Loss of Heterozygosity (LOH) Affecting HLA Genes in Breast Cancer: Clinical Relevance and Therapeutic Opportunities
Abstract
1. Introduction
2. Importance of HLA Class-I Loss in Tumor Rejection and Immune Escape
3. HLA Class-I Alterations and Tumor Immunophenotypes in Breast Cancer
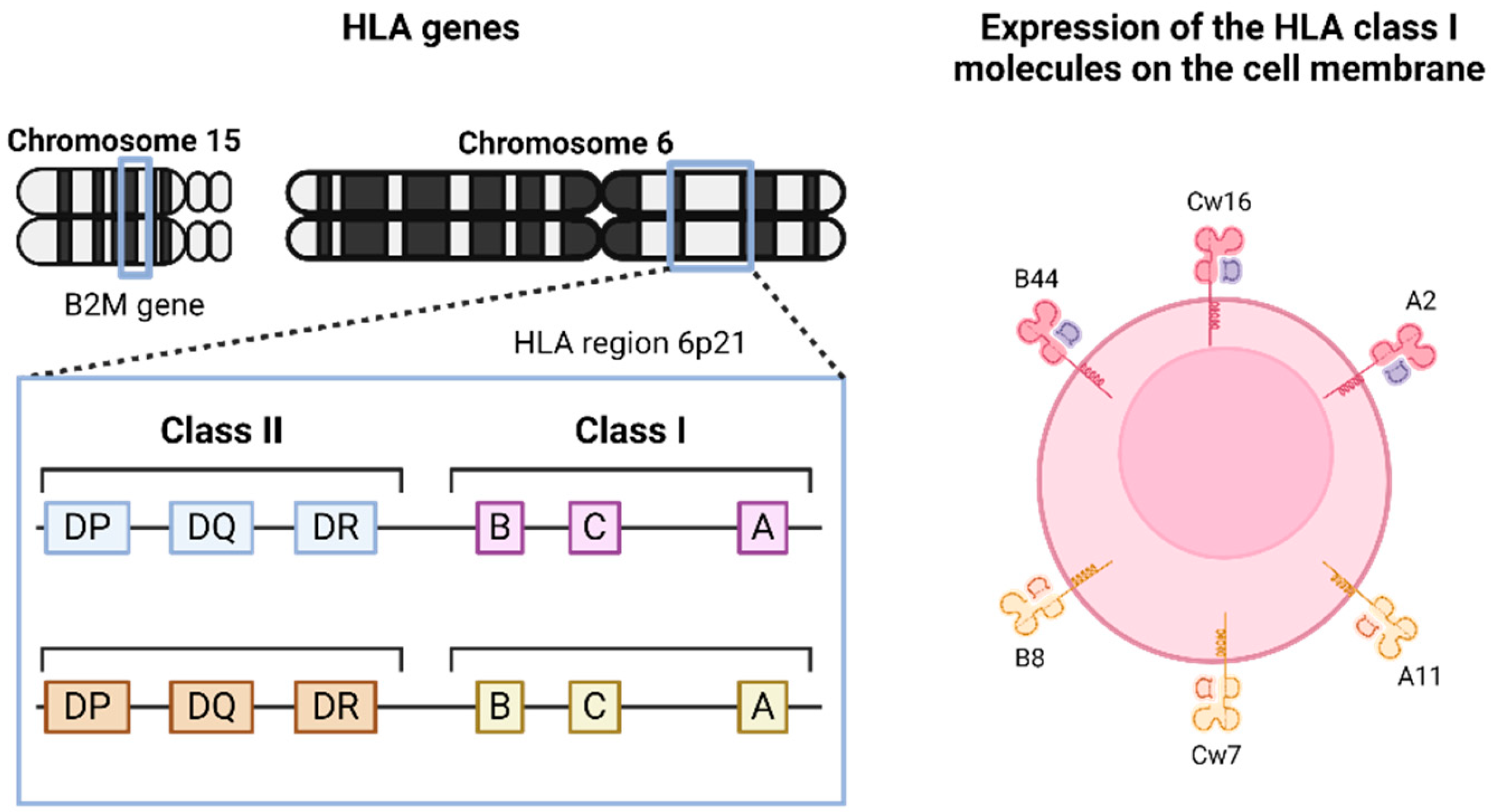
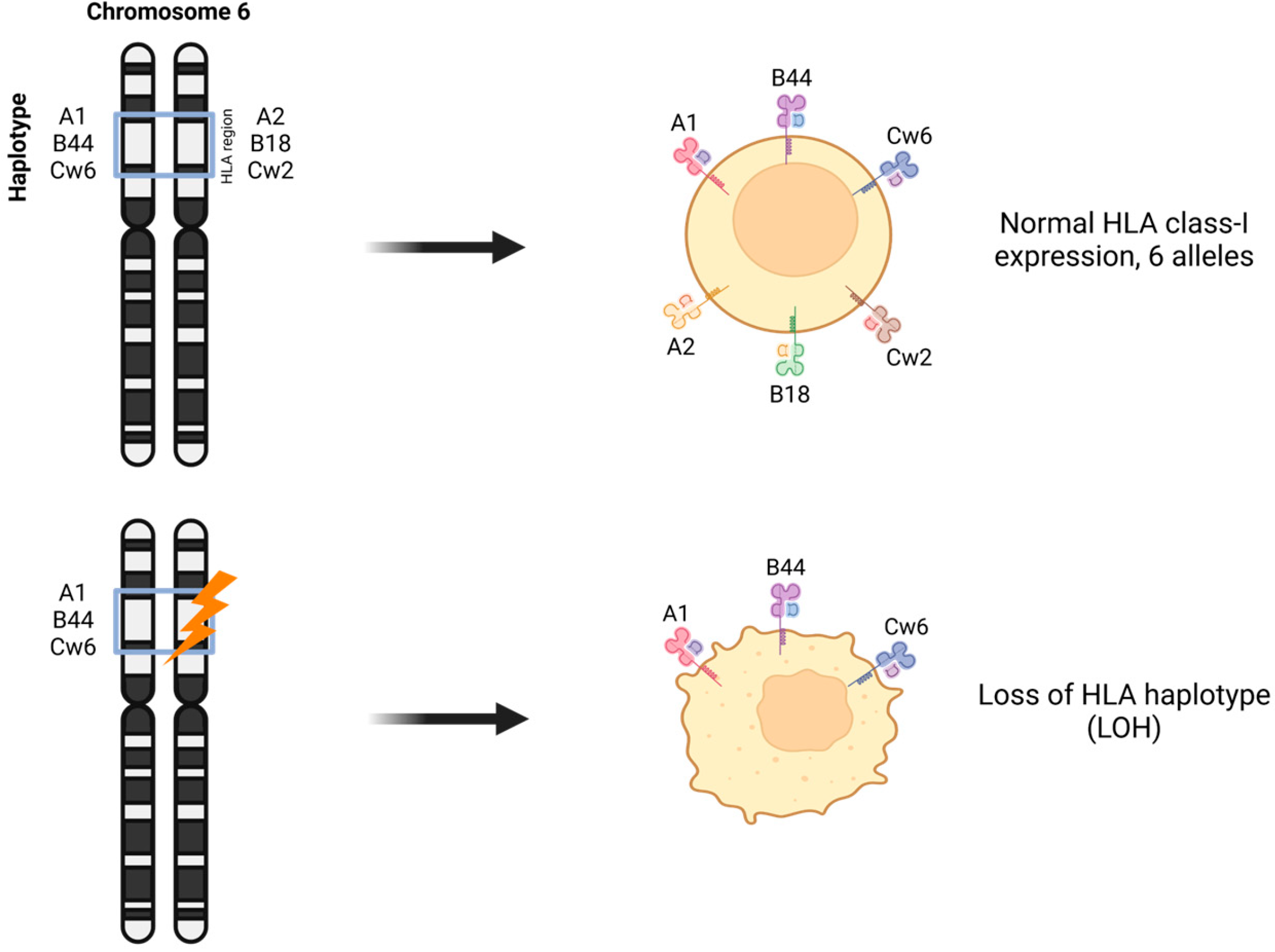
4. Chromosome Instability and LOH at Chromosome 6 in Breast Cancer and Other Malignancies
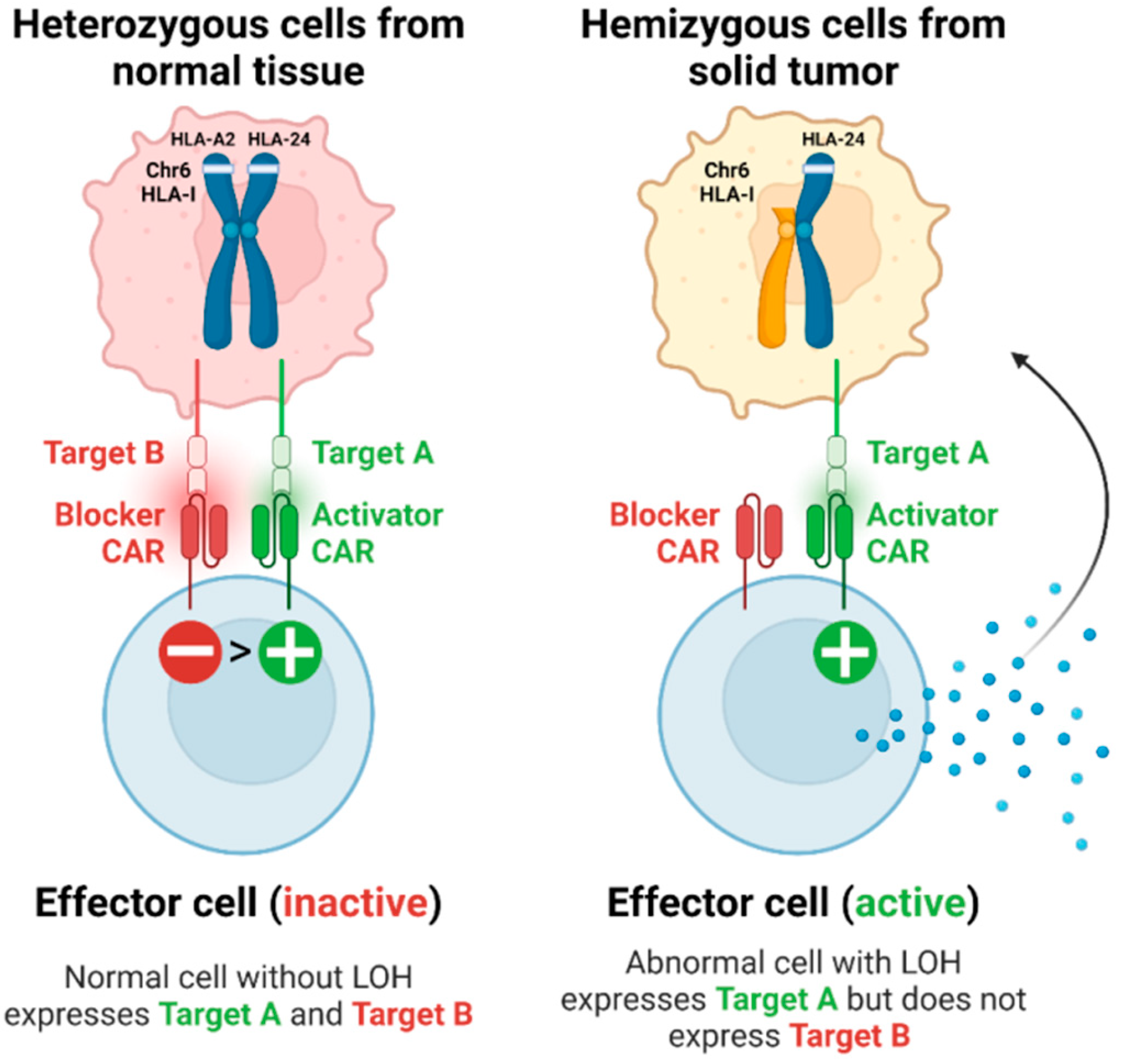
5. Therapeutic Opportunities for Targeting LOH-HLA in Tumor Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Garrido, F.; Ruiz-Cabello, F.; Cabrera, T.; Perez-Villar, J.J.; Lopez-Botet, M.; Duggan-Keen, M.; Stern, P.L. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol. Today 1997, 18, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Aptsiauri, N.; Carretero, R.; Garcia-Lora, A.; Real, L.M.; Cabrera, T.; Garrido, F. Regressing and progressing metastatic lesions: Resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol. Immunother. 2008, 57, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Maleno, I.; Aptsiauri, N.; Cabrera, T.; Gallego, A.; Paschen, A.; López-Nevot, M.A.; Garrido, F. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics 2010, 63, 65–71. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.K.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J.; et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 2017, 171, 1259–1271.e11. [Google Scholar] [CrossRef]
- Marincola, F.M.; Jaffee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of human solid tumors from T–cell recognition: Molecular mechanisms and functional significance. Adv. Immunol. 2000, 74, 181–273. [Google Scholar] [PubMed]
- Aptsiauri, N.; Garrido, F. The Challenges of HLA Class I Loss in Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2022, 28, 5021–5029. [Google Scholar] [CrossRef]
- Seliger, B.; Cabrera, T.; Garrido, F.; Ferrone, F. HLA class I antigen abnormalities and immune escape by malignant cells. Semin. Cancer Biol. 2002, 12, 3–13. [Google Scholar] [CrossRef]
- Flores-Martín, J.F.; Perea, F.; Exposito-Ruiz, M.; Carretero, F.J.; Rodriguez, T.; Villamediana, M.; Ruiz-Cabello, F.; Garrido, F.; Cózar-Olmo, J.M.; Aptsiauri, N. A Combination of Positive Tumor HLA-I and Negative PD-L1 Expression Provides an Immune Rejection Mechanism in Bladder Cancer. Ann. Surg. Oncol. 2019, 26, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, T.; Pedrajas, G.; Cozar, J.M.; Garrido, A.; Vicente, J.; Tallada, M.; Garrido, F. HLA class-I expresión in bladder carcinomas. Tissue Antigens 2003, 62, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Ishigami, S.; Kijima, Y.; Funasako, Y.; Hirata, M.; Okumura, H.; Shinchi, H.; Koriyama, C.; Ueno, S.; Yoshinaka, H.; et al. Clinical implication of HLA class I expression in breast cancer. BMC Cancer 2011, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Michos, G.D.; Xanthopoulou, E.; Koukourakis, M.I. HLA-class-I expression loss, tumor microenvironment and breast cancer prognosis. Cell. Immunol. 2024, 399–400, 104816. [Google Scholar] [CrossRef]
- Koopman, L.A.; Corver, W.E.; van der Slik, A.R.; Giphart, M.J.; Fleuren, G.J. Multiple genetic alterations cause frequent and hetero-geneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J. Exp. Med. 2000, 191, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Jordanova, E.S.; Kenter, G.G.; Ferrone, S.; Fleuren, G.J. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol. Immunother. 2007, 57, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.M.; Jiménez, P.; Cabrera, T.; Esparza, C.; Ruiz-Cabello, F.; Garrido, F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: Beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregu-lation in MSI-negative tumors. Tissue Antigens 2003, 61, 211–219. [Google Scholar] [PubMed]
- Kloor, M.; Michel, S.; von Knebel Doeberitz, M. Immune evasion of microsatelite unstable colorectal cancers. Int. J. Cancer 2010, 127, 1001–1010. [Google Scholar] [PubMed]
- So, T.; Takenoyama, M.; Mizukami, M.; Ichiki, Y.; Sugaya, M.; Hanagiri, T.; Sugio, K.; Yasumoto, K. Haplotype loss of HLA class I antigens as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005, 65, 5945–5952. [Google Scholar]
- Perea, F.; Bernal, M.; Sanchez-Palencia, A.; Carretero, J.; Torres, C.; Bayarri, C.; Gomez-Morales, G.F.; Ruiz- Cabello, F. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int. J. Cancer 2017, 140, 888–899. [Google Scholar]
- Paschen, A.; Méndez, R.M.; Jimenez, P.; Sucker, A.; Ruiz-Cabello, F.; Song, M.; Garrido, F.; Schadendorf, D. Complete loss of HLA class I antigen expression on melanoma cells: A result of successive mutational events. Int. J. Cancer 2002, 103, 759–767. [Google Scholar] [CrossRef]
- Chang, C.-C.; Pirozzi, G.; Wen, S.-H.; Chung, I.-H.; Chiu, B.-L.; Errico, S.; Luongo, M.; Lombardi, M.L.; Ferrone, S. Multiple structural and epigenetic defects in the human leukocyte antigen class I antigen presentation pathway in a recurrent metastatic melanoma following immunotherapy. J. Biol. Chem. 2015, 290, 26562–26575. [Google Scholar] [CrossRef]
- Hiraoka, N.; Ino, Y.; Hori, S.; Yamazaki-Itoh, R.; Naito, C.; Shimasaki, M.; Esaki, M.; Nara, S.; Kishi, Y.; Shimada, K.; et al. Expression of classical human leukocyte antigen class I antigens, HLA-E and HLA-G, is adversely prognostic in pancreatic cancer patients. Cancer Sci. 2020, 111, 3057–3070. [Google Scholar] [CrossRef] [PubMed]
- Ryschich, E.; Notzel, T.; Hinz, U.; Autschbach, F.; Ferguson, J.; Simon, I.; Weitz, J.; Frohlich, B.; Klar, E.; Buchler, M.; et al. Control of T cell mediated immune response by HLA class I in human pancreatic carcinoma. Clin. Cancer Res. 2005, 11, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Stoehr, R.; Handke, D.; Mueller, A.; Ferrone, S.; Wullich, B.; Tannapfel, A.; Hofstaedter, F.; Hartmann, A. Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol. Immunother. 2009, 59, 529–540. [Google Scholar] [CrossRef]
- Carretero, F.J.; del Campo, A.B.; Flores-Martín, J.F.; Mendez, R.; García-Lopez, C.; Cozar, J.M.; Adams, V.; Ward, S.; Cabrera, T.; Ruiz-Cabello, F.; et al. Frequent HLA class I alterations in human prostate cancer: Molecular mechanisms and clinical relevance. Cancer Immunol. Immunother. 2015, 65, 47–59. [Google Scholar] [CrossRef]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; LoPresti, J.S.; Epstein, A.L. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin. Cancer Res. 2014, 20, 6034–6044. [Google Scholar] [CrossRef] [PubMed]
- Challa-Malladi, M.; Lieu, Y.K.; Califano, O.; Holmes, A.B.; Bhagat, G.; Murty, V.V.; Dominguez-Sola, D.; Pasqualucci, L.; Dalla-Favera, R. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell 2011, 20, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Hamilton, R.; Ohnishi, K.; Ikeura, M.; Potter, D.; Nikiforova, M.; Ferrone, S.; Jakacki, R.; Pollack, I.; Okada, H. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin. Cancer Res. 2013, 19, 1816–1826. [Google Scholar]
- Beck, S.; Trowsdale, J. The human major histocompatibility complex: Lessons from the DNA sequence. Annu. Rev. Genom. Hum. Genet. 2000, 1, 117–137. [Google Scholar] [CrossRef]
- Garrido, F.; Algarra, I. MHC antigens and tumor escape from immune surveillance. Adv. Cancer Res. 2001, 83, 117–158. [Google Scholar]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Maeurer, M.J.; Ferrone, S. Antigen-processing machinery breakdown and tumor growth. Immunol. Today 2000, 21, 455–464. [Google Scholar] [CrossRef]
- Garrido, F.; Cabrera, T.; Aptsiauri, N. “Hard” and “Soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer 2010, 127, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Algarra, I.; García-Lora, A.M. The escape of cancer from T lymphocytes: Immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol. Immunother. 2010, 59, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Tanzarella, S.; Lionello, I.; Mendez, R.; Traversari, C.; Ruiz-Cabello, F.; Garrido, F. Reexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-20-deoxycytidine treatment. Int. J. Cancer 2001, 94, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.A.; Rodriguez, T.; Zinchenko, S.; Maleno, I.; Ruiz-Cabello, F.; Concha, A.; Olea, N.; Garrido, F.; Aptsiauri, N. HLA class I alterations in breast carcinoma are associated with a high frequency of the loss of heterozygosity at chromosomes 6 and 15. Immunogenetics 2018, 70, 647–659. [Google Scholar] [CrossRef]
- Bombonati, A.; Sgroi, D.C. The Molecular Pathology of Breast Cancer Progression. J. Pathol. 2010, 223, 308–318. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Zhou, E.; Chen, G.; Qian, K.; Wu, X.; Miao, X.; Tang, Z. Intratumoral CD8+ Cytotoxic Lymphocyte Is a Favorable Prognostic Marker in Node-Negative Breast Cancer. PLoS ONE 2014, 9, e95475. [Google Scholar] [CrossRef]
- Redondo, M.; García, J.; Villar, E.; Rodrigo, I.; Perea-Milla, E.; Serrano, A.; Morell, M. Major histocompatibility complex status in breast carcinogenesis and relationship to apoptosis. Hum. Pathol. 2003, 34, 1283–1289. [Google Scholar] [CrossRef]
- de Kruijf, E.M.; van Nes, J.G.; Sajet, A.; Tummers, Q.R.; Putter, H.; Osanto, S.; Speetjens, F.M.; Smit, V.T.; Liefers, G.J.; van de Velde, C.J.; et al. The Predictive Value of HLA Class I Tumor Cell Expression and Presence of Intratumoral Tregs for Chemotherapy in Patients with Early Breast Cancer. Clin. Cancer Res. 2010, 16, 1272–1280. [Google Scholar] [CrossRef]
- Dusenbery, A.C.; Maniaci, J.L.; Hillerson, N.D.; Dill, E.A.; Bullock, T.N.; Mills, A.M. MHC Class I Loss in Triple-negative Breast Cancer: A Potential Barrier to PD-1/PD-L1 Checkpoint Inhibitors. Am. J. Surg. Pathol. 2021, 45, 701–707. [Google Scholar] [PubMed]
- Steven, A.; Seliger, B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care 2018, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Critchley-Thornea, R.J.; Simonsa, D.L.; Yana, N.; Miyahiraa, A.K.; Dirbasc, F.M.; Johnsonc, D.L.; Swetterd, S.M.; Carlsone, R.W.; Fishere, G.A.; Koongf, A.; et al. Impaired interferon signaling is a common immuneedefect in human cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 9010–9015. [Google Scholar]
- Ding, X.-H.; Xiao, Y.; Chen, F.; Liu, C.-L.; Fu, T.; Shao, Z.-M.; Jiang, Y.-Z. The HLA-I landscape confers prognosis and antitumor immunity in breast cancer. Brief. Bioinform. 2024, 25, bbae151. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, S.; Wirtz, R.; Sütterlin, M.; Karic, U.; Schneeweiss, A.; Deutsch, T.M.; Wallwiener, M. Cut-off analysis of HLA-A and HLA-B/C expression as a potential prognosticator of favorable survival in patients with meta-static breast cancer. Anticancer. Res. 2023, 43, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.H.; Hood, B.L.; Beck, H.C.; Conrads, T.P.; Ditzel, H.J.; Leth-Larsen, R. Downregulation of antigen presentation-associated pathway proteins is linked to poor outcome in triple-negative breast cancer patient tumors. OncoImmunology 2017, 6, e1305531. [Google Scholar] [CrossRef] [PubMed]
- Hammerl, D.; Smid, M.; Timmermans, A.; Sleijfer, S.; Martens, J.; Debets, R. Breast cancer genomics and immuno-oncological markers to guide immune therapies. Semin. Cancer 2017, 52, 178–188. [Google Scholar] [CrossRef]
- Han, S.-H.; Kim, M.; Chung, Y.R.; Woo, J.W.; Choi, H.Y.; Park, S.Y. Expression of HLA class I is associated with immune cell infiltration and patient outcome in breast cancer. Sci. Rep. 2022, 27, 20367. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Kirtane, K.; John, M.S.; Fuentes-Bayne, H.; Patel, S.P.; Mardiros, A.; Xu, H.; Ng, E.W.; Go, W.Y.; Wong, D.J.; Sunwoo, J.B.; et al. Genomic Immune Evasion: Diagnostic and Therapeutic Opportunities in Head and Neck Squamous Cell Carcinomas. J. Clin. Med. 2022, 11, 7259. [Google Scholar] [CrossRef]
- Chowell, D.; Yoo, S.-K.; Valero, C.; Pastore, A.; Krishna, C.; Lee, M.; Hoen, D.; Shi, H.; Kelly, D.W.; Patel, N.; et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat. Biotechnol. 2021, 40, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer im-munotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Kim, H.S.; Cha, H.; Kim, S.; Kim, T.M.; Anagnostou, V.; Choi, Y.L.; Jung, H.A.; Sun, J.M.; Ahn, J.S.; et al. HLA-corrected tumor mutation burden and ho-mologous recombination deficiency for the prediction of response to PD-(L)1 blockade in advanced non-small-cell lung cancer patients. Ann. Oncol. 2020, 31, 902–911. [Google Scholar] [PubMed]
- Hendrickx, W.; Simeone, I.; Anjum, S.; Mokrab, Y.; Bertucci, F.; Finetti, P.; Curigliano, G.; Seliger, B.; Cerulo, L.; Tomei, S.; et al. Identification of genetic determinants of breast cancer immune phenotypes by inte-grative genome-scale analysis. Oncoimmunology 2017, 6, e1253654. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Cabrera, T.; Gil, H.; Saenz-Lopez, P.; Maleno, I.; Aptsiauri, N.; Cozar, J.M.; Garrido, F. Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int. J. Cancer 2010, 129, 839–846. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. N Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. New Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef]
- Kumar, Y.; Yang, J.; Hu, T.; Chen, L.; Xu, Z.; Xu, L.; Hu, X.-X.; Tang, G.; Wang, J.-M.; Li, Y.; et al. Massive interstitial copy-neutral loss-of-heterozygosity as evidence for cancer being a disease of the DNA-damage response. BMC Med. Genom. 2015, 8, 42. [Google Scholar] [CrossRef][Green Version]
- Thiagalingam, S.; Laken, S.; Willson, J.K.V.; Markowitz, S.D.; Kinzler, K.W.; Vogelstein, B.; Lengauer, C. Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc. Natl. Acad. Sci. USA 2001, 98, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Maleno, I.; López-Nevot, M.; Cabrera, T.; Salinero, J.; Garrido, F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: High frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol. Immunother. 2002, 51, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Maleno, I.; Cabrera, C.M.; Cabrera, T.; Paco, L.; Lopez-Nevot, M.A.; Collado, A.; Ferron, A.; Garrido, F. Distribution of HLA class I altered phenotypes in colorectal carcinomas: High frequency of HLA haplotype loss associated with loss of heterozygosity in chro-mosome region 6p21. Immunogenetics 2004, 56, 244–253. [Google Scholar] [PubMed]
- Maleno, I.; Romero, J.M.; Cabrera, T.; Paco, L.; Aptsiauri, N.; Cozar, J.M.; Tallada, M.; López-Nevot, M.A.; Garrido, F. LOH at 6p21.3 region and HLA class altered phenotypes in bladder carcinomas. Immunogenetics 2006, 58, 503–510. [Google Scholar] [CrossRef]
- Garrido, M.A.; Perea, F.; Vilchez, J.R.; Rodríguez, T.; Anderson, P.; Garrido, F.; Ruiz-Cabello, F.; Aptsiauri, N. Copy Neutral LOH Affecting the Entire Chromosome 6 Is a Frequent Mechanism of HLA Class I Alterations in Cancer. Cancers 2021, 13, 5046. [Google Scholar] [CrossRef] [PubMed]
- Puttick, C.; Jones, T.P.; Leung, M.M.; Galvez-Cancino, F.; Liu, J.; Varas-Godoy, M.; Rowan, A.; Pich, O.; Martinez-Ruiz, C.; Bentham, R.; et al. MHC Hammer reveals genetic and non-genetic HLA disruption in cancer evolution. Nat. Genet. 2024, 56, 2121–2131. [Google Scholar] [CrossRef]
- Maleno, I.; Lopez-Nevot, M.A.; Seliger, B.; Garrido, F. Low frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21 in clear renal cell carcinomas. Int. J. Cancer 2004, 109, 636–638. [Google Scholar]
- Montesion, M.; Murugesan, K.; Jin, D.X.; Sharaf, R.; Sanchez, N.; Guria, A.; Minker, M.; Li, G.; Fisher, V.; Sokol, E.S.; et al. Somatic HLA Class I Loss Is a Widespread Mechanism of Immune Evasion Which Refines the Use of Tumor Mutational Burden as a Biomarker of Checkpoint Inhibitor Response. Cancer Discov. 2021, 11, 282–292. [Google Scholar] [CrossRef]
- Chhibber, A.; Huang, L.; Zhang, H.; Xu, J.; Cristescu, R.; Liu, X.; Mehrotra, D.V.; Shen, J.; Shaw, P.M.; Hellmann, M.D.; et al. Germline HLA landscape does not predict efficacy of pembrolizumab monotherapy across solid tumor types. Immunity 2022, 55, 56–64.e4. [Google Scholar] [CrossRef]
- Tamaki, T.; Karube, K.; Sakihama, S.; Tsuruta, Y.; Awazawa, R.; Hayashi, M.; Nakada, N.; Matsumoto, H.; Yagi, N.; Ohshiro, K.; et al. A Comprehensive Study of the Immunophenotype and its Clinicopathologic Significance in Adult T-Cell Leuke-mia/Lymphoma. Mod. Pathol. 2023, 36, 100169. [Google Scholar] [CrossRef]
- Demanet, C.; Mulder, A.; Deneys, V.; Worsham, M.J.; Maes, P.; Claas, F.H.; Ferrone, S. Down-regulation of HLA-A and HLA-Bw6 but not HLA-Bw4 allospecificities in leukemic cells: An escape mechanism from CTLs and NK attack. Blood 2004, 103, 3122–3130. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.; Luo, H.; Lu, H.; Pan, C.; Yu, H.; Zhang, L.; Guan, Y.; Yu, L.; Chu, H.; et al. Integrated investigation of the prognostic role of HLA LOH in advanced lung cancer patients with immunotherapy. Front. Genet. 2022, 13, 1066636. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Xiao, Y.; Jin, X.; Di, G.-H.; Jiang, Y.-Z.; Shao, Z.-M. Integrated analysis reveals prognostic value of HLA-I LOH in triple-negative breast cancer. J. Immunother. Cancer 2021, 9, e003371. [Google Scholar] [CrossRef] [PubMed]
- Sammut, S.J.; Crispin-Ortuzar, M.; Chin, S.F.; Provenzano, E.; Bardwell, H.A.; Ma, W.; Cope, W.; Dariush, A.; Dawson, S.J.; Abraham, J.E. Multi-omic machine learning predictor of breast cancer therapy response. Nature 2022, 601, 623–629. [Google Scholar] [CrossRef]
- Aptsiauri, N.; Ruiz-Cabello, F.; Garrido, F. The transition from HLA-I positive to HLA-I negative primary tumors: The road to escape from T-cell responses. Curr. Opin. Immunol. 2018, 51, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Ocón, A.; Cabo-Zabala, L.; Brinkmann, B.; López-Sánchez, A.; Paschen, A.; Garrido, F.; Ruiz-Cabello, F.; Aptsiauri, N. Liquid biopsy for the detection of HLA-I alterations in cancer. HLA 2022, 99, 430. [Google Scholar]
- Nichols, C.A.; Gibson, W.J.; Brown, M.S.; Kosmicki, J.A.; Busanovich, J.P.; Wei, H.; Urbanski, L.M.; Curimjee, N.; Berger, A.C.; Gao, G.F.; et al. Loss of heterozygosity of essential genes represents a widespread class of potential cancer vulnerabilities. Nat. Commun. 2020, 11, 2517. [Google Scholar] [CrossRef]
- Hwang, M.S.; Mog, B.J.; Douglass, J.; Pearlman, A.H.; Hsiue, E.H.; Paul, S.; DiNapoli, S.R.; Konig, M.F.; Pardoll, D.M.; Gabelli, S.B.; et al. Targeting loss of heterozygosity for cancer-specific immunotherapy. Proc. Natl. Acad. Sci. USA 2021, 118, e2022410118. [Google Scholar] [CrossRef]
- Hamburger, A.E.; DiAndreth, B.; Cui, J.; Daris, M.E.; Munguia, M.L.; Deshmukh, K.; Mock, J.-Y.; Asuelime, G.E.; Lim, E.D.; Kreke, M.R.; et al. Engineered T cells directed at tumors with defined allelic loss. Mol. Immunol. 2020, 128, 298–310. [Google Scholar] [CrossRef]
- Tokatlian, T.; Asuelime, G.E.; Mock, J.-Y.; DiAndreth, B.; Sharma, S.; Warshaviak, D.T.; E Daris, M.; Bolanos, K.; Luna, B.L.; Naradikian, M.S.; et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J. Immunother. Cancer 2022, 10, e003826. [Google Scholar] [CrossRef]
- Sandberg, M.L.; Wang, X.; Martin, A.D.; Nampe, D.P.; Gabrelow, G.B.; Li, C.Z.; McElvain, M.E.; Lee, W.-H.; Shafaattalab, S.; Martire, S.; et al. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci. Transl. Med. 2022, 14, eabm0306. [Google Scholar] [CrossRef] [PubMed]
- Bassan, D.; Weinberger, L.; Yi, J.; Kim, T.; Weist, M.R.; Adams, G.B.; Foord, O.; Chaim, N.; Tabak, S.; Bujanover, N.; et al. HER2 and HLA-A*02 dual CAR-T cells utilize LOH in a NOT logic gate to address on-target off-tumor toxicity. J. Immunother. Cancer 2023, 11, e007426. [Google Scholar] [CrossRef]
- Messaoudene, M.; Mourikis, T.; Michels, J.; Fu, Y.; Bonvalet, M.; Lacroix-Trikki, M.; Routy, B.; Fluckiger, A.; Rusakiewicz, S.; Roberti, M.; et al. T-cell bispecific antibodies in node-positive breast cancer: Novel therapeutic avenue for MHC class I loss variants. Ann. Oncol. 2019, 30, 934–944. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, M.A.; Navarro-Ocón, A.; Ronco-Díaz, V.; Olea, N.; Aptsiauri, N. Loss of Heterozygosity (LOH) Affecting HLA Genes in Breast Cancer: Clinical Relevance and Therapeutic Opportunities. Genes 2024, 15, 1542. https://doi.org/10.3390/genes15121542
Garrido MA, Navarro-Ocón A, Ronco-Díaz V, Olea N, Aptsiauri N. Loss of Heterozygosity (LOH) Affecting HLA Genes in Breast Cancer: Clinical Relevance and Therapeutic Opportunities. Genes. 2024; 15(12):1542. https://doi.org/10.3390/genes15121542
Chicago/Turabian StyleGarrido, María Antonia, Alba Navarro-Ocón, Víctor Ronco-Díaz, Nicolás Olea, and Natalia Aptsiauri. 2024. "Loss of Heterozygosity (LOH) Affecting HLA Genes in Breast Cancer: Clinical Relevance and Therapeutic Opportunities" Genes 15, no. 12: 1542. https://doi.org/10.3390/genes15121542
APA StyleGarrido, M. A., Navarro-Ocón, A., Ronco-Díaz, V., Olea, N., & Aptsiauri, N. (2024). Loss of Heterozygosity (LOH) Affecting HLA Genes in Breast Cancer: Clinical Relevance and Therapeutic Opportunities. Genes, 15(12), 1542. https://doi.org/10.3390/genes15121542