, S.; Kronenberger, B.; et al. Differential stability of cell-free circulating microRNAs: Implications for their utilization as biomarkers. PLoS ONE 2013, 8, e75184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sahu, S.; Kumari, P.; Gopi, S.R.; Malhotra, R.; Biswas, S. Genome-wide identification and functional annotation of miRNAs in anti-inflammatory plant and their cross-kingdom regulation in Homo sapiens. J. Biomol. Struct. Dyn. 2017, 35, 1389–1400. [Google Scholar] [CrossRef]
- Zhou, L.K.; Zhou, Z.; Jiang, X.M.; Zheng, Y.; Chen, X.; Fu, Z.; Xiao, G.; Zhang, C.Y.; Zhang, L.K.; Yi, Y. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020, 6, 54. [Google Scholar] [CrossRef]
- Ji, L.; Chen, X. Regulation of small RNA stability: Methylation and beyond. Cell Res. 2012, 22, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, A.; Potestà, M.; Gismondi, A.; Pirrò, S.; Cirilli, M.; Gattabria, F.; Galgani, A.; Sessa, L.; Mattei, M.; Canini, A.; et al. Olea europaea small RNA with functional homology to human miR34a in cross-kingdom interaction of anti-tumoral response. Sci. Rep. 2018, 8, 12413. [Google Scholar] [CrossRef]
- Lamonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; Nicchitta, C.V.; et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; Van Eijndhoven, M.A.J.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Würdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef]
- Pastrello, C.; Tsay, M.; McQuaid, R.; Abovsky, M.; Pasini, E.; Shirdel, E.; Angeli, M.; Tokar, T.; Jamnik, J.; Kotlyar, M.; et al. Circulating plant miRNAs can regulate human gene expression in vitro. Sci. Rep. 2016, 6, 32773. [Google Scholar] [CrossRef]
- Wang, M.L.; Morris, J.B. Flavonoid content in seeds of guar germplasm using HPLC. Plant Genet. Res. 2007, 5, 96–99. [Google Scholar] [CrossRef]
- Gaikwad, K.; Ramakrishna, G.; Srivastava, H.; Saxena, S.; Kaila, T.; Tyagi, A.; Sharma, P.; Sharma, S.; Sharma, R.; Mahla, H.R.; et al. The chromosome-scale genome assembly of cluster bean provides molecular insight into edible gum (galactomannan) biosynthesis family genes. Sci. Rep. 2023, 13, 9941. [Google Scholar] [CrossRef]
- Mukhtar, H.M.; Ansari, S.H.; Bhat, Z.A.; Naved, T. Antihyperglycemic activity of Cyamopsis tetragonoloba beans on blood glucose levels in alloxan-induced diabetic rats. Pharm. Biol. 2006, 44, 10–13. [Google Scholar] [CrossRef]
- Tyagi, A.; Nigam, D.; SV, A.M.; Solanke, A.U.; Singh, N.K.; Sharma, T.R.; Gaikwad, K. Genome-wide discovery of tissue-specific miRNAs in clusterbean (Cyamopsis tetragonoloba) indicates their association with galactomannan biosynthesis. Plant Biotechnol. J. 2018, 16, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Rao, A.R.; Pandey, J.; Gaikwad, K.; Ghoshal, S.; Mohapatra, T. Genome-wide identification and characterization of lncRNAs and miRNAs in cluster bean (Cyamopsis tetragonoloba). Gene 2018, 667, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. PsRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Pirrò, S.; Minutolo, A.; Galgani, A.; Potestà, M.; Colizzi, V.; Montesano, C. Bioinformatics prediction and experimental validation of microRNAs involved in cross-kingdom interaction. J. Comput. Biol. 2016, 23, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.J.; Chakraborty, S.; Deb, B.; Rajwanshi, R. Computational identification and functional annotation of microRNAs and their targets from expressed sequence tags (ESTs) and genome survey sequences (GSSs) of coffee (Coffea arabica L.). Plant Gene 2016, 1, 30–42. [Google Scholar] [CrossRef]
- Supriya, P.; Kumar, A.; Archak, S.; Bhat, K.V. Computational identification of microRNAs and their target genes in sesame (Sesamum indicum L.). Indian J. Genet. Plant Breed. 2022, 82, 469–473. [Google Scholar]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 15, 2628–2629. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG database. Novartis Found. Symp. 2002, 247, 91–101. [Google Scholar] [PubMed]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A.; et al. WEGO 2.0: A web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Strande, N.T.; Riggs, E.R.; Buchanan, A.H.; Ceyhan-Birsoy, O.; DiStefano, M.; Dwight, S.S.; Goldstein, J.; Ghosh, R.; Seifert, B.A.; Sneddon, T.P.; et al. Evaluating the clinical validity of gene-disease associations: An evidence-based framework developed by the clinical genome resource. Am. J. Hum. Genet. 2017, 100, 895–906. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Ayachit, G.; Bhairappanavar, S.B.; Ansari, A.; Sharma, P.; Soni, S.; Das, J. Cross-kingdom regulation of putative miRNAs derived from happy tree in cancer pathway: A systems biology approach. Int. J. Mol. Sci. 2017, 18, 1191. [Google Scholar] [CrossRef]
- Chin, A.R.; Fong, M.Y.; Somlo, G.; Wu, J.; Swiderski, P.; Wu, X.; Wang, S.E. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016, 26, 217–228. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.X.; Luo, S.Q.; Xiong, D.H.; Sun, S.; Wang, Y.P.; Bu, X.F.; Liu, J.; Hu, J. Promoter methylation induced epigenetic silencing of DAZAP2, a downstream effector of p38/MAPK pathway, in multiple myeloma cells. Cell Signal 2019, 60, 136–145. [Google Scholar] [CrossRef]
- Chen, Z.; Picaud, S.; Filippakopoulos, P.; D’Angiolella, V.; Bullock, A.N. Structural Basis for Recruitment of DAPK1 to the KLHL20 E3 Ligase. Structure 2019, 27, 1395–1404.e4. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Thawornkuno, C.; Chavalitshewinkoon-Petmitr, P.; Punyarit, P.; Petmitr, S. Quantitative Real-Time RT-PCR of ITGA7, SVEP1, TNS1, LPHN3, SEMA3G, KLB and MMP13 mRNA Expression in Breast Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 5879–5882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheidecker, S.; Etard, C.; Pierce, N.W.; Geoffroy, V.; Schaefer, E.; Muller, J.; Chennen, K.; Flori, E.; Pelletier, V.; Poch, O.; et al. Exome sequencing of Bardet-Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18). J. Med. Genet. 2014, 51, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.S.; Papandreou, A.; Drury, S.; Boustred, C.; Yue, W.W.; Wedatilake, Y.; Beesley, C.; Jacques, T.S.; Anderson, G.; Abulhoul, L.; et al. Advantages and pitfalls of an extended gene panel for investigating complex neurometabolic phenotypes. Brain J. Neurol. 2016, 139, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Ulveling, D.; Le Clerc, S.; Cobat, A.; Labib, T.; Noirel, J.; Laville, V.; Coulonges, C.; Carpentier, W.; Nalpas, B.; Heim, M.H.; et al. A new 3p25 locus is associated with liver fibrosis progression in human immunodeficiency virus/hepatitis C virus-coinfected patients. Hepatology 2016, 64, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, Y.; Kawai, Y.; Naito, T.; Hirano, A.; Umeno, J.; Fuyuno, Y.; Liu, Z.; Li, D.; Nakano, T.; Izumiyama, Y.; et al. A Genome-wide Association Study Identifying RAP1A as a Novel Susceptibility Gene for Crohn’s Disease in Japanese Individuals. J. Crohn’s Colitis 2021, 13, 648–658. [Google Scholar] [CrossRef]
- Appay, R.; Fina, F.; Macagno, N.; Padovani, L.; Colin, C.; Barets, D.; Ordioni, J.; Scavarda, D.; Giangaspero, F.; Badiali, M.; et al. Duplications of KIAA1549 and BRAF screening by Droplet Digital PCR from formalin-fixed paraffin-embedded DNA is an accurate alternative for KIAA1549-BRAF fusion detection in pilocytic astrocytomas. Mod. Pathol. 2018, 31, 1490–1501. [Google Scholar] [CrossRef]
- Vine, A.E.; McQuillin, A.; Bass, N.J.; Pereira, A.; Kandaswamy, R.; Robinson, M.; Lawrence, J.; Anjorin, A.; Sklar, P.; Gurling, H.M.; et al. No evidence for excess runs of homozygosity in bipolar disorder. Psychiatr. Genet. 2009, 19, 165–170. [Google Scholar] [CrossRef]
- Han, X.; Huang, S.; Xue, P.; Fu, J.; Liu, L.; Zhang, C.; Yang, L.; Xia, L.; Sun, L.; Huang, S.K.; et al. LncRNA PTPRE-AS1 modulates M2 macrophage activation and inflammatory diseases by epigenetic promotion of PTPRE. Sci. Adv. 2019, 5, eaax9230. [Google Scholar] [CrossRef]
- Fan, S.J.; Snell, C.; Turley, H.; Li, J.L.; McCormick, R.; Perera, S.M.; Heublein, S.; Kazi, S.; Azad, A.; Wilson, C.; et al. PAT4 levels control amino-acid sensitivity of rapamycin-resistant mTORC1 from the Golgi and affect clinical outcome in colorectal cancer. Oncogene 2016, 35, 3004–3015. [Google Scholar] [CrossRef]
- AbdulHameed, M.D.; Tawa, G.J.; Kumar, K.; Ippolito, D.L.; Lewis, J.A.; Stallings, J.D.; Wallqvist, A. Systems level analysis and identification of pathways and networks associated with liver fibrosis. PLoS ONE 2014, 9, e112193. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Sidiq, T.; Yousuf, S.; van den Elsen, P.J.; Kobayashi, K.S. Class I transactivator, NLRC5: A central player in the MHC class I pathway and cancer immune surveillance. Immunogenetics 2019, 71, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M.; Seielstad, M.; Padyukov, L.; Lee, A.T.; Remmers, E.F.; Ding, B.; Liew, A.; Khalili, H.; Chandrasekaran, A.; Davies, L.R.; et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—A genomewide study. N. Engl. J. Med. 2007, 357, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Bradbury, N.A. Lemur Tyrosine Kinase 2, a novel target in prostate cancer therapy. Oncotarget 2015, 6, 14233–14246. [Google Scholar] [CrossRef] [PubMed]
- Schomisch, S.J.; Murdock, D.G.; Hedayati, N.; Carino, J.L.; Lesnefsky, E.J.; Cmolik, B.L. Cardioplegia prevents ischemia-induced transcriptional alterations of cytoprotective genes in rat hearts: A DNA microarray study. J. Thorac. Cardiovasc. Surg. 2005, 130, 1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cusanovich, D.A.; Billstrand, C.; Zhou, X.; Chavarria, C.; De Leon, S.; Michelini, K.; Pai, A.A.; Ober, C.; Gilad, Y. The combination of a genome-wide association study of lymphocyte count and analysis of gene expression data reveals novel asthma candidate genes. Hum. Mol. Genet. 2012, 21, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Makise, N.; Sekimizu, M.; Kobayashi, E.; Yoshida, H.; Fukayama, M.; Kato, T.; Kawai, A.; Ichikawa, H.; Yoshida, A. Low-grade endometrial stromal sarcoma with a novel MEAF6-SUZ12 fusion. Virchows Arch. Int. J. Pathol. 2019, 475, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.D.; Jen, J.C.; Choi, S.Y.; Shin, J.H.; Kim, H.S.; Kim, H.J.; Kim, J.S.; Choi, J.H. Late-onset episodic ataxia associated with SLC1A3 mutation. J. Hum. Genet. 2017, 62, 443–446. [Google Scholar] [CrossRef]
- Albash, B.; Imtiaz, F.; Al-Zaidan, H.; Al-Manea, H.; Banemai, M.; Allam, R.; Al-Suheel, A.; Al-Owain, M. Novel PHKG2 mutation causing GSD IX with prominent liver disease: Report of three cases and review of literature. Eur. J. Pediatr. 2014, 173, 647–653. [Google Scholar] [CrossRef]
- Ogushi, K.; Hattori, A.; Suzuki, E.; Shima, H.; Izawa, M.; Yagasaki, H.; Horikawa, R.; Uetake, K.; Umezawa, A.; Ishii, T.; et al. DNA Methylation Status of SHOX-Flanking CpG Islands in Healthy Individuals and Short Stature Patients with Pseudoautosomal Copy Number Variations. Cytogenet. Genome Res. 2019, 158, 56–62. [Google Scholar] [CrossRef]
- Rosales-Reynoso, M.A.; Ochoa-Hernández, A.B.; Aguilar-Lemarroy, A.; Jave-Suárez, L.F.; Troyo-Sanromán, R.; Barros-Núñez, P. Gene expression profiling identifies WNT7A as a possible candidate gene for decreased cancer risk in fragile X syndrome patients. Arch. Med. Res. 2010, 41, 110–118.e2. [Google Scholar] [CrossRef] [PubMed]
- Cali, F.; Ragalmuto, A.; Chiavetta, V.; Calabrese, G.; Fichera, M.; Vinci, M.; Ruggeri, G.; Schinocca, P.; Sturnio, M.; Romano, S.; et al. Novel deletion of the E3A ubiquitin protein ligase gene detected by multiplex ligation-dependent probe amplification in a patient with Angelman syndrome. Exp. Mol. Med. 2010, 12, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Tickoo, M.; Gupta, R.; Vaswani, M.; Ambekar, A.; Grover, T.; Sharma, A. Association of serotonin and GABA pathway gene polymorphisms with alcohol dependence: A preliminary study. Asian J. Psychiatry 2019, 39, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Loor, J.J.; Dann, H.M.; Everts, R.E.; Oliveira, R.; Green, C.A.; Guretzky, N.A.; Rodriguez-Zas, S.L.; Lewin, H.A.; Drackley, J.K. Temporal gene expression profiling of liver from periparturient dairy cows reveals complex adaptive mechanisms in hepatic function. Physiol. Genom. 2005, 23, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lee, J.E.; Kang, J.W.; Shin, H.Y.; Lee, J.B.; Jin, D.I. Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in mammalian oocyte maturation and preimplantation embryo development. Int. J. Mol. Sci. 2019, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Goo, Y.A. Stromal Mesenchyme Cell Genes in Prostate Cancer Development: Epigenetic Markers for Cancer and Potential Targets for Therapy. Univ. Washington Report, 2006. Available online: https://apps.dtic.mil/sti/citations/ADA467588 (accessed on 29 June 2023).
- Wurmser, C.; Tardif, S.; Price, M.; Küry, P. Developmental disorders associated with EMX1 mutations. Mol. Syndromol. 2017, 8, 41–47. [Google Scholar]
- Rupp, R.; Hernandez, A.; Mallard, B.A.; Brockman, F.J. Association of variation in the SEC14L5 gene with susceptibility to mastitis in dairy cattle. J. Dairy Sci. 2019, 102, 8083–8094. [Google Scholar]
- Van der Stede, Y.; Vanderbeke, E.; Bauwens, W.; Mintiens, K.; De Leeuw, I. Association between DHRS11 and susceptibility to bovine viral diarrhea virus infection in cattle. Vet. Res. 2018, 49, 1–8. [Google Scholar]
- McGill, J.L.; Kelly, S.M.; Guerra-Maupome, M.; Prewitt, A.K.; Bass, B.E. Galectin-9 upregulation in the lungs of cattle with bovine respiratory disease contributes to neutrophil and CD4+ T cell recruitment. Vet. Immunol. Immunopathol. 2020, 228, 110090. [Google Scholar]
- Kametani, Y.; Kawamoto, T.; Furuichi, T.; Ikegawa, S.; Okazaki, Y. A novel homozygous ALDH18A1 mutation in a patient with hereditary spastic paraplegia and developmental delay. Gene 2014, 544, 137–142. [Google Scholar]
- Casas, E.; Lunstra, D.D.; Stone, R.T.; Franke, D.E.; Kappes, S.M.; Beever, J.E. A missense mutation in the bovine SPTAN1 gene causes congenital cerebellar ataxia in Angus cattle. BMC Genet. 2003, 4, 18. [Google Scholar]
- Kim, J.H.; Yoon, D.H.; Kim, B.H.; Lim, D.J.; Kim, Y.M.; Lee, S.S. Associations of SNPs in genes related to reproduction and milk production traits in Holstein cows. Asian-Australas. J. Anim. Sci. 2021, 34, 1306–1313. [Google Scholar]
- Neibergs, H.L.; Seabury, C.M.; Wojtowicz, A.J.; Wang, Z.; Scraggs, E.; Kiser, J.N.; Neupane, M.; Womack, J.E.; Eenennaam, A.V.; Hagevoort, G.R.; et al. Susceptibility loci revealed for bovine respiratory disease complex in pre-weaned holstein calves. BMC Genom. 2014, 15, 1164. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Pal, K.; Sharma, A.K.; Dutta, S.K.; Lau, J.S.; Yan, I.K.; Wang, E.; Elkhanany, A.; Alkharfy, K.M.; Sanyal, A.; et al. GAIP interacting protein C-terminus regulates autophagy and exosome biogenesis of pancreatic cancer through metabolic pathways. PLoS ONE 2014, 9, e114409. [Google Scholar] [CrossRef] [PubMed]
- Hojo, T.; Al-Zi’abi, M.O.; Komiyama, J.; Manabe, N.; Acosta, T.J.; Okuda, K. Expression and localization of cFLIP, an anti-apoptotic factor, in the bovine corpus luteum. J. Reprod. Dev. 2010, 56, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; Quarcoo, D.; Frossard, N.; Fischer, A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy 2004, 59, 1139–1152. [Google Scholar] [CrossRef]
- Hayes, H.C.; Popescu, P.; Dutrillaux, B. Comparative gene mapping of lactoperoxidase, retinoblastoma, and alpha-lactalbumin genes in cattle, sheep, and goats. Mamm. Genome 1993, 4, 593–597. [Google Scholar] [CrossRef]
- Rossier, B.C. Epithelial Sodium Channel (ENaC) and the Control of Blood Pressure. Curr. Opin. Pharmacol. 2015, 21, 76–87. [Google Scholar] [CrossRef]
- Jiang, M.; Sang, X.; Hong, Z. Beyond nutrients: Food-derived microRNAs provide cross-kingdom regulation. Bioessays 2012, 34, 280–284. [Google Scholar] [CrossRef]
- Zhang, S.; Sang, X.; Hou, D.; Chen, J.; Gu, H.; Zhang, Y.; Li, J.; Yang, D.; Zhu, H.; Yang, X.; et al. Plant-derived RNAi therapeutics: A strategic inhibitor of HBsAg. Biomaterials 2019, 210, 83–93. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Yang, Y.; Liu, J.; Li, H.; Li, R.; Cao, C.; Shi, L.; Wu, W.; He, K. A timely review of cross-kingdom regulation of plant-derived MicroRNAs. Front. Genet. 2021, 12, 613197. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, A.N.; Vasavada, A.R.; Johar, S.K. Recent trends in miRNA therapeutics and the application of plant miRNA for prevention and treatment of human diseases. Future J. Pharm. Sci. 2022, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sanchita, R.S.; Singh, R.; Srivastava, G.; Sharma, A. Comparative Study of Withanolide Biosynthesis-Related miRNAs in Root and Leaf Tissues of Withania somnifera. Appl. Biochem. Biotechnol. 2018, 185, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.; Ferro, V.A.; Tate, R.J. Determination of the potential bioavailability of plant microRNAs using a simulated human digestion process. Mol. Nutr. Food Res. 2015, 59, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, C.; Clayton, A.; Newbury, L.J.; Corish, P.; Jenkins, R.H.; Phillips, A.O.; Fraser, D.J.; Bowen, T. Stabilization of urinary microRNAs by association with exosomes and argonaute 2 protein. Non-Coding RNA 2015, 1, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef]
- Min, H.; Yoon, S. Got target?: Computational methods for microRNA target prediction and their extension. Exp. Mol. Med. 2010, 42, 233–244. [Google Scholar] [CrossRef]
- Patel, M.; Mangukia, N.; Jha, N.; Gadhavi, H.; Shah, K.; Patel, S.; Mankad, A.; Pandya, H.; Rawal, R. Computational identification of miRNA and their cross kingdom targets from expressed sequence tags of Ocimum basilicum. Mol. Biol. Rep. 2019, 1, 2979–2995. [Google Scholar] [CrossRef]
- Bellato, M.; De Marchi, D.; Gualtieri, C.; Sauta, E.; Magni, P.; Macovei, A.; Pasotti, L. A bioinformatics approach to explore microRNAs as tools to bridge pathways between plants and animals. Is DNA damage response (DDR) a potential target process? Front. Plant Sci. 2019, 10, 1535. [Google Scholar] [CrossRef]
- Gismondi, A.; Nanni, V.; Monteleone, V.; Colao, C.; Di Marco, G.; Canini, A. Plant miR171 modulates mTOR pathway in HEK293 cells by targeting GNA12. Mol. Biol. Rep. 2021, 48, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Halder, L.D.; Babych, S.; Palme, D.I.; Mansouri-Ghahnavieh, E.; Ivanov, L.; Ashonibare, V.; Langenhorst, D.; Prusty, B.; Rambach, G.; Wich, M.; et al. Candida albicans induces cross-kingdom miRNA trafficking in human monocytes to promote fungal growth. mBio 2022, 13, e03563-21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romo, D.; Hernández-Vásquez, C.I.; Pereyra-Alférez, B.; García-García, J.H. Identification of potential target genes in Homo sapiens, by miRNA of Triticum aestivum: A cross kingdom computational approach. Non-Coding RNA Res. 2022, 7, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Karlebach, G.; Shamir, R. Modelling and analysis of gene regulatory networks. Nat. Rev. Mol. Cell Biol. 2008, 9, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Park, G.C.; Kim, J.M.; Shin, S.C.; Cheon, Y.I.; Sung, E.S.; Lee, M.; Lee, J.C.; Lee, B.J. Tensin Regulates Fundamental Biological Processes by Interacting with Integrins of Tonsil-Derived Mesenchymal Stem Cells. Cells 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Chen, L.; Guo, S. Knockdown of SLC34A2 inhibits cell proliferation, metastasis, and elevates chemosensitivity in glioma. J. Cell. Biochem. 2019, 120, 10205–10214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, L.J.; Fan, C.C.; Chang, H.C.; Shih, H.A.; Min, M.Y.; Chang, M.S. Important roles of Vilse in dendritic architecture and synaptic plasticity. Sci. Rep. 2017, 7, 45646. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, Z.; Bassel-Duby, R.; Olson, E.N. Genetic and epigenetic regulation of cardiomyocytes in development, regeneration and disease. Development 2018, 145, dev171983. [Google Scholar] [CrossRef]
- Vos, M.J.; Zijlstra, M.P.; Kanon, B.; van Waarde-Verhagen, M.A.; Brunt, E.R.; Oosterveld-Hut, H.M.; Carra, S.; Sibon, O.C.; Kampinga, H.H. HSPB7 is the most potent polyQ aggregation suppressor within the HSPB family of molecular chaperones. Hum. Mol. Genet. 2010, 19, 4677–4693. [Google Scholar] [CrossRef]
- Shao, L.; Cui, Y.; Li, H.; Liu, Y.; Zhao, H.; Wang, Y.; Zhang, Y.; Ng, K.M.; Han, W.; Ma, D.; et al. CMTM5 exhibits tumor suppressor activities and is frequently silenced by methylation in carcinoma cell lines. Clin. Cancer Res. 2007, 13, 5756–5762. [Google Scholar] [CrossRef]
- Oh, W.J.; Gu, C. The role and mechanism-of-action of Sema3E and Plexin-D1 in vascular and neural development. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2013; Volume 24, pp. 156–162. [Google Scholar]
- Bruick, R.K.; McKnight, S.L. Differential interaction of the hypoxia-inducible factor-alpha (HIF-alpha) subunits with ARNT2. Mol. Cell Biol. 1995, 15, 4073–4082. [Google Scholar]
- East, M.P.; Christopher, R.M.A. CDC42BPA/MRCK [alpha]: A kinase target for brain, ovarian and skin cancers. Nat. Rev. Drug Discov. 2021, 20, 167–168. [Google Scholar] [CrossRef]
- Gadhavi, H.; Patel, M.; Mangukia, N.; Shah, K.; Bhadresha, K.; Patel, S.K.; Rawal, R.M.; Pandya, H.A. Transcriptome-wide miRNA identification of Bacopa monnieri: A cross-kingdom approach. Plant Signal. Behav. 2020, 2, 1699265. [Google Scholar] [CrossRef]
- Trivedi, T.S.; Mangukia, N.; Bhavsar, M.; Mankad, A.U.; Rawal, R.M.; Patel, S.K. A novel insight of Picrorhiza kurroa miRNAs in human cystic fibrosis: A transcriptome-wide cross-kingdom study. Hum. Gene 2023, 35, 201153. [Google Scholar] [CrossRef]
- Zhao, X.; Mo, D.; Li, A.; Gong, W.; Xiao, S.; Zhang, Y.; Li, J. Identification and characterization of long non-coding RNAs in subcutaneous adipose tissue from castrated and intact full-sib pair Huai pig breeds. Genomics 2018, 110, 190–199. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).