Melatonin Improves H2O2-Induced Oxidative Stress in Sertoli Cells Through Nrf2-Keap1 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Culture and Treatment
2.3. Cell Viability Assay and MDA Determination
2.4. The Detection of ROS Production, GSH Content, and SOD Activity
2.5. Real-Time Quantitative PCR Analysis
2.6. Western-Blot Analysis
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
3.1. Effects of H2O2 and Melatonin on Cell Viability and MDA Content
3.2. The Effects of H2O2 or/and MLT on ROS, GSH, and SOD in SCs
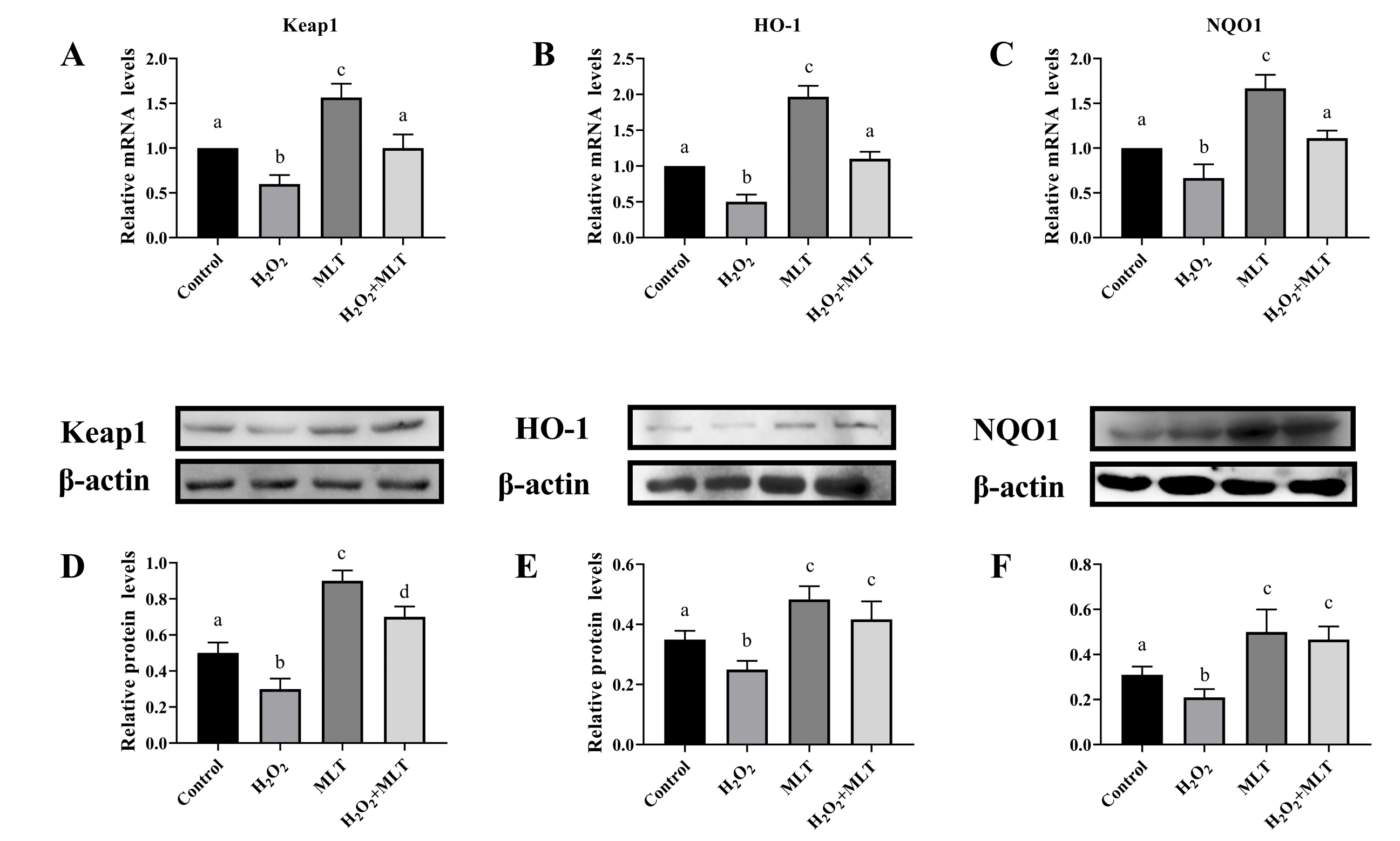
3.3. The Effect of H2O2 or/and MLT on the Keap1, HO-1, and NQO1
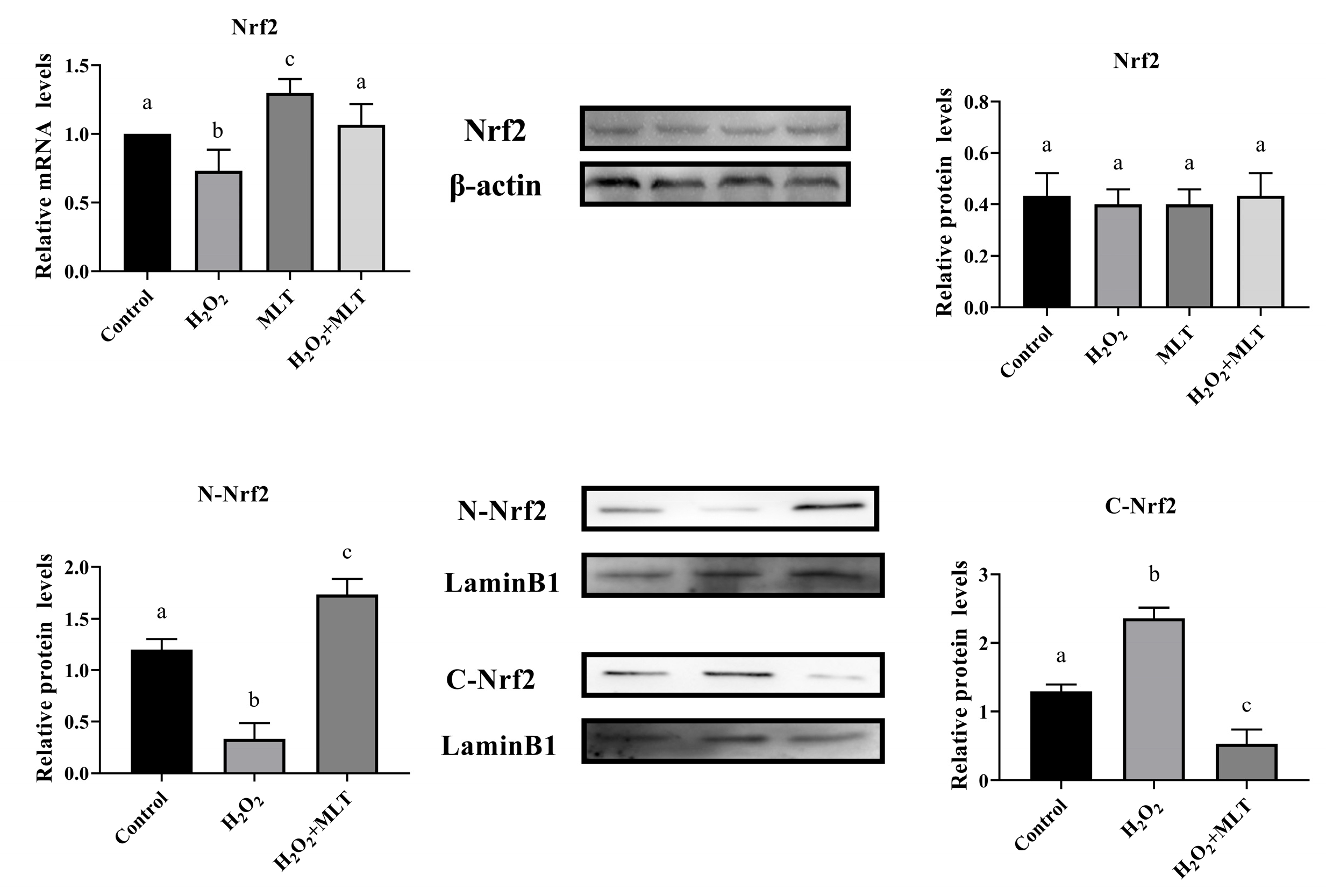
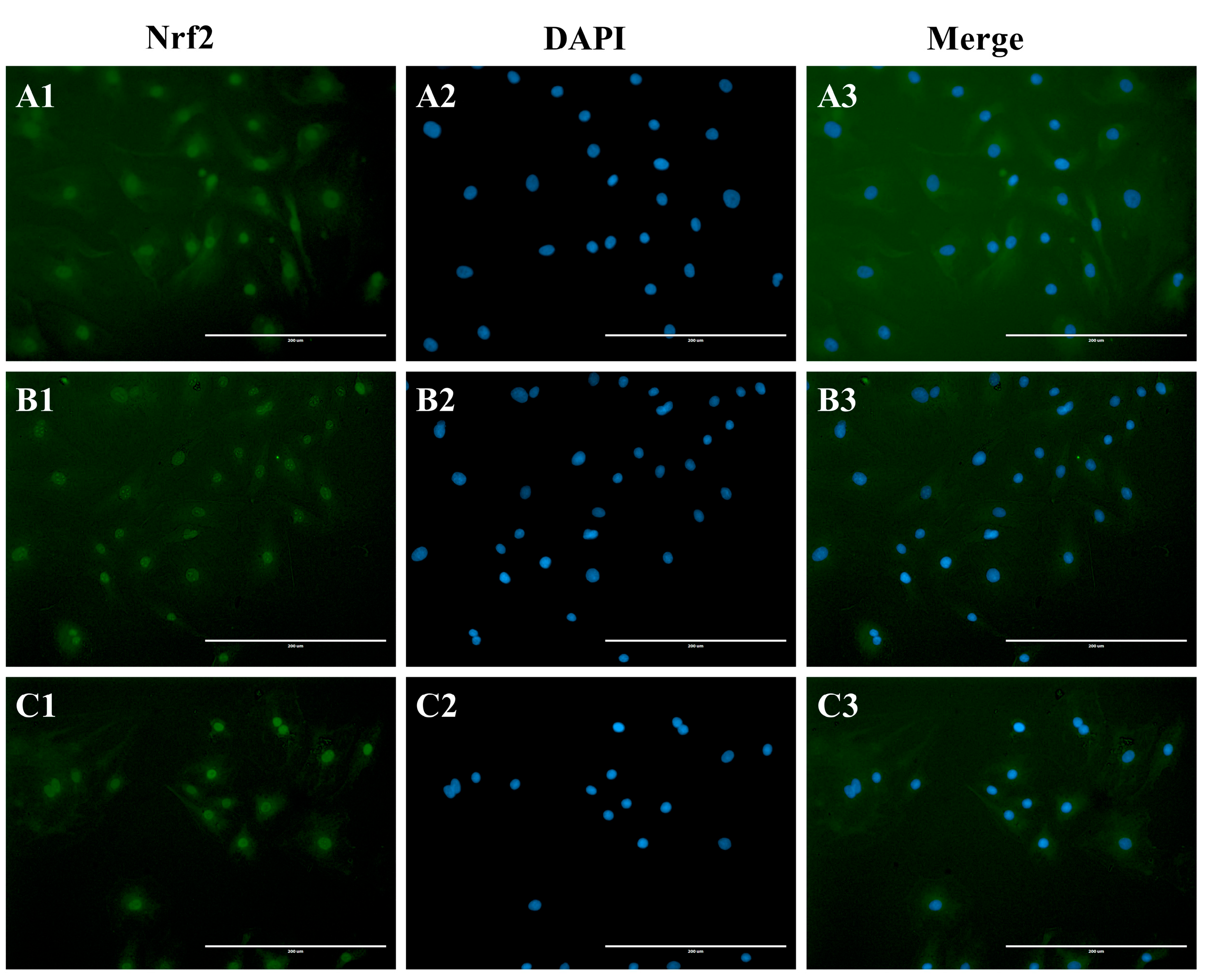
3.4. The Effect of H2O2 or/and MLT on Nrf2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orrenius, S.; Nicotera, P.; Zhivotovsky, B. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 2011, 119, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-X.; Cao, Q.-Q.; Zhang, C.-D.; Xu, T.-T.; Yue, K.; Li, Q.; Liu, F.; Wang, X.; Dong, H.-J.; Huang, S.-C.; et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol. Environ. Saf. 2022, 232, 113225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Liao, J.; Chen, K.; Javed, M.T.; Qiao, N.; Zeng, Q.; Liu, B.; Yi, J.; Tang, Z.; et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ. Sci. Pollut. Res. Int. 2021, 28, 55140–55153. [Google Scholar] [CrossRef] [PubMed]
- Escorcia, M.; Sánchez-Godoy, F.; Ramos-Vidales, D.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Effect of the Age and Body Weight of the Broiler Breeders Male on the Presentation of Oxidative Stress and Its Correlation with the Quality of Testicular Parenchyma and Physiological Antioxidant Levels. Vet. Sci. 2020, 7, 69. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Lin, R.; Pian, Y.; Zhang, C.; Zhou, L.; Ren, X. N-acetylcysteine alleviates cadmium-induced testicular interstitial cell apoptosis by activating protein kinase B pathway. Wei Sheng Yan Jiu 2022, 51, 632–637. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant Activities and Protective Effects of Dendropachol, a New Bisbibenzyl Compound from Dendrobium pachyglossum, on Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef]
- Oroojan, A.A.; Chenani, N.; An’aam, M. Antioxidant Effects of Eugenol on Oxidative Stress Induced by Hydrogen Peroxide in Islets of Langerhans Isolated from Male Mouse. Int. J. Hepatol. 2020, 2020, 5890378. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, P.; Adeniran, S.; Ma, M.; Huang, F.; Adegoke, E.; Zhang, G. Thyroid hormone (T3) is involved in inhibiting the proliferation of newborn calf Sertoli cells via the PI3K/Akt signaling pathway in vitro. Theriogenology 2019, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, G. A Sertoli cell-specific knock-out of the androgen receptor. Andrologia 2005, 37, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gao, D.; Zhang, J.; Li, C.; Wu, M.; Xiao, Y.; Yang, L.; Ma, T.; Wang, X.; Zhang, M.; et al. Age-related endoplasmic reticulum stress represses testosterone synthesis via attenuation of the circadian clock in Leydig cells. Theriogenology 2022, 189, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-F.; Li, Y.-K.; Ren, K.; Xie, Y.-J.; Yin, W.-D.; Mo, Z.-C. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis (Review). Mol. Med. Rep. 2018, 17, 705–713. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Y.; Tang, D.; Xu, X.; Zhu, X.; Wu, S.; Yu, H.; Cheng, H.; Luo, T.; Shen, Q.; et al. Activation of MT1/MT2 to Protect Testes and Leydig Cells against Cisplatin-Induced Oxidative Stress through the SIRT1/Nrf2 Signaling Pathway. Cells 2022, 11, 1690. [Google Scholar] [CrossRef]
- BBartolini, D.; Arato, I.; Mancuso, F.; Giustarini, D.; Bellucci, C.; Vacca, C.; Aglietti, M.C.; Stabile, A.M.; Rossi, R.; Cruciani, G.; et al. Melatonin modulates Nrf2 activity to protect porcine pre-pubertal Sertoli cells from the abnormal H2O2 generation and reductive stress effects of cadmium. J. Pineal Res. 2022, 73, e12806. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Lv, Y.; Zeng, W. Melatonin protects rabbit spermatozoa from cryo-damage via decreasing oxidative stress. Cryobiology 2019, 88, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Sun, B.-X.; Wang, D.-L.; Liu, Y.; Qi, J.-J.; Nie, X.-W.; Bai, C.-Y.; Zhang, J.-B.; Liang, S. Melatonin ameliorates cypermethrin-induced impairments by regulating oxidative stress, DNA damage and apoptosis in porcine Sertoli cells. Theriogenology 2021, 167, 67–76. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Zhu, Q.; Liu, H.; Wang, J.; Lu, W. Melatonin inhibits the apoptosis of rooster Leydig cells by suppressing oxidative stress via AKT-Nrf2 pathway activation. Free Radic. Biol. Med. 2020, 160, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Adeniran, S.O.; Huang, F.; Ma, M.; Zhang, H.; Li, X.; Zheng, P.; Zhang, G. Wilms’ tumour 1 (WT1) negatively regulates the expression of connexin 43 via a non-canonical Wnt signalling pathway in cultured bovine Sertoli cells. Reprod. Fertil. Dev. 2020, 32, 522–530. [Google Scholar] [CrossRef]
- Sakib, S.; Lara, N.d.L.e.M.; Huynh, B.C.; Dobrinski, I. Organotypic Rat Testicular Organoids for the Study of Testicular Maturation and Toxicology. Front. Endocrinol. 2022, 13, 892342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; You, X.; Zhu, T.; Gao, S.; Wang, Y.; Wang, R.; Yu, H.; Qian, B. Silica nanoparticles enhance germ cell apoptosis by inducing reactive oxygen species (ROS) formation in Caenorhabditis elegans. J. Toxicol. Sci. 2020, 45, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.-M.; Zheng, W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Yang, F.; Peng, S.; Wang, D.; Lai, X.; Su, B.; Xie, H. Adiponectin Attenuates Splenectomy-Induced Cognitive Deficits by Neuroinflammation and Oxidative Stress via TLR4/MyD88/NF-κb Signaling Pathway in Aged Rats. ACS Chem. Neurosci. 2023, 14, 1799–1809. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Y.; Chen, Y.; Li, B.; Wang, H.; Liu, S.; Adeniran, S.O.; Zheng, P. Melatonin Regulates the Expression of VEGF and HOXA10 in Bovine Endometrial Epithelial Cells through the SIRT1/PI3K/AKT Pathway. Animals 2024, 14, 2771. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, J.; Li, T.; Zhang, Q.; Bu, S.; Wang, Q.; Lai, D. Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-κB/iNOS and Nrf2/HO-1 signaling pathway. Sci. Rep. 2017, 7, 9599. [Google Scholar] [CrossRef]
- Pastorek, M.; Müller, P.; Vojtěšek, B. Nrf2—Two Faces of Antioxidant System Regulation. Klin. Onkol. 2015, 28 (Suppl. S2), 2S26–2S31. [Google Scholar] [CrossRef]
- Fan, Z.; Wirth, A.-K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2, INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Flodström, M.; Karlsen, A.E.; Welsh, N. The harmony of the spheres: Inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia 1996, 39, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Estaras, M.; Gonzalez-Portillo, M.R.; Martinez, R.; Garcia, A.; Estevez, M.; Fernandez-Bermejo, M.; Mateos, J.M.; Vara, D.; Blanco-Fernández, G.; Lopez-Guerra, D.; et al. Melatonin Modulates the Antioxidant Defenses and the Expression of Proinflammatory Mediators in Pancreatic Stellate Cells Subjected to Hypoxia. Antioxidants 2021, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Yu, M.; Yin, S.; Ji, Y.; Shi, T.; Li, N. Analysis of the Renal Protection and Antioxidative Stress Effects of Panax notoginseng Saponins in Diabetic Nephropathy Mice. J. Immunol. Res. 2022, 2022, 3610935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Wang, Y.; Wu, J.; Li, J. Higenamine promotes M2 macrophage activation and reduces Hmgb1 production through HO-1 induction in a murine model of spinal cord injury. Int. Immunopharmacol. 2014, 23, 681–687. [Google Scholar] [CrossRef]
- Qin, T.; Feng, D.; Zhou, B.; Bai, L.; Yin, Y. Melatonin Suppresses LPS-Induced Oxidative Stress in Dendritic Cells for Inflammatory Regulation via the Nrf2/HO-1 Axis. Antioxidants 2022, 11, 2012. [Google Scholar] [CrossRef]


| Genes | GenBank Accession No. | Primer Sequences (5′→3′) | Product Length/bp |
|---|---|---|---|
| NQO1 | NM_001098104.1 | F:CACTCTGCACTTCTGTGGCTTCC R:CAGGCGTTTCTTCCATCCTTCCAG | 278 |
| HO-1 | NM_001046332.1 | F:CCGCTACCTGGGAGACCTGTC R:ACTTGGTGGCACTGGCGATATTG | 202 |
| Nrf2 | NM_001205732.1 | F:TCAGCCAGCACAACACATACCATC R:ACGGGAATGTCTCTGCCAAAAGC | 112 |
| Keap1 | NM_001002763.1 | F:CGCCCTGGGAATTACCGTTCAC R:AACACTCCACGCTGTCCAAGAATG | 387 |
| β-actin | NM_173979.3 | F:GCGGCATTCACGAAACTACCTT R:TCCTGCTTGCTGATCCACATCT | 268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Wang, Z.; Chen, Y.; Wang, J.; Wang, H.; Li, B.; Liu, B.; Zheng, P. Melatonin Improves H2O2-Induced Oxidative Stress in Sertoli Cells Through Nrf2-Keap1 Signaling Pathway. Genes 2024, 15, 1544. https://doi.org/10.3390/genes15121544
Tang Y, Wang Z, Chen Y, Wang J, Wang H, Li B, Liu B, Zheng P. Melatonin Improves H2O2-Induced Oxidative Stress in Sertoli Cells Through Nrf2-Keap1 Signaling Pathway. Genes. 2024; 15(12):1544. https://doi.org/10.3390/genes15121544
Chicago/Turabian StyleTang, Ying, Ziming Wang, Yanru Chen, Junying Wang, Hongzhan Wang, Bo Li, Bojing Liu, and Peng Zheng. 2024. "Melatonin Improves H2O2-Induced Oxidative Stress in Sertoli Cells Through Nrf2-Keap1 Signaling Pathway" Genes 15, no. 12: 1544. https://doi.org/10.3390/genes15121544
APA StyleTang, Y., Wang, Z., Chen, Y., Wang, J., Wang, H., Li, B., Liu, B., & Zheng, P. (2024). Melatonin Improves H2O2-Induced Oxidative Stress in Sertoli Cells Through Nrf2-Keap1 Signaling Pathway. Genes, 15(12), 1544. https://doi.org/10.3390/genes15121544






