Hotspots for Disease-Causing Mutations in the Mitochondrial TIM23 Import Complex
Abstract
1. Introduction
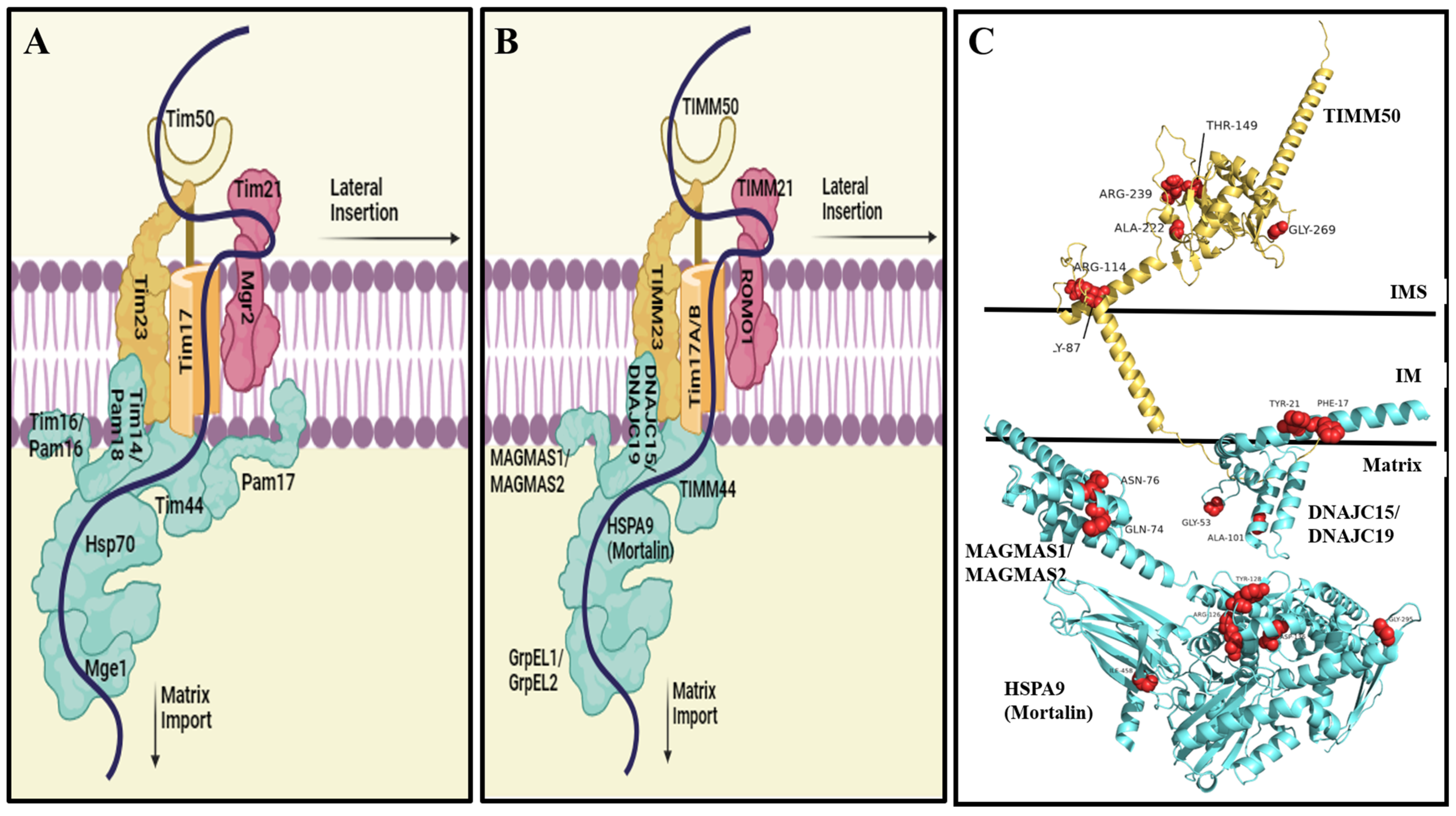
2. The Yeast TIM23 and Human TIMM23 Complexes
- In humans, due to the presence of isoforms of Tim17 and DnaJC (the homolog of yeast Tim14/Pam18), three TIMM23MOTOR complex formations are presently known, namely, (i) translocase A consisting of Tim17a and DnaJC15, (ii) translocase B1 consisting of Tim17b1 and DnaJC19, and (iii) translocase B2 consisting of Tim17b2 and DnaJC19 [49,50]. Similar to the yeast complex, translocases B1 and B2 are expected to play major roles in protein import via the presequence pathway [50]. Interestingly, high expression of Tim17A mRNA has been reported in patients suffering from breast cancer [68,69]. Such elevated expression is closely linked to the aggressive growth of cancerous cells and adverse pathological and clinical outcomes [68,69]. Therefore, Tim17A is thought to be a prognostic biomarker for human breast cancer and a potential target for therapeutic developments.
- Interestingly, no homolog of Pam17 has yet been identified in humans. As discussed above, Pam17 plays a supportive role within the PAM complex. Although not essential, Pam17 is crucial for optimizing the activity of the import motor. Therefore, it would be worthwhile to identify those human subunits that fulfill the same function.
- In yeast, Tim50 contains an extra, so-called presequence-binding domain (PBD) at its C-terminus (residues 395 to 476) thought to contribute to four major functions: (i) interaction with Tom22IMS [22]; (ii) interaction with the presequence [71]; (iii) interaction with the conserved core domain of Tim50 (residues 164 to 361) [72]; and (iv) interaction with Tim21IMS [73]. This suggests that Tim50 plays a highly dynamic and intricate role, especially the PBD, in IM and matrix protein import. However, in humans, Timm50 lacks the PBD. It is thus not surprising that human Timm50 was unable to complement its yeast homolog [74].
3. Genetic Variants in the TIM23 Complex
3.1. Genetic Diseases Associated with Tim50
3.2. Genetic Diseases Associated with mHsp70
3.3. Genetic Diseases Associated with Tim14 and Tim16
4. Future Perspectives
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Sinha, D. The complexities of human mitochondrial inner-membrane protein translocases in the maintenance of organeller function. Proc. Indian Natl. Sci. Acad. 2017, 83, 877–891. [Google Scholar]
- Shoshan-Barmatz, V.; Gincel, D. The voltage-dependent anion channel: Characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem. Biophys. 2003, 39, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Chacinska, A.; Koehler, C.M.; Milenkovic, D.; Lithgow, T.; Pfanner, N. Importing mitochondrial proteins: Machineries and mechanisms. Cell 2009, 138, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Prosdocimi, E.; Carrer, A.; Checchetto, V.; Szabò, I. Mitochondrial ion channels of the inner membrane and their regulation in cell death signaling. Front. Cell Dev. Biol. 2021, 8, 620081. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Montoya, J.; Pacheu-Grau, D. Molecular insights into mitochondrial protein translocation and human disease. Genes 2021, 12, 1031. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, M.I.; Falkenberg, M.; Rantanen, A.; Park, C.B.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.M.; Larsson, N.-G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.F.; Gray, M.W.; Burger, G. Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet. 1999, 33, 351–397. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Stiller, S.B.; Lübbert, P.; Peikert, C.D.; Dannenmaier, S.; Drepper, F.; Weill, U.; Höß, P.; Feuerstein, R.; Gebert, M. Definition of a high-confidence mitochondrial proteome at quantitative scale. Cell Rep. 2017, 19, 2836–2852. [Google Scholar] [CrossRef] [PubMed]
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2. 0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Robinson, A.J. MitoMiner v3. 1, an update on the mitochondrial proteomics database. Nucleic Acids Res. 2016, 44, D1258–D1261. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.; Burkhart, J.M.; Gonczarowska-Jorge, H.; Kücükköse, C.; Taskin, A.A.; Kopczynski, D.; Ahrends, R.; Mossmann, D.; Sickmann, A.; Zahedi, R.P. Landscape of submitochondrial protein distribution. Nat. Commun. 2017, 8, 290. [Google Scholar] [CrossRef]
- Chaudhuri, M.; Tripathi, A.; Gonzalez, F.S. Diverse functions of Tim50, a component of the mitochondrial inner membrane protein translocase. Int. J. Mol. Sci. 2021, 22, 7779. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Lill, R. Function and biogenesis of iron–sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.W.; Burger, G.; Lang, B.F. Mitochondrial evolution. Science 1999, 283, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Ordureau, A.; Heo, J.-M. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; García-Sáez, A.J. Bax and Bak pores: Are we closing the circle? Trends Cell Biol. 2017, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Zhou, C.; Jin, E.; Kucharavy, A.; Zhang, Y.; Wen, Z.; Florens, L.; Li, R. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017, 543, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Schulte, U.; den Brave, F.; Haupt, A.; Gupta, A.; Song, J.; Müller, C.S.; Engelke, J.; Mishra, S.; Mårtensson, C.; Ellenrieder, L.; et al. Mitochondrial complexome reveals quality-control pathways of protein import. Nature 2023, 614, 153–159. [Google Scholar] [CrossRef]
- Waegemann, K.; Popov-Čeleketić, D.; Neupert, W.; Azem, A.; Mokranjac, D. Cooperation of TOM and TIM23 complexes during translocation of proteins into mitochondria. J. Mol. Biol. 2015, 427, 1075–1084. [Google Scholar] [CrossRef]
- Dekker, P.J.; Ryan, M.T.; Brix, J.; Muller, H.; Honlinger, A.; Pfanner, N. Preprotein translocase of the outer mitochondrial membrane: Molecular dissection and assembly of the general import pore complex. Mol. Cell. Biol. 1998, 18, 6515–6524. [Google Scholar] [CrossRef]
- Kozjak, V.; Wiedemann, N.; Milenkovic, D.; Lohaus, C.; Meyer, H.E.; Guiard, B.; Meisinger, C.; Pfanner, N. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J. Biol. Chem. 2003, 278, 48520–48523. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Pfannschmidt, S.; Guiard, B.; Stojanovski, D.; Milenkovic, D.; Kutik, S.; Pfanner, N.; Meisinger, C.; Wiedemann, N. Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors. J. Biol. Chem. 2008, 283, 120–127. [Google Scholar] [CrossRef]
- Sirrenberg, C.; Bauer, M.F.; Guiard, B.; Neupert, W.; Brunner, M. Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 1996, 384, 582–585. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.A.; Heimes, M.; Sinning, I. Structural and molecular mechanisms for membrane protein biogenesis by the Oxa1 superfamily. Nat. Struct. Mol. Biol. 2021, 28, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Brix, J.; Dietmeier, K.; Pfanner, N. Differential recognition of preproteins by the purified cytosolic domains of the mitochondrial import receptors Tom20, Tom22, and Tom70. J. Biol. Chem. 1997, 272, 20730–20735. [Google Scholar] [CrossRef] [PubMed]
- Shiota, T.; Mabuchi, H.; Tanaka-Yamano, S.; Yamano, K.; Endo, T. In vivo protein-interaction mapping of a mitochondrial translocator protein Tom22 at work. Proc. Natl. Acad. Sci. USA 2011, 108, 15179–15183. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J.; Rehling, P.; van der Laan, M. Mitochondrial protein import: Common principles and physiological networks. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Dukanovic, J.; Dimmer, K.S.; Bonnefoy, N.; Krumpe, K.; Rapaport, D. Genetic and functional interactions between the mitochondrial outer membrane proteins Tom6 and Sam37. Mol. Cell. Biol. 2009, 29, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Guiard, B.; Thornton, N.; Zufall, N.; Stroud, D.A.; Wiedemann, N.; Pfanner, N. Assembly of the mitochondrial protein import channel: Role of Tom5 in two-stage interaction of Tom40 with the SAM complex. Mol. Biol. Cell 2010, 21, 3106–3113. [Google Scholar] [CrossRef]
- Stojanovski, D.; Guiard, B.; Kozjak-Pavlovic, V.; Pfanner, N.; Meisinger, C. Alternative function for the mitochondrial SAM complex in biogenesis of α-helical TOM proteins. J. Cell Biol. 2007, 179, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Doan, K.N.; Grevel, A.; Mårtensson, C.U.; Ellenrieder, L.; Thornton, N.; Wenz, L.-S.; Opaliński, Ł.; Guiard, B.; Pfanner, N.; Becker, T. The mitochondrial import complex MIM functions as main translocase for α-helical outer membrane proteins. Cell Rep. 2020, 31, 107567. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Ramming, T.; Müller, J.M.; Wenz, L.-S.; Gebert, N.; Schulze-Specking, A.; Stojanovski, D.; Rospert, S.; Chacinska, A. Identification of the signal directing Tim9 and Tim10 into the intermembrane space of mitochondria. Mol. Biol. Cell 2009, 20, 2530–2539. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Q.; Guan, Z.; Wu, Y.; Shen, C.; Hong, S.; Cao, J.; Zhang, X.; Yan, C.; Yin, P. Cryo-EM structure of the human mitochondrial translocase TIM22 complex. Cell Res. 2021, 31, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Stuart, R.A. Insertion of proteins into the inner membrane of mitochondria: The role of the Oxa1 complex. Biochim. Biophys. Acta Mol. Cell Res. 2002, 1592, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, N.; Fiumera, H.L.; Dujardin, G.; Fox, T.D. Roles of Oxa1-related inner-membrane translocases in assembly of respiratory chain complexes. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Szyrach, G.; Ott, M.; Bonnefoy, N.; Neupert, W.; Herrmann, J.M. Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 2003, 22, 6448–6457. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.-N.; Wortelkamp, S.; Zahedi, R.P.; Becker, D.; Leidhold, C.; Gevaert, K.; Kellermann, J.; Voos, W.; Sickmann, A.; Pfanner, N. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 2009, 139, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Chacinska, A.; Lind, M.; Frazier, A.E.; Dudek, J.; Meisinger, C.; Geissler, A.; Sickmann, A.; Meyer, H.E.; Truscott, K.N.; Guiard, B. Mitochondrial presequence translocase: Switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 2005, 120, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.S.; Brandt, A.; Cunningham, K.; Müller, S.; Hallberg, R.L.; Schatz, G. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell 1992, 69, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Hutu, D.P.; Guiard, B.; Chacinska, A.; Becker, D.; Pfanner, N.; Rehling, P.; Van Der Laan, M. Mitochondrial protein import motor: Differential role of Tim44 in the recruitment of Pam17 and J-complex to the presequence translocase. Mol. Biol. Cell 2008, 19, 2642–2649. [Google Scholar] [CrossRef]
- Pfanner, N.; Geissler, A. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2001, 2, 339–349. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, M.; Hutu, D.P.; Rehling, P. On the mechanism of preprotein import by the mitochondrial presequence translocase. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 732–739. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, M.; Meinecke, M.; Dudek, J.; Hutu, D.P.; Lind, M.; Perschil, I.; Guiard, B.; Wagner, R.; Pfanner, N.; Rehling, P. Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat. Cell Biol. 2007, 9, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Esaki, M.; Kanamori, T.; Tamura, Y.; Nishikawa, S.-I.; Endo, T. Tim50 is a subunit of the TIM23 complex that links protein translocation across the outer and inner mitochondrial membranes. Cell 2002, 111, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gevorkyan-Airapetov, L.; Zohary, K.; Popov-Čeleketić, D.; Mapa, K.; Hell, K.; Neupert, W.; Azem, A.; Mokranjac, D. Interaction of Tim23 with Tim50 is essential for protein translocation by the mitochondrial TIM23 complex. J. Biol. Chem. 2009, 284, 4865–4872. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.F.; Gempel, K.; Reichert, A.S.; Rappold, G.A.; Lichtner, P.; Gerbitz, K.-D.; Neupert, W.; Brunner, M.; Hofmann, S. Genetic and structural characterization of the human mitochondrial inner membrane translocase. J. Mol. Biol. 1999, 289, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Srivastava, S.; Krishna, L.; D’Silva, P. Unraveling the intricate organization of mammalian mitochondrial presequence translocases: Existence of multiple translocases for maintenance of mitochondrial function. Mol. Cell. Biol. 2014, 34, 1757–1775. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Karnezis, A.N.; Murphy, S.P.; Hoang, T.; Freeman, B.C.; Phillips, B.; Morimoto, R.I. Cloning and Subcellular Localization of Human Mitochondrial hsp70 (∗). J. Biol. Chem. 1995, 270, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.C.; Fischer, H.; Craievich, A.F.; Hansen, L.D.; Ramos, C.H. Free human mitochondrial GrpE is a symmetric dimer in solution. J. Biol. Chem. 2003, 278, 35337–35344. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Joshi, N.; Chittoor, B.; Samji, P.; D’Silva, P. Role of Magmas in protein transport and human mitochondria biogenesis. Hum. Mol. Genet. 2010, 19, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Mick, D.U.; Dennerlein, S.; Wiese, H.; Reinhold, R.; Pacheu-Grau, D.; Lorenzi, I.; Sasarman, F.; Weraarpachai, W.; Shoubridge, E.A.; Warscheid, B. MITRAC links mitochondrial protein translocation to respiratory-chain assembly and translational regulation. Cell 2012, 151, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Srivastava, S.; D’Silva, P. Functional diversity of human mitochondrial J-proteins is independent of their association with the inner membrane presequence translocase. J. Biol. Chem. 2016, 291, 17345–17359. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Dennerlein, S.; Nikolov, M.; Jans, D.C.; Naumenko, N.; Aich, A.; MacVicar, T.; Linden, A.; Jakobs, S.; Urlaub, H. ROMO1 is a constituent of the human presequence translocase required for YME1L protease import. J. Cell Biol. 2019, 218, 598–614. [Google Scholar] [CrossRef] [PubMed]
- Günsel, U.; Mokranjac, D. A journey along the TIM23 complex, the major protein translocase of the mitochondrial inner membrane. Biol. Serbica 2020, 41. [Google Scholar]
- Mossmann, D.; Meisinger, C.; Vögtle, F.-N. Processing of mitochondrial presequences. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 1098–1106. [Google Scholar] [CrossRef]
- Mokranjac, D.; Sichting, M.; Popov-Čeleketić, D.; Mapa, K.; Gevorkyan-Airapetov, L.; Zohary, K.; Hell, K.; Azem, A.; Neupert, W. Role of Tim50 in the transfer of precursor proteins from the outer to the inner membrane of mitochondria. Mol. Biol. Cell 2009, 20, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Van Der Laan, M.; Chacinska, A.; Lind, M.; Perschil, I.; Sickmann, A.; Meyer, H.E.; Guiard, B.; Meisinger, C.; Pfanner, N.; Rehling, P. Pam17 is required for architecture and translocation activity of the mitochondrial protein import motor. Mol. Cell. Biol. 2005, 25, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Popov-Čeleketić, D.; Mapa, K.; Neupert, W.; Mokranjac, D. Active remodelling of the TIM23 complex during translocation of preproteins into mitochondria. EMBO J. 2008, 27, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Harada, Y.; Shiota, T.; Yamano, K.; Watanabe, K.; Yokota, M.; Yamamoto, H.; Sesaki, H.; Endo, T. Tim23–Tim50 pair coordinates functions of translocators and motor proteins in mitochondrial protein import. J. Cell Biol. 2009, 184, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.I.; Chen, Y.; Lynch, D.L.; Gumbart, J.C.; Park, E. Structural basis of mitochondrial protein import by the TIM23 complex. Nature 2023, 621, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, Y.; Wang, G.; Wang, S.; Sun, D.; Ou, X.; Lian, Y.; Li, L. Molecular pathway of mitochondrial preprotein import through the TOM-TIM23 supercomplex. bioRxiv 2023, preprint. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, J.; Huang, R.; Guan, Z.; Yan, L.; Hong, S.; Zhang, L.; Huang, C.; Liu, Z.; Yin, P. The architecture of substrate-engaged TOM–TIM23 supercomplex reveals preprotein proximity sites for mitochondrial protein translocation. Cell Discov. 2024, 10, 19. [Google Scholar] [CrossRef]
- Sokol, A.M.; Sztolsztener, M.E.; Wasilewski, M.; Heinz, E.; Chacinska, A. Mitochondrial protein translocases for survival and wellbeing. FEBS Lett. 2014, 588, 2484–2495. [Google Scholar] [CrossRef]
- Chacinska, A.; van der Laan, M.; Mehnert, C.S.; Guiard, B.; Mick, D.U.; Hutu, D.P.; Truscott, K.N.; Wiedemann, N.; Meisinger, C.; Pfanner, N. Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol. Cell. Biol. 2010, 30, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Salhab, M.; Patani, N.; Jiang, W.; Mokbel, K. High TIMM17A expression is associated with adverse pathological and clinical outcomes in human breast cancer. Breast Cancer 2012, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Si, Y.; Tao, T.; Martin, T.A.; Cheng, S.; Yu, H.; Li, J.; He, J.; Jiang, W.G. The impact of TIMM17A on aggressiveness of human breast cancer cells. Anticancer. Res. 2016, 36, 1237–1241. [Google Scholar] [PubMed]
- Kan, K.T.; Wilcock, J.; Lu, H. Role of Yme1 in mitochondrial protein homeostasis: From regulation of protein import, OXPHOS function to lipid synthesis and mitochondrial dynamics. Biochem. Soc. Trans. 2024, 52, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Lytovchenko, O.; Melin, J.; Chacinska, A.; Guiard, B.; Neumann, P.; Ficner, R.; Jahn, O.; Schmidt, B.; Rehling, P. Tim50’s presequence receptor domain is essential for signal driven transport across the TIM23 complex. J. Cell Biol. 2011, 195, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Rahman, B.; Kawano, S.; Yunoki-Esaki, K.; Anzai, T.; Endo, T. NMR analyses on the interactions of the yeast Tim50 C-terminal region with the presequence and Tim50 core domain. FEBS Lett. 2014, 588, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Gomkale, R.; Linden, A.; Neumann, P.; Schendzielorz, A.B.; Stoldt, S.; Dybkov, O.; Kilisch, M.; Schulz, C.; Cruz-Zaragoza, L.D.; Schwappach, B. Mapping protein interactions in the active TOM-TIM23 supercomplex. Nat. Commun. 2021, 12, 5715. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, M.; Staretz-Chacham, O.; Dayan, D.; Stephen, J.; Weech, A.; Damseh, N.; Pri Chen, H.; Edvardson, S.; Mazaheri, S.; Saada, A. Mitochondrial epileptic encephalopathy, 3-methylglutaconic aciduria and variable complex V deficiency associated with TIMM50 mutations. Clin. Genet. 2017, 91, 690–696. [Google Scholar] [CrossRef]
- Reyes, A.; Melchionda, L.; Burlina, A.; Robinson, A.J.; Ghezzi, D.; Zeviani, M. Mutations in TIMM50 compromise cell survival in OxPhos-dependent metabolic conditions. EMBO Mol. Med. 2018, 10, e8698. [Google Scholar] [CrossRef]
- ClinVar. NM_145261.4(DNAJC19):c.158G>A (p.Gly53Glu). Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/993012/ (accessed on 9 July 2024).
- Moosa, S.; Fano, V.; Obregon, M.G.; Altmüller, J.; Thiele, H.; Nürnberg, P.; Nishimura, G.; Wollnik, B. A novel homozygous PAM16 mutation in a patient with a milder phenotype and longer survival. Am. J. Med. Genet. A 2016, 170, 2436–2439. [Google Scholar] [CrossRef] [PubMed]
- Royer-Bertrand, B.; Castillo-Taucher, S.; Moreno-Salinas, R.; Cho, T.J.; Chae, J.H.; Choi, M.; Kim, O.H.; Dikoglu, E.; Campos-Xavier, B.; Girardi, E.; et al. Mutations in the heat-shock protein A9 (HSPA9) gene cause the EVEN-PLUS syndrome of congenital malformations and skeletal dysplasia. Sci. Rep. 2015, 5, 17154. [Google Scholar] [CrossRef] [PubMed]
- Tort, F.; Ugarteburu, O.; Texidó, L.; Gea-Sorlí, S.; García-Villoria, J.; Ferrer-Cortès, X.; Arias, Á.; Matalonga, L.; Gort, L.; Ferrer, I. Mutations in TIMM50 cause severe mitochondrial dysfunction by targeting key aspects of mitochondrial physiology. Hum. Mutat. 2019, 40, 1700–1712. [Google Scholar] [CrossRef]
- Mir, A.; Hadab, S.; Sammak, M.; Alhazmi, R.; Housawi, Y.; Bashir, S. Complete resolution of epileptic spasms with vigabatrin in a patient with 3-methylglutaconic aciduria caused by TIMM50 gene mutation. Clin. Genet. 2020, 98, 102–103. [Google Scholar] [CrossRef]
- ClinVar. NM_001001563.5(TIMM50):c.664G>A (p.Ala222Thr). Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/488622/ (accessed on 9 July 2024).
- ClinVar. NM_001001563.5(TIMM50):c.715C>T (p.Arg239Trp). Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/488623/ (accessed on 9 July 2024).
- Plutino, M.; Chaussenot, A.; Rouzier, C.; Ait-El-Mkadem, S.; Fragaki, K.; Paquis-Flucklinger, V.; Bannwarth, S. Targeted next generation sequencing with an extended gene panel does not impact variant detection in mitochondrial diseases. BMC Med. Genet. 2018, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Ucar, S.K.; Mayr, J.A.; Feichtinger, R.G.; Canda, E.; Çoker, M.; Wortmann, S.B. Previously Unreported Biallelic Mutation in DNAJC19: Are Sensorineural Hearing Loss and Basal Ganglia Lesions Additional Features of Dilated Cardiomyopathy and Ataxia (DCMA) Syndrome? JIMD Rep. 2017, 35, 39–45. [Google Scholar] [CrossRef]
- Davey, K.M.; Parboosingh, J.S.; McLeod, D.R.; Chan, A.; Casey, R.; Ferreira, P.; Snyder, F.F.; Bridge, P.J.; Bernier, F.P. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J. Med. Genet. 2006, 43, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Marinakis, N.M.; Svingou, M.; Veltra, D.; Kekou, K.; Sofocleous, C.; Tilemis, F.N.; Kosma, K.; Tsoutsou, E.; Fryssira, H.; Traeger-Synodinos, J. Phenotype-driven variant filtration strategy in exome sequencing toward a high diagnostic yield and identification of 85 novel variants in 400 patients with rare Mendelian disorders. Am. J. Med. Genet. A 2021, 185, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Ojala, T.; Polinati, P.; Manninen, T.; Hiippala, A.; Rajantie, J.; Karikoski, R.; Suomalainen, A.; Tyni, T. New mutation of mitochondrial DNAJC19 causing dilated and noncompaction cardiomyopathy, anemia, ataxia, and male genital anomalies. Pediatr. Res. 2012, 72, 432–437. [Google Scholar] [CrossRef]
- Mehawej, C.; Delahodde, A.; Legeai-Mallet, L.; Delague, V.; Kaci, N.; Desvignes, J.P.; Kibar, Z.; Capo-Chichi, J.M.; Chouery, E.; Munnich, A.; et al. The impairment of MAGMAS function in human is responsible for a severe skeletal dysplasia. PLoS Genet. 2014, 10, e1004311. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Abe, K.; Ciesielski, S.J.; Schmidt, P.J.; Campagna, D.R.; Rahimov, F.; Schilke, B.A.; Cuijpers, M.; Rieneck, K.; Lausen, B.; Linenberger, M.L.; et al. Congenital sideroblastic anemia due to mutations in the mitochondrial HSP70 homologue HSPA9. Blood 2015, 126, 2734–2738. [Google Scholar] [CrossRef]
- van Waveren Hogervorst, G.D.; van Roermund, H.P.; Snijders, P.J. Hereditary sideroblastic anaemia and autosomal inheritance of erythrocyte dimorphism in a Dutch family. Eur. J. Haematol. 1987, 38, 405–409. [Google Scholar] [CrossRef]
- Paz, E.; Jain, S.; Gottfried, I.; Staretz-Chacham, O.; Mahajnah, M.; Bagchi, P.; Seyfried, N.; Ashery, U.; Azem, A. Biochemical and neurophysiological effects of deficiency of the mitochondrial import protein TIMM50. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Crameri, J.J.; Palmer, C.S.; Stait, T.; Jackson, T.D.; Lynch, M.; Sinclair, A.; Frajman, L.E.; Compton, A.G.; Coman, D.; Thorburn, D.R.; et al. Reduced Protein Import via TIM23 SORT Drives Disease Pathology in TIMM50-Associated Mitochondrial Disease. Mol. Cell. Biol. 2024, 44, 226–244. [Google Scholar] [CrossRef]
- Iosefson, O.; Sharon, S.; Goloubinoff, P.; Azem, A. Reactivation of protein aggregates by mortalin and Tid1—The human mitochondrial Hsp70 chaperone system. Cell Stress Chaperones 2012, 17, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.-F.; Hayashi, M.; Woo-Kim, S.; Tian, B.; Huang, J.-F.; Fearns, C.; Takayama, S.; Zapata, J.M.; Yang, Y.; Lee, J.-D. Tid1, a Cochaperone of the Heat Shock 70 Protein and the Mammalian Counterpart of the Drosophila Tumor Suppressor l(2)tid, Is Critical for Early Embryonic Development and Cell Survival. Mol. Cell. Biol. 2004, 24, 2226–2236. [Google Scholar] [CrossRef]
- Banerjee, S.; Chaturvedi, R.; Singh, A.; Kushwaha, H.R. Putting human Tid-1 in context: An insight into its role in the cell and in different disease states. Cell Commun. Signal. 2022, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Al Teneiji, A.; Siriwardena, K.; George, K.; Mital, S.; Mercimek-Mahmutoglu, S. Progressive cerebellar atrophy and a novel homozygous pathogenic DNAJC19 variant as a cause of dilated cardiomyopathy ataxia syndrome. Pediatr. Neurol. 2016, 62, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef] [PubMed]
- Jubinsky, P.; Messer, A.; Short, M. Characterization of magmas, a novel gene involved in gm-csf signal transduction. Exp. Hematol. 2000, 28, 86. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, D.; Saha, P.; Marthala, H.; D’silva, P. Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis. 2014, 5, e1394. [Google Scholar] [CrossRef] [PubMed]
| Protein | Pathogenic/Likely Pathogenic Mutation * | Symptoms | ClinVar Accession No. | First Deposited in ClinVar | Reference |
|---|---|---|---|---|---|
| TIM23 core components | |||||
| Tim23 | NA | ||||
| Tim17A | NA | ||||
| Tim17B | NA | ||||
| Tim50 | NM_001001563.5(TIMM50):c.26C>A (p.Ser9Ter) | Mitochondrial encephalopathy, Reduced TIMM50 mRNA levels, OXPHOS malfunction, Failure to thrive, Lactic acidosis | RCV000677434.1, RCV001328000.2 | 24 August 2018 | [75] |
| NM_001001563.5(TIMM50):c.260G>C (p.Gly87Ala) | Mitochondrial encephalopathy, Reduced TIMM50 mRNA levels, OXPHOS malfunction, Failure to thrive, Lactic acidosis | RCV000677433.1, RCV001328001.1 | 24 August 2018 | [75] | |
| NM_001001563.5(TIMM50):c.341G>A (p.Arg114Gln) | Encephalopathy, Decreased complex I, II, IV and V levels, Abnormality of visual evoked potentials, Strabismus, Scoliosis | RCV001812628.1, RCV003120700.4 | 19 January 2022 | [79] | |
| NM_001001563.5(TIMM50):c.340C>T (p.Arg114Trp) | Epileptic encephalopathy, Decreased complex V activity, Elevated CSF lactate levels, Myoclonic jerks, Cachectic | RCV000509033.3, RCV001367110.6 | 9 October 2017 | [74] | |
| NM_001001563.5(TIMM50):c.446C>T (p.Thr149Met) | Epileptic spasms, Hypsarrhythmia, Bilateral optic atrophy, Abnormal EEG, Developmental delay | RCV000509024.3 | 9 October 2017 | [74,80] | |
| NM_001001563.5(TIMM50):c.664G>A (p.Ala222Thr) | 3-methylglutaconic aciduria type 9 | RCV000578358.5 | 8 February 2018 | [81] | |
| NM_001001563.5(TIMM50):c.715C>T (p.Arg239Trp) | 3-methylglutaconic aciduria type 9 | RCV000578437.5, RCV002529040.2 | 8 February 2018 | [82] | |
| NM_001001563.5(TIMM50):c.805G>A (p.Gly269Ser) | Encephalopathy, Failure to thrive, Spastic tetraparesia with dystonia, Piramidalism, Elevated CSF lactate levels | RCV000190713.5, RCV001812182.1 | 14 September 2015 | [79] | |
| TIM23 lateral-sorting components | |||||
| Tim21 | NA | ||||
| Mgr2 | NA | ||||
| TIM23 motor components/PAM complex | |||||
| Tim44 | NA | ||||
| Tim14 (Isoform 1) | NM_145261.4(DNAJC19):c.51del (p.Phe17fs) | Dilated cardiomyopathy with ataxia, Lipidosis, 3-methylglutaconic aciduria type 5 | RCV001231277.9 | 16 July 2020 | [83,84,85] |
| NM_145261.4(DNAJC19):c.63del (p.Arg20_Tyr21insTer) | Dilated cardiomyopathy with ataxia, 3-methylglutaconic aciduria type 5 | RCV001780991.5 | 29 November 2021 | [84,85] | |
| NM_145261.4(DNAJC19):c.63C>G (p.Tyr21Ter) | Dilated cardiomyopathy with ataxia, Failure to thrive, Optic atrophy, 3-methylglutaconic aciduria type 5, 3-methylglutaconic aciduria type 3 | RCV001206673.7, RCV001824933.1 | 16 July 2020 | [84,85] | |
| NM_145261.4(DNAJC19):c.62dup (p.Tyr21Ter) | Dilated cardiomyopathy with ataxia, 3-methylglutaconic aciduria type 5 | RCV001729987.3 | 16 October 2021 | [84,85,86] | |
| NM_145261.4(DNAJC19):c.158G>A (p.Gly53Glu) | Dilated cardiomyopathy with ataxia, 3-methylglutaconic aciduria type 5 | RCV001283818.1 | 26 January 2021 | [76] | |
| NM_145261.4(DNAJC19):c.300del (p.Ala101fs) | Dilated cardiomyopathy with ataxia, Noncompaction cardiomyopathy, 3-methylglutaconic aciduria type 5 | RCV000106304.5 | 24 March 2014 | [87] | |
| Tim14 (Isoform 2) | NA | ||||
| Pam16 | NM_016069.11(PAM16):c.221A>C (p.Gln74Pro) | Autosomal recessive spondylometaphyseal dysplasia, Megarbane type, Macrocephaly, Developmental delay, Hypotonia, Narrow spinal cord | RCV000788051.3 | 22 July 2019 | [77] |
| NM_016069.11(PAM16):c.226A>G (p.Asn76Asp) | Autosomal recessive spondylometaphyseal dysplasia, Megarbane type, Developmental delay, Prominent abdomen, Square iliac bones, Respiratory insufficiency | RCV000167551.4 | 29 March 2015 | [88] | |
| mHsp70 | NM_004134.7(HSPA9):c.376C>T (p.Arg126Trp) | EVEN-plus syndrome (EVPLS) [Epiphyseal and vertebral dysplasia, microtia, and flat nose, plus associated malformations] | RCV000210028.3 | 14 March 2016 | [78] |
| NM_004134.7(HSPA9):c.383A>G (p.Tyr128Cys) | EVEN-plus syndrome (EVPLS) [Epiphyseal and vertebral dysplasia, microtia, and flat nose, plus associated malformations], Premature termination predicted to abolish half the protein | RCV000209966.4 | 14 March 2016 | [78] | |
| NM_004134.7(HSPA9):c.409_410del (p.Asp136_Ile137insTer) | Autosomal dominant sideroblastic anemia, 50% of HSPA9 mRNA and 80% of HSPA9 protein | RCV000209839.4 | 12 March 2016 | [89,90] | |
| NM_004134.7(HSPA9):c.882_883del (p.Gly295_Val296insTer) | EVEN-plus syndrome (EVPLS) [Epiphyseal and vertebral dysplasia, microtia, and flat nose, plus associated malformations], Predicted to result in premature protein termination, Developmental delay | RCV000209995.6, RCV001781629.4, RCV003387515.2 | 12 March 2016 | [78] | |
| NM_004134.7(HSPA9):c.1373_1378del (p.Ile458_Asn459del) | Autosomal dominant sideroblastic anemia, 50% of HSPA9 mRNA and 80% of HSPA9 protein | RCV000209862.4 | 12 March 2016 | [89] | |
| Mge 1 (Isoform 1) | NA | ||||
| Mge 1 (Isoform 2)_ | NA | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.; Paz, E.; Azem, A. Hotspots for Disease-Causing Mutations in the Mitochondrial TIM23 Import Complex. Genes 2024, 15, 1534. https://doi.org/10.3390/genes15121534
Jain S, Paz E, Azem A. Hotspots for Disease-Causing Mutations in the Mitochondrial TIM23 Import Complex. Genes. 2024; 15(12):1534. https://doi.org/10.3390/genes15121534
Chicago/Turabian StyleJain, Sahil, Eyal Paz, and Abdussalam Azem. 2024. "Hotspots for Disease-Causing Mutations in the Mitochondrial TIM23 Import Complex" Genes 15, no. 12: 1534. https://doi.org/10.3390/genes15121534
APA StyleJain, S., Paz, E., & Azem, A. (2024). Hotspots for Disease-Causing Mutations in the Mitochondrial TIM23 Import Complex. Genes, 15(12), 1534. https://doi.org/10.3390/genes15121534





