The Effect of Maternal Diet and Physical Activity on the Epigenome of the Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Risk Assessment
3. Results
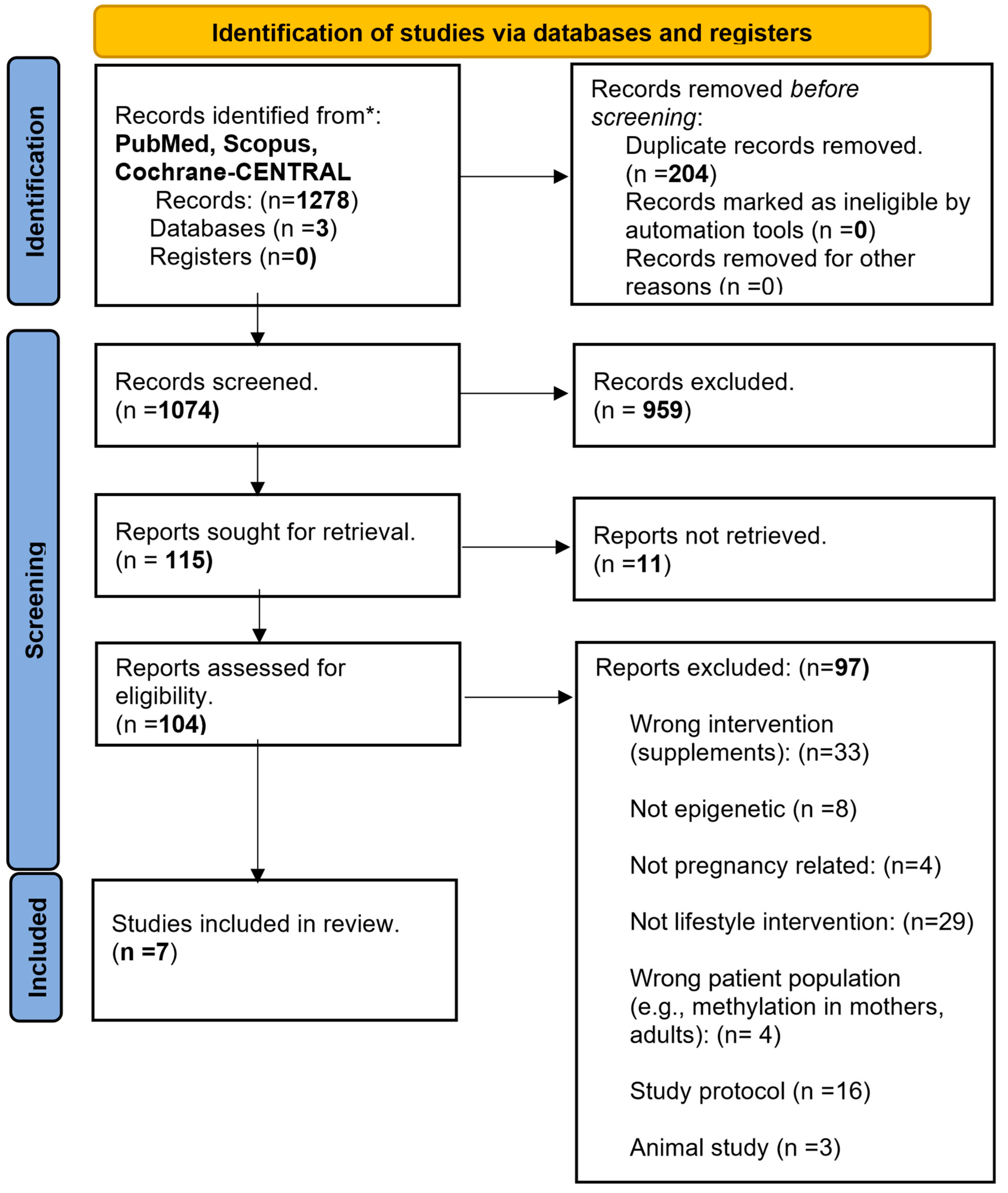
3.1. Search Results
3.2. Study Characteristics
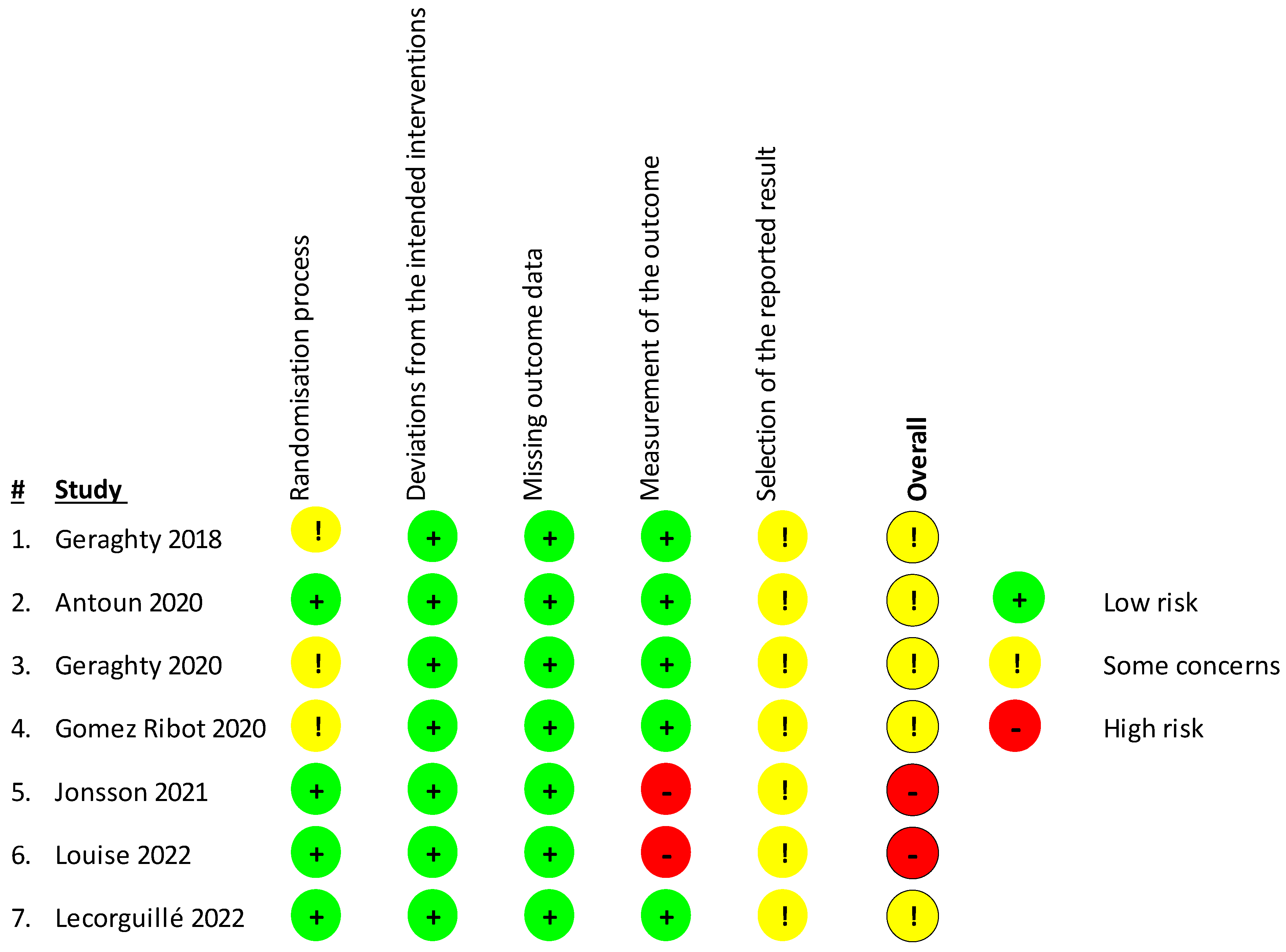
3.3. Risk Assessment of Included Studies
3.4. Synthesis of Results
4. Discussion
4.1. Principal Findings
4.2. Comparison with Existing Literature
4.3. Interpretation of Results
4.4. Strengths and Limitations
4.4.1. Strengths and Limitations of Included Studies
4.4.2. Strengths and Limitations of the Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Column 1 | Column 2 | Column 3 | ||
|---|---|---|---|---|
| Pregnan * | Epigen * | Lifestyle | ||
| Gestation * | DNA methylation | Diet * | ||
| Periconception* | AND | Methylation | AND | Physical activity |
| Preconception * | Histone | Stress | ||
| Mother | Acetylation | Sedentary | ||
| Matern * | Phosphorylation | |||
| Infant | miRNA | |||
| Offspring |
- PubMed on 8/7/2023
- (pregnan* OR gestation OR mother OR matern* OR infant OR offspring OR periconception* OR preconception*) AND (epigen* OR “DNA methylation” OR methylation OR methyl* OR miRNA OR histone OR acetylation OR modification OR phosphorylation) AND (lifestyle OR diet* OR Mediterranean OR food OR nutri* OR metal* OR pollution OR “physical activity” OR sedentary OR stress)
- + setting filters to “Clinical trials” and “Randomized controlled trials” → 562 results
- Scopus on 8/7/2023
- TITLE-ABS-KEY (pregnan* AND epigen* AND (lifestyle OR diet* OR “physical activity” OR stress OR pollution) AND trial) AND (LIMIT-TO (SRCTYPE, “j”)) AND (LIMIT-TO (SUBJAREA, “MEDI”) OR LIMIT-TO (SUBJAREA, “BIOC”) OR LIMIT-TO (SUBJAREA, “IMMU”) OR LIMIT-TO (SUBJAREA, “PHAR”) OR LIMIT-TO (SUBJAREA, “NEUR”) OR LIMIT-TO (SUBJAREA, “PSYC”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (EXACTKEYWORD, “Human”)) → 172 results
- Cochrane-CENTRAL in 8/7/2023
- (pregnan* OR gestation OR mother OR matern* OR infant OR offspring) AND (epigen* OR “DNA methylation” OR methylation OR methyl* OR histone) AND (lifestyle OR diet* OR pollution OR “physical activity” OR stress)
- + filters “Trials” and “Clinical answers” → 544 results
- In total: 1278 articles
References
- Reijnders, I.F.; Mulders, A.G.M.G.J.; van der Windt, M.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. The Impact of Periconceptional Maternal Lifestyle on Clinical Features and Biomarkers of Placental Development and Function: A Systematic Review. Hum. Reprod. Update 2018, 25, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Zerfu, T.A.; Pinto, E.; Baye, K. Consumption of Dairy, Fruits and Dark Green Leafy Vegetables Is Associated with Lower Risk of Adverse Pregnancy Outcomes (APO): A Prospective Cohort Study in Rural Ethiopia. Nutr. Diabetes 2018, 8, 52. [Google Scholar] [CrossRef]
- Moody, L.; Chen, H.; Pan, Y.-X. Early-Life Nutritional Programming of Cognition—The Fundamental Role of Epigenetic Mechanisms in Mediating the Relation between Early-Life Environment and Learning and Memory Process. Adv. Nutr. Int. Rev. J. 2017, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.; Barker, D. Type 2 (Non-Insulin-Dependent) Diabetes Mellitus: The Thrifty Phenotype Hypothesis. Int. J. Epidemiol. 2013, 42, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal Programming of the Metabolic Syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Marshall, M.R.; Paneth, N.; Gerlach, J.; Mudd, L.M.; Biery, L.; Ferguson, D.P.; Pivarnik, J.M. Differential Methylation of Insulin-like Growth Factor 2 in Offspring of Physically Active Pregnant Women. J. Dev. Orig. Health Dis. 2018, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The Fetal and Infant Origins of Adult Disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Barker, D.J.P. The Origins of the Developmental Origins Theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; Picardi, A.; Buonocore, G. Fetal Programming and Early Identification of Newborns at High Risk of Free Radical-Mediated Diseases. World J. Clin. Pediatr. 2016, 5, 172. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, Epigenetics and Gestational Diabetes: Consequences in Mother and Child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From Basics to Birth and Beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Han, H.; De Carvalho, D.D.; Lay, F.D.; Jones, P.A.; Liang, G. Gene Body Methylation Can Alter Gene Expression and Is a Therapeutic Target in Cancer. Cancer Cell 2014, 26, 577–590. [Google Scholar] [CrossRef] [PubMed]
- OSBORNE-MAJNIK, A.; FU, Q.; LANE, R.H. Epigenetic Mechanisms in Fetal Origins of Health and Disease. Clin. Obstet. Gynecol. 2013, 56, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Eckert, J.J.; Denisenko, O. The Role of Maternal Nutrition during the Periconceptional Period and Its Effect on Offspring Phenotype. Adv. Exp. Med. Biol. 2017, 1014, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Ross, J.W. Epigenetics in Fertilization and Preimplantation Embryo Development. Prog. Biophys. Mol. Biol. 2013, 113, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M. DNA Methylation—Introduction. In DNA Methylation and Complex Human Disease; Academic Press: Cambridge, MA, USA, 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Cutter, A.R.; Hayes, J.J. A Brief Review of Nucleosome Structure. FEBS Lett. 2015, 589 Pt A, 2914–2922. [Google Scholar] [CrossRef]
- Wilczynska, A.; Bushell, M. The Complexity of MiRNA-Mediated Repression. Cell Death Differ. 2014, 22, 22–33. [Google Scholar] [CrossRef]
- Chang, G.; Mouillet, J.F.; Mishima, T.; Chu, T.; Sadovsky, E.; Coyne, C.B.; Parks, W.T.; Surti, U.; Sadovsky, Y. Expression and Trafficking of Placental MicroRNAs at the Feto-Maternal Interface. FASEB J. 2017, 31, 2760–2770. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, Y.; Liu, J.; Fei, X.; Zhang, Z.; Li, M.; Chen, X.; Xu, J.; Zhu, Q.; Zhou, W.; et al. Epigenetic Alternations of MicroRNAs and DNA Methylation Contribute to Gestational Diabetes Mellitus. J. Cell. Mol. Med. 2020, 24, 13899–13912. [Google Scholar] [CrossRef]
- Chen, P.; Piaggi, P.; Traurig, M.; Bogardus, C.; Knowler, W.C.; Baier, L.J.; Hanson, R.L. Differential Methylation of Genes in Individuals Exposed to Maternal Diabetes in Utero. Diabetologia 2017, 60, 645–655. [Google Scholar] [CrossRef]
- Awamleh, Z.; Butcher, D.T.; Hanley, A.; Retnakaran, R.; Haertle, L.; Haaf, T.; Hamilton, J.; Weksberg, R. Exposure to Gestational Diabetes Mellitus (GDM) Alters DNA Methylation in Placenta and Fetal Cord Blood. Diabetes Res. Clin. Pract. 2021, 174, 108690. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, K.; Hou, X.; Lu, J. Comprehensive Analysis of DNA Methylation and Gene Expression Profiles in Gestational Diabetes Mellitus. Medicine 2021, 100, e26497. [Google Scholar] [CrossRef] [PubMed]
- Gemma, C.; Sookoian, S.; Alvariñas, J.; García, S.I.; Quintana, L.; Kanevsky, D.; González, C.D.; Pirola, C.J. Maternal Pregestational BMI Is Associated with Methylation of thePPARGC1APromoter in Newborns. Obesity 2009, 17, 1032–1039. [Google Scholar] [CrossRef]
- Oelsner, K.T.; Guo, Y.; To, S.B.-C.; Non, A.L.; Barkin, S.L. Maternal BMI as a Predictor of Methylation of Obesity-Related Genes in Saliva Samples from Preschool-Age Hispanic Children At-Risk for Obesity. BMC Genom. 2017, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Nogues, P.; Dos Santos, E.; Jammes, H.; Berveiller, P.; Arnould, L.; Vialard, F.; Dieudonné, M.-N. Maternal Obesity Influences Expression and DNA Methylation of the Adiponectin and Leptin Systems in Human Third-Trimester Placenta. Clin. Epigenetics 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Berglind, D.; Müller, P.; Willmer, M.; Sinha, I.; Tynelius, P.; Näslund, E.; Dahlman-Wright, K.; Rasmussen, F. Differential Methylation in Inflammation and Type 2 Diabetes Genes in Siblings Born before and after Maternal Bariatric Surgery. Obesity 2015, 24, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Guenard, F.; Deshaies, Y.; Cianflone, K.; Kral, J.G.; Marceau, P.; Vohl, M.-C. Differential Methylation in Glucoregulatory Genes of Offspring Born before vs. after Maternal Gastrointestinal Bypass Surgery. Proc. Natl. Acad. Sci. USA 2013, 110, 11439–11444. [Google Scholar] [CrossRef]
- Robinson, S.L.; Mumford, S.L.; Guan, W.; Zeng, X.; Kim, K.; Radoc, J.G.; Trinh, M.-H.; Flannagan, K.; Schisterman, E.F.; Yeung, E. Maternal Fatty Acid Concentrations and Newborn DNA Methylation. Am. J. Clin. Nutr. 2019, 111, 613–621. [Google Scholar] [CrossRef]
- Fan, C.; Fu, H.; Dong, H.; Lu, Y.; Lu, Y.; Qi, K. Maternal N-3 Polyunsaturated Fatty Acid Deprivation during Pregnancy and Lactation Affects Neurogenesis and Apoptosis in Adult Offspring: Associated with DNA Methylation of Brain-Derived Neurotrophic Factor Transcripts. Nutr. Res. 2016, 36, 1013–1021. [Google Scholar] [CrossRef]
- Cinquina, V.; Calvigioni, D.; Farlik, M.; Halbritter, F.; Fife-Gernedl, V.; Shirran, S.L.; Fuszard, M.A.; Botting, C.H.; Poullet, P.; Piscitelli, F.; et al. Life-Long Epigenetic Programming of Cortical Architecture by Maternal “Western” Diet during Pregnancy. Mol. Psychiatry 2019, 25, 22–36. [Google Scholar] [CrossRef]
- House, J.A.; Mendez, M.A.; Maguire, R.L.; Gonzalez-Nahm, S.; Huang, Z.; Daniels, J.L.; Murphy, S.K.; Fuemmeler, B.F.; Wright, F.A.; Hoyo, C. Periconceptional Maternal Mediterranean Diet Is Associated with Favorable Offspring Behaviors and Altered CpG Meth-ylation of Imprinted Genes. Front. Cell Dev. Biol. 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nahm, S.; Mendez, M.; Robinson, W.; Murphy, S.K.; Hoyo, C.; Hogan, V.; Rowley, D. Low Maternal Adherence to a Mediterranean Diet Is Associated with Increase in Methylation at the MEG3-IG Differentially Methylated Region in Female Infants. Environ. Epigenetics 2017, 3, dvx007. [Google Scholar] [CrossRef] [PubMed]
- Küpers, L.K.; Fernández-Barrés, S.; Nounu, A.; Friedman, C.; Fore, R.; Mancano, G.; Dabelea, D.; Rifas-Shiman, S.L.; Mulder, R.H.; Oken, E.; et al. Maternal Mediterranean Diet in Pregnancy and Newborn DNA Methylation: A Meta-Analysis in the PACE Consortium. Epigenetics 2022, 17, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, S.L.; Eliot, M.N.; Whitsel, E.A.; Huang, Y.-T.; Kelsey, K.T.; Marsit, C.J.; Wellenius, G.A. Maternal Residential Proximity to Major Roadways, Birth Weight, and Placental DNA Methylation. Environ. Int. 2016, 92–93, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ladd-Acosta, C.; Feinberg, J.I.; Brown, S.C.; Lurmann, F.W.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Feinberg, A.P.; Fallin, M.D.; Volk, H.E. Epigenetic Marks of Prenatal Air Pollution Exposure Found in Multiple Tissues Relevant for Child Health. Environ. Int. 2019, 126, 363–376. [Google Scholar] [CrossRef]
- Brooks, S.A.; Fry, R.C. Cadmium Inhibits Placental Trophoblast Cell Migration via MiRNA Regulation of the Transforming Growth Factor Beta (TGF-β) Pathway. Food Chem. Toxicol. 2017, 109, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Rager, J.E.; Bailey, K.A.; Smeester, L.; Miller, S.K.; Parker, J.S.; Drobná, Z.; Currier, J.M.; Douillet, C.; Olshan, A.F.; Rubio-Andrade, M.; et al. Prenatal Arsenic Exposure and the Epigenome: Altered MicroRNAs Associated with Innate and Adaptive Immune Signaling in Newborn Cord Blood. Environ. Mol. Mutagen. 2014, 55, 196–208. [Google Scholar] [CrossRef]
- Sood, S.; Shekhar, S.; Santosh, W. Dimorphic Placental Stress: A Repercussion of Interaction between Endocrine Disrupting Chemicals (EDCs) and Fetal Sex. Med. Hypotheses 2017, 99, 73–75. [Google Scholar] [CrossRef]
- Gillet, V.; Hunting, D.J.; Takser, L. Turing Revisited: Decoding the MicroRNA Messages in Brain Extracellular Vesicles for Early Detection of Neurodevelopmental Disorders. Curr. Environ. Health Rep. 2016, 3, 188–201. [Google Scholar] [CrossRef]
- Hompes, T.; Izzi, B.; Gellens, E.; Morreels, M.; Fieuws, S.; Pexsters, A.; Schops, G.; Dom, M.; Van Bree, R.; Freson, K.; et al. Investigating the Influence of Maternal Cortisol and Emotional State during Pregnancy on the DNA Methylation Status of the Glucocorticoid Receptor Gene (NR3C1) Promoter Region in Cord Blood. J. Psychiatr. Res. 2013, 47, 880–891. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal Exposure to Maternal Depression, Neonatal Methylation of Human Glucocorticoid Receptor Gene (NR3C1) and Infant Cortisol Stress Responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.; Sexton-Oates, A.; O’Brien, E.; Alberdi, G.; Fransquet, P.; Saffery, R.; McAuliffe, F. A Low Glycaemic Index Diet in Pregnancy Induces DNA Methylation Variation in Blood of Newborns: Results from the ROLO Randomised Controlled Trial. Nutrients 2018, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Antoun, E.; Kitaba, N.T.; Titcombe, P.; Dalrymple, K.V.; Garratt, E.S.; Barton, S.J.; Murray, R.; Seed, P.T.; Holbrook, J.D.; Kobor, M.S.; et al. Maternal Dysglycaemia, Changes in the Infant’s Epigenome Modified with a Diet and Physical Activity Intervention in Pregnancy: Secondary Analysis of a Randomised Control Trial. PLoS Med. 2020, 17, e1003229. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.A.; Sexton-Oates, A.; O’Brien, E.C.; Saffery, R.; McAuliffe, F.M. Epigenetic Patterns in Five-Year-Old Children Exposed to a Low Glycemic Index Dietary Intervention during Pregnancy: Results from the ROLO Kids Study. Nutrients 2020, 12, 3602. [Google Scholar] [CrossRef] [PubMed]
- Gomez Ribot, D.; Diaz, E.; Fazio, M.V.; Gómez, H.L.; Fornes, D.; Macchi, S.B.; Gresta, C.A.; Capobianco, E.; Jawerbaum, A. An Extra Virgin Olive Oil-Enriched Diet Improves Maternal, Placental and Cord Blood Parameters in GDM Pregnancies. Diabetes/Metab. Res. Rev. 2020, 36, e3349. [Google Scholar] [CrossRef]
- Jönsson, J.; Renault, K.M.; García-Calzón, S.; Perfilyev, A.; Estampador, A.C.; Nørgaard, K.; Lind, M.V.; Vaag, A.; Hjort, L.; Michaelsen, K.F.; et al. Lifestyle Intervention in Pregnant Women with Obesity Impacts Cord Blood DNA Methylation, Which Associates with Body Composition in the Offspring. Diabetes 2021, 70, 854–866. [Google Scholar] [CrossRef]
- Louise, J.; Deussen, A.R.; Koletzko, B.; Owens, J.A.; Saffery, R.; Dodd, J.M. Effect of an Antenatal Diet and Lifestyle Intervention and Maternal BMI on Cord Blood DNA Methylation in Infants of Overweight and Obese Women: The LIMIT Randomised Controlled Trial. PLoS ONE 2022, 17, e0269723. [Google Scholar] [CrossRef]
- Lecorguillé, M.; McAuliffe, F.M.; Twomey, P.J.; Viljoen, K.; Mehegan, J.; Kelleher, C.C.; Suderman, M.; Phillips, C.M. Maternal Glycaemic and Insulinemic Status and Newborn DNA Methylation: Findings in Women with Overweight and Obesity. J. Clin. Endocrinol. Metab. 2022, 108, 85–98. [Google Scholar] [CrossRef]
- Tobi, E.W.; Juvinao-Quintero, D.L.; Ronkainen, J.; Ott, R.; Alfano, R.; Canouil, M.; Khamis, A.; Küpers, L.K.; Lim, I.Y.; Perron, P.; et al. Maternal Glycemic Dysregulation during Pregnancy and Neonatal Blood DNA Methylation: Meta-Analyses of Epigenome-Wide Association Studies. Diabetes Care 2022, 45, 614–623. [Google Scholar] [CrossRef]
- Howe, C.G.; Cox, B.; Fore, R.; Jungius, J.; Kvist, T.; Lent, S.; Miles, H.E.; Salas, L.A.; Rifas-Shiman, S.; Starling, A.P.; et al. Maternal Gestational Diabetes Mellitus and Newborn DNA Methylation: Findings from the Pregnancy and Childhood Epigenetics Consortium. Diabetes Care 2020, 43, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, M.L.; Vincent; Gaillard, R.; Felix, J.F. Associations of Maternal Early-Pregnancy Blood Glucose and Insulin Concentrations with DNA Methylation in Newborns. Clin. Epigenetics 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Canouil, M.; Khamis, A.; Keikkala, E.; Hummel, S.; Lobbens, S.; Bonnefond, A.; Delahaye, F.; Tzala, E.; Mustaniemi, S.; Vääräsmäki, M.; et al. Epigenome-Wide Association Study Reveals Methylation Loci Associated with Offspring Gestational Diabetes Mellitus Exposure and Maternal Methylome. Diabetes Care 2021, 44, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Knorr, S.; Antoniussen, C.S.; Bruun, J.M.; Ovesen, P.G.; Fuglsang, J.; Kampmann, U. The Impact of Lifestyle, Diet and Physical Activity on Epigenetic Changes in the Offspring—A Systematic Review. Nutrients 2021, 13, 2821. [Google Scholar] [CrossRef] [PubMed]
- Ruchat, S.-M.; Houde, A.-A.; Voisin, G.; St-Pierre, J.; Perron, P.; Baillargeon, J.-P.; Gaudet, D.; Hivert, M.-F.; Brisson, D.; Bouchard, L. Gestational Diabetes Mellitus Epigenetically Affects Genes Predominantly Involved in Metabolic Diseases. Epigenetics 2013, 8, 935–943. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Sheppard, A.; Gluckman, P.D.; Lillycrop, K.A.; Burdge, G.C.; McLean, C.; Rodford, J.; Slater-Jefferies, J.L.; Garratt, E.; Crozier, S.R.; et al. Epigenetic Gene Promoter Methylation at Birth Is Associated with Child’s Later Adiposity. Diabetes 2011, 60, 1528–1534. [Google Scholar] [CrossRef]
- El Hajj, N.; Pliushch, G.; Schneider, E.; Dittrich, M.; Muller, T.; Korenkov, M.; Aretz, M.; Zechner, U.; Lehnen, H.; Haaf, T. Metabolic Programming of MEST DNA Methylation by Intrauterine Exposure to Gestational Diabetes Mellitus. Diabetes 2012, 62, 1320–1328. [Google Scholar] [CrossRef]
- Côté, S.; Gagné-Ouellet, V.; Guay, S.-P.; Allard, C.; Houde, A.-A.; Perron, P.; Baillargeon, J.-P.; Gaudet, D.; Guérin, R.; Brisson, D.; et al. PPARGC1α Gene DNA Methylation Variations in Human Placenta Mediate the Link between Maternal Hyperglycemia and Leptin Levels in Newborns. Clin. Epigenetics 2016, 8, 72. [Google Scholar] [CrossRef]
- McCullough, L.E.; Mendez, M.A.; Miller, E.E.; Murtha, A.P.; Murphy, S.K.; Hoyo, C. Associations between Prenatal Physical Activity, Birth Weight, and DNA Methylation at Genomically Imprinted Domains in a Multiethnic Newborn Cohort. Epigenetics 2015, 10, 597–606. [Google Scholar] [CrossRef]
- Hopkins, S.A.; Baldi, J.C.; Cutfield, W.S.; McCowan, L.; Hofman, P.L. Exercise Training in Pregnancy Reduces Offspring Size without Changes in Maternal Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2010, 95, 2080–2088. [Google Scholar] [CrossRef]
- Hopkins, S.A.; Baldi, J.C.; Cutfield, W.S.; McCowan, L.; Hofman, P.L. Effects of Exercise Training on Maternal Hormonal Changes in Pregnancy. Clin. Endocrinol. 2011, 74, 495–500. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.G.; Hackney, A.C.; Guion, W.K.; Katz, V.L. Metabolic and Hormonal Responses to Low-Impact Aerobic Dance during Pregnancy. Med. Sci. Sports Exerc. 1996, 28, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A.; Campagna, P.D.; Gilchrist, L.; Beresford, P. Substrate and Hormonal Responses during Exercise Classes at Selected Stages of Pregnancy. Can. J. Appl. Physiol. 1995, 20, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute Exercise Remodels Promoter Methylation in Human Skeletal Muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef]
- Sharp, G.C.; Lawlor, D.A.; Richmond, R.C.; Fraser, A.; Simpkin, A.; Suderman, M.; Shihab, H.A.; Lyttleton, O.; McArdle, W.; Ring, S.M.; et al. Maternal Pre-Pregnancy BMI and Gestational Weight Gain, Offspring DNA Methylation and Later Offspring Adiposity: Findings from the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2015, 44, 1288–1304. [Google Scholar] [CrossRef]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J. Embryos, DOHaD and David Barker. J. Dev. Orig. Health Dis. 2015, 6, 377–383. [Google Scholar] [CrossRef]
- Franzago, M.; Rovere, M.L.; Franchi, P.G.; Vitacolonna, E.; Stuppia, L. Epigenetics and Human Reproduction: The Primary Prevention of the Noncommunicable Diseases. Epigenomics 2019, 11, 1441–1460. [Google Scholar] [CrossRef]
| Author | Country | Participants | Intervention | Intervention Period | Control | Characteristics | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| 1. | Geraghty (2018) [44] RCT | Ireland | n = 60 sex-matched neonates (30 intervention 30 control from the ROLO study) ROLO study: women ≥ 18 who previously gave birth to a macrosomic baby | n = 30 Dietary advice about healthy eating and a low glycemic index diet given in second trimester. | Three times in pregnancy (18, 28 and 34 weeks). | n = 30 No specific dietary advice |
|
|
| 2. | Antoun (2020) [45] RCT | UK | n = 557 (buffy coat samples from) pregnant women from the UPBEAT RCT with BMI ≥ 30 pre pregnancy randomized between 15 + 0 and 18 + 6 to standard care or intervention. | n = 263 Low glycemic index diet, low saturated fat, increased physical activity. | Eight hourly sessions once/week for 8 weeks | n = 294 Treated using standard protocols. (159 of them were diagnosed with GDM) |
|
|
| 3. | Geraghty (2020) [46] RCT | Ireland | n = 63 5 year-olds from the ROLO study | n = 31 Dietary advice about healthy eating and a low glycemic index diet given in second trimester. | Three times in pregnancy (18, 28 and 34 weeks). | n = 32 No specific dietary advice |
|
|
| 4. | Gomez Ribot (2020) [47] RCT | Argentina | n = 60 Pregnant women with singleton pregnancy and GDM at 24–28 wks divided in three groups: Control, GDM and GDM-EVOO. Results available for: Control (n = 15), GDM (n = 15), GDM-EVOO (n = 15) | Three tablespoons of uncooked EVOO daily (36 g/day) within the meals. All women were given dietary advice to follow: 2100–2400 Kcal/day; carbohydrates 48–50%, proteins 18–20% and lipids 30–32%. | Enrollment at 24–28 wks. | None to one tablespoon of EVOO daily (0–12 g/day). All women were given dietary advice to follow: 2100–2400 Kcal/day; carbohydrates 48–50%, proteins 18–20% and lipids 30–32%. | Not stated |
|
| 5. | Jönsson (2021) [48] RCT | Denmark | n = 208 DNA samples from obese pregnant women (BMI ≥ 30 kg/m2) in TOP study | Two groups: Physical activity (assessed with pedometer) + diet (n = 76) Physical activity (assessed with pedometer) (n = 59) Women of both groups were recommended to walk 11,000 steps/day, maintain a low-fat Mediterranean-style diet of 1200–1675 kcal and keep GWG to ≤5 kg. | Enrollment at 11–14 weeks. Only the PA + D group received follow-up appointments every 2 weeks | n = 73 Standard of care + recommendation of keeping a low-fat Mediterranean-style diet of 1200–1675 kcal and limit GWG to ≤5 kg. |
|
|
| 6. | Louise (2022) [49] RCT | Australia | n = 645 randomly selected and balanced between the two groups cord blood samples from participants of the LIMIT RCT. Pregnant women with BMI ≥ 25 kg/m2 | n = 325 Dietary and lifestyle advice (healthy alternatives to sugar and achievable goals in physical activity) | Enrollment at 10–20 weeks and follow-up sessions with the dietitian or research assistants at 22, 24, 28, 32, 36 weeks | n = 320 Standard care. No specific advice on diet or activity. |
|
|
| 7. | Lecorguillé (2022) [50] RCT | Ireland | n = 172 Cord blood samples from participants of the PEARS RCT. Singleton pregnancy women BMI ≥ 25 kg/m2 and ≤39.9 kg/m2 | n = 96 Dietary advice about a low GI diet and recommendation on portion sizes of carbohydrates + moderate exercise of 30 min for 5–7 days/week. Smartphone app and emails every 2 weeks. | Enrollment at 10–15 weeks. Follow-up sessions for the intervention group at 28 and 34 weeks. | Standard care without consistent dietary, exercise of gestational weight gain advice. |
|
|
| Authors Name | Country | Study | Intervention | Outcome | Tissue of Interest | Tool Used | |
|---|---|---|---|---|---|---|---|
| 1. | Geraghty et al., 2018 [44] | Ireland | ROLO | Dietary advice | Epigenome-wide DNA methylation of offspring at birth | Cord blood | Illumina Infinum MethylationEPIC BeadChip array (HM850K) |
| 2. | Antoun et al., 2020 [45] | UK | UPBEAT | Dietary advice + physical activity | Epigenome-wide DNA methylation of offspring at birth | Cord blood | Infinium Human MethylationEPIC BeadChip array (HM850K) |
| 3. | Geraghty et al., 2020 [46] | Ireland | ROLO | Dietary advice | Epigenome-wide DNA methylation of offspring at 5 years of age | Saliva | Illumina MethylationEPIC array (HM850K) |
| 4. | Gomez Ribot et al., 2020 [47] | Argentina | [nameless] | Three tablespoons of EVOO | miRNA expression | Placenta | TaqMan MicroRNA reverse transcription kit |
| 5. | Jönsson et al., 2021 [48] | Denmark | TOP | Two groups:
| Epigenome-wide DNA methylation of offspring at birth | Cord blood | Illumina Infinium Human Methylation450 BeadChip array |
| 6. | Louise et al., 2022 [49] | Australia | LIMIT | Dietary advice + physical activity | Epigenome-wide DNA methylation of offspring + methylation at specific genes at birth | Cord blood | Illumina Infinium Human Methylation 450 BeadChip array |
| 7. | Lecorguillé et al., 2022 [50] | Ireland | PEARS | Dietary advice + moderate exercise | Epigenome-wide DNA methylation of offspring at birth | Cord blood | Illumina Infinium Human MethylationEPIC array (HM850K) |
| Results | Studies |
|---|---|
| Statistically significant differences between intervention-control groups | |
| Intervention altered the pathology-related methylation/miRNA expression | |
| Statistically non-significant differences between intervention-control groups |
| Study | Outcome Measures | Findings | |
|---|---|---|---|
| 1. | Geraghty et al., 2018 [44] (RCT) | Cord blood genome-wide DNAm changes in offspring of mothers participating in the ROLO study of a low-glycemic-index diet intervention in pregnancy. |
|
| 2. | Antoun et al., 2020 [45] (RCT) | Cord blood DNAm in offspring of obese mothers (BMI ≥ 30) with dysglycemia at 24–28 weeks and effect of a dietary and physical activity intervention to it. (Part of the UPBEAT RCT) |
|
| 3. | Geraghty et al., 2020 [46] (RCT) | Genome-wide DNAm in saliva samples of 5-year-old children whose mothers participated in the ROLO study taking dietary advice about healthy eating and a low glycemic index diet in the second trimester. Adiposity and body composition at birth, six months, 2, and 5 years of age. |
|
| 4. | Gomez Ribot et al., 2020 [47] (RCT) | Expression of miR-518d and miR-130a (miRNAs that target PPARα and PPARγ) in placentas of women taking three tablespoons of extra virgin olive oil at 24–28 wks of pregnancy. |
|
| 5. | Jönsson et al., 2021 [48] (RCT) | Genome-wide cord blood DNAm changes in offspring of obese mothers participating in the TOP study, studying the effect of physical activity with or without a diet intervention (low energy/low-fat Mediterranean-style diet) in pregnancy. Association of DNAm to lean mass at birth, growth and body composition at birth, 9 and 36 months of age. |
|
| 6. | Louise et al., 2022 [49] (RCT) | Cord blood DNAm and methylation of specific candidate genes in offspring of mothers (BMI ≥ 25 kg/m2) who participated in the LIMIT study getting dietary and lifestyle advice (healthy alternatives to sugar and achievable goals in physical activity). The genes assessed were related to obesity, metabolism, adiposity, and growth (PPARGC1A, IGF2, RXRA, or MEST) |
|
| 7. | Lecorguillé et al., 2022 [50] (RCT) | Genome-wide DNAm in cord blood of offspring of mothers with BMI ≥ 25 kg/m2 and ≤39.9 kg/m2 who participated in a RCT of dietary advice and moderate exercise in pregnancy. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotidou, A.; Chatzakis, C.; Ververi, A.; Eleftheriades, M.; Sotiriadis, A. The Effect of Maternal Diet and Physical Activity on the Epigenome of the Offspring. Genes 2024, 15, 76. https://doi.org/10.3390/genes15010076
Panagiotidou A, Chatzakis C, Ververi A, Eleftheriades M, Sotiriadis A. The Effect of Maternal Diet and Physical Activity on the Epigenome of the Offspring. Genes. 2024; 15(1):76. https://doi.org/10.3390/genes15010076
Chicago/Turabian StylePanagiotidou, Anastasia, Christos Chatzakis, Athina Ververi, Makarios Eleftheriades, and Alexandros Sotiriadis. 2024. "The Effect of Maternal Diet and Physical Activity on the Epigenome of the Offspring" Genes 15, no. 1: 76. https://doi.org/10.3390/genes15010076
APA StylePanagiotidou, A., Chatzakis, C., Ververi, A., Eleftheriades, M., & Sotiriadis, A. (2024). The Effect of Maternal Diet and Physical Activity on the Epigenome of the Offspring. Genes, 15(1), 76. https://doi.org/10.3390/genes15010076







