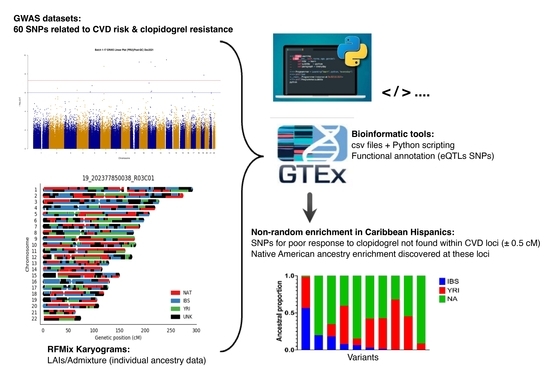
Non-Random Enrichment of Single-Nucleotide Polymorphisms Associated with Clopidogrel Resistance within Risk Loci Linked to the Severity of Underlying Cardiovascular Diseases: The Role of Admixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Identification of Variants
2.3. Local-Ancestry Inference
2.4. Python Scripts
2.4.1. Ancestry Search
2.4.2. Random Search
2.4.3. Bracket Search
2.5. Statistical Analysis
3. Results
3.1. Data Cohort
3.2. Expression Quantitative Trait Locus (eQTL) SNPs
3.3. Non-Random Enrichment of SNPs
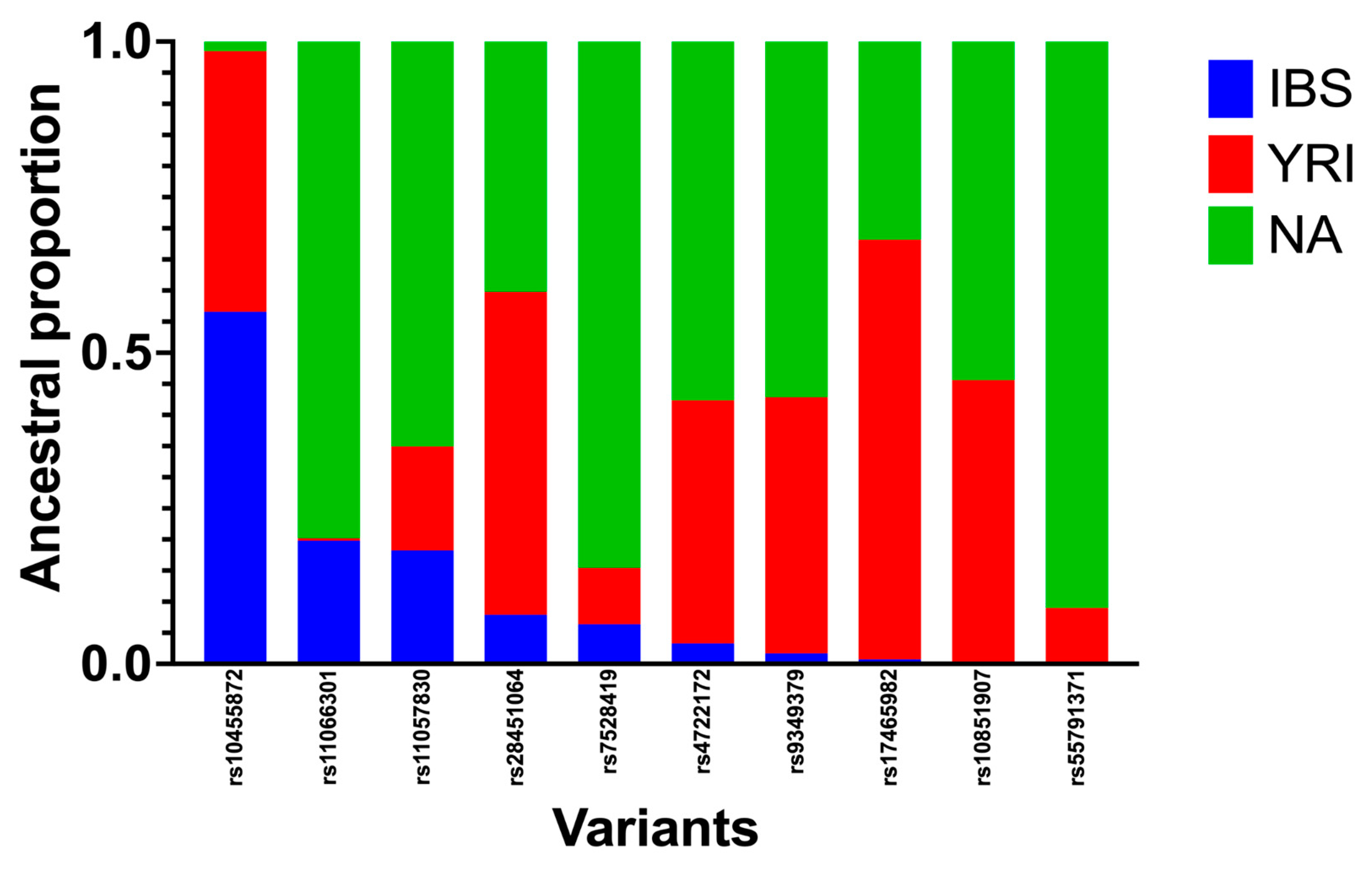
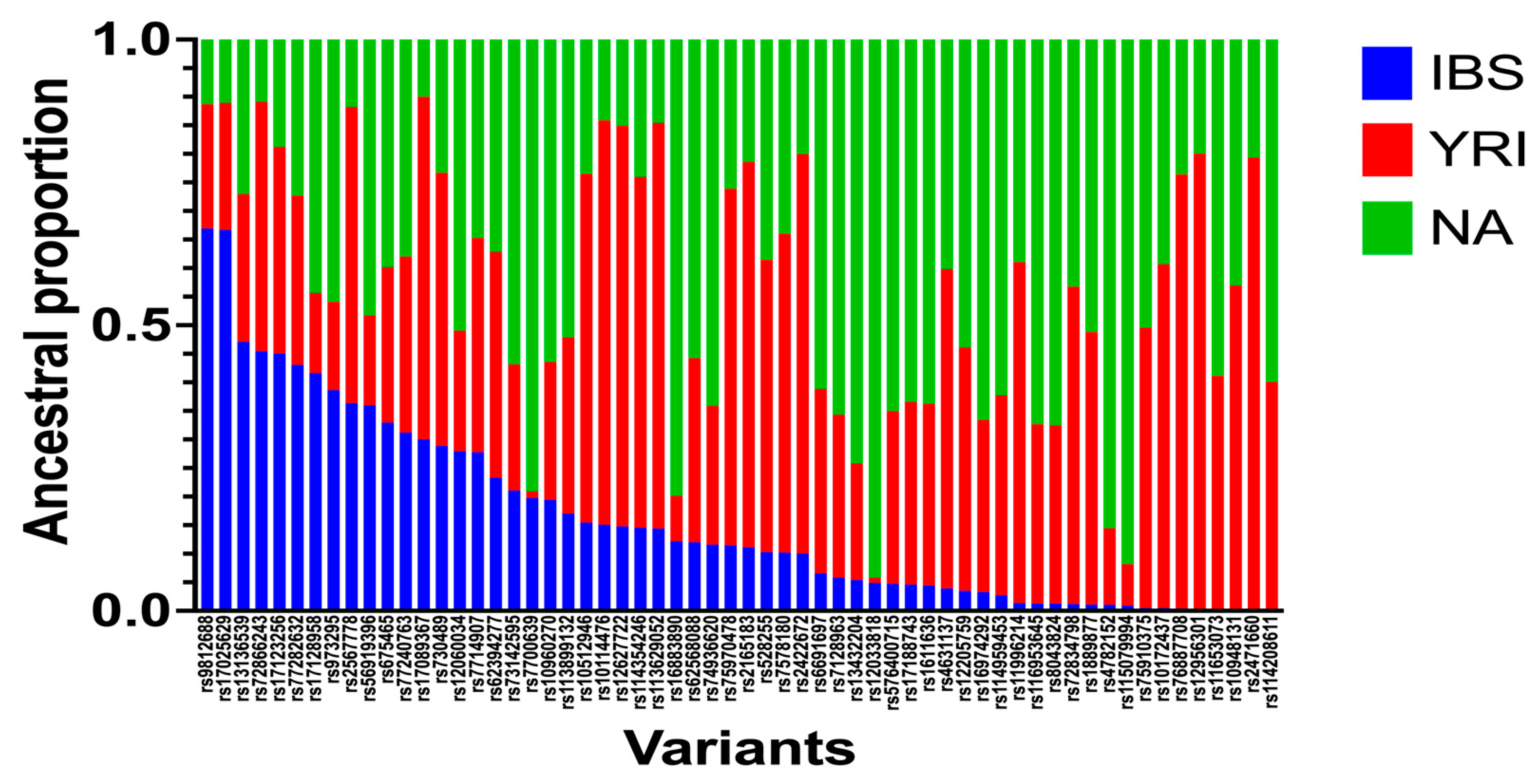
3.4. Local-Ancestry Inferences (LAIs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| CAD | coronary artery disease |
| CV | cardiovascular |
| CHR | chromosome |
| cM | centimorgan |
| DAPT | dual antiplatelet therapy |
| eQTL | expression quantitative trait loci |
| GWAS | genome-wide association study |
| Hct | hematocrit |
| HGDP | Human Genome Diversity Project |
| HTPR | high on-treatment platelet reactivity |
| IBS | Iberian |
| LA | local ancestry |
| LAI | local-ancestry inference |
| LDL | low-density lipoprotein |
| MEGA | Multi-Ethnic AMR/AFR Genotyping Array |
| MI | myocardial infarction |
| NA | Native American |
| PAD | peripheral artery disease |
| PHACTR1 | phosphatase and actin regulator 1 |
| POS | position |
| SNP | single-nucleotide polymorphism |
| TIA | transient ischemic attack |
| YRI | Yoruba |
References
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef]
- Evangelou, E.; Program, T.M.V.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Johnson, A.D.; Yanek, L.R.; Chen, M.-H.; Faraday, N.; Larson, M.G.; Tofler, G.; Lin, S.J.; Kraja, A.T.; Province, M.A.; Yang, Q.; et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat. Genet. 2010, 42, 608–613. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; Botton, M.R.; Scott, S.; Tomey, M.; Garcia, M.J.; Wiley, J.; Villablanca, P.; Melin, K.; Lopez-Candales, A.; Renta, J.Y.; et al. Pharmacogenetic association study on clopidogrel response in Puerto Rican Hispanics with cardiovascular disease: A novel characterization of a Caribbean population. Pharmacogenomics Pers. Med. 2018, 11, 95–106. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; Scott, S.A.; Tomey, M.I.; Melin, K.; Lopez-Candales, A.; Buckley, C.E.; Duconge, J. Clinical determinants of clopidogrel responsiveness in a heterogeneous cohort of Puerto Rican Hispanics. Ther. Adv. Cardiovasc. Dis. 2017, 11, 235–241. [Google Scholar] [CrossRef]
- Tcheng, J.E.; Lim, I.H.; Srinivasan, S.; Jozic, J.; Gibson, C.M.; O’Shea, J.C.; Puma, J.A.; Simon, D. Stent parameters predict major adverse clinical events and the response to platelet glycoprotein IIb/IIIa blockade: Findings of the ESPRIT trial. Circ. Cardiovasc. Interv. 2009, 2, 43–51. [Google Scholar] [CrossRef]
- Janssen, P.W.A.; Bergmeijer, T.O.; Vos, G.-J.A.; Kelder, J.C.; Qaderdan, K.; Godschalk, T.C.; Breet, N.J.; Deneer, V.H.M.; Hackeng, C.M.; Berg, J.M.T. Tailored P2Y12 inhibitor treatment in patients undergoing non-urgent PCI—The POPular Risk Score study. Eur. J. Clin. Pharmacol. 2019, 75, 1201–1210. [Google Scholar] [CrossRef]
- Via, M.; Gignoux, C.R.; Roth, L.A.; Fejerman, L.; Galanter, J.; Choudhry, S.; Toro-Labrador, G.; Viera-Vera, J.; Oleksyk, T.K.; Beckman, K.; et al. History Shaped the Geographic Distribution of GenomicAdmixture on the Island of Puerto Rico. PLoS ONE 2011, 6, e16513. [Google Scholar] [CrossRef]
- Hernández, C.L.; Pita, G.; Cavadas, B.; López, S.; Sánchez-Martínez, L.J.; Dugoujon, J.-M.; Novelletto, A.; Cuesta, P.; Pereira, L.; Calderón, R. Human Genomic Diversity Where the Mediterranean Joins the Atlantic. Mol. Biol. Evol. 2020, 37, 1041–1055. [Google Scholar] [CrossRef]
- Shriner, D.; Adeyemo, A.; Ramos, E.; Chen, G.; Rotimi, C.N. Mapping of disease-associated variants in admixed populations. Genome Biol. 2011, 12, 223. [Google Scholar] [CrossRef][Green Version]
- Torgerson, D.G.; Gignoux, C.R.; Galanter, J.M.; Drake, K.A.; Roth, L.A.; Eng, C.; Huntsman, S.; Torres, R.; Avila, P.C.; Chapela, R.; et al. Case-control admixture mapping in Latino populations enriches for known asthma-associated genes. J. Allergy Clin. Immunol. 2012, 130, 76–82.e12. [Google Scholar]
- Baran, Y.; Pasaniuc, B.; Sankararaman, S.; Torgerson, D.G.; Gignoux, C.; Eng, C.; Rodriguez-Cintron, W.; Chapela, R.; Ford, J.G.; Avila, P.C.; et al. Fast and accurate inference of local ancestry in Latino populations. Bioinformatics 2012, 28, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Uren, C.; Hoal, E.G.; Möller, M. Putting RFMix and ADMIXTURE to the test in a complex admixed population. BMC Genet. 2020, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y. Detecting Structure of Haplotypes and Local Ancestry. Genetics 2014, 196, 625–642. [Google Scholar] [CrossRef]
- Salter-Townshend, M.; Myers, S. Fine-scale inference of ancestry segments without prior knowledge of admixing groups. Genetics 2019, 212, 869–889. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Andaleon, A.; Wheeler, H.E. Comparing local ancestry inference models in populations of two- and three-way admixture. PeerJ 2020, 8, e10090. [Google Scholar] [CrossRef]
- Maples, B.K.; Gravel, S.; Kenny, E.E.; Bustamante, C.D. RFMix: A Discriminative Modeling Approach for Rapid and Robust Local-Ancestry Inference. Am. J. Hum. Genet. 2013, 93, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Browning, S.R.; Grinde, K.; Plantinga, A.; Gogarten, S.M.; Stilp, A.M.; Kaplan, R.C.; Avilés-Santa, M.L.; Browning, B.L.; Laurie, C.C. Local ancestry inference in a large US-based Hispanic/Latino study: Hispanic community health study/study of Latinos (HCHS/SOL). G3 Genes Genomes Genet. 2016, 6, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Sofer, T.; Baier, L.J.; Browning, S.R.; Thornton, T.A.; Talavera, G.A.; Wassertheil-Smoller, S.; Daviglus, M.L.; Hanson, R.; Kobes, S.; Cooper, R.S.; et al. Admixture mapping in the Hispanic Community Health Study/Study of Latinos reveals regions of genetic associations with blood pressure traits. PLoS ONE 2017, 12, e0188400. [Google Scholar] [CrossRef] [PubMed]
- Duconge, J.; Santiago, E.; Hernandez-Suarez, D.F.; Moneró, M.; López-Reyes, A.; Rosario, M.; Renta, J.Y.; González, P.; Fernández-Morales, L.I.; Vélez-Figueroa, L.A.; et al. Pharmacogenomic polygenic risk score for clopidogrel responsiveness among Caribbean Hispanics: A candidate gene approach. Clin. Transl. Sci. 2021, 14, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yao, M.; Wong, J.Y.Y.; Liu, Z.; Huang, T. Shared genetic etiology and causality between body fat percentage and cardiovascular diseases: A large-scale genome-wide cross-trait analysis. BMC Med. 2021, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.; Li, L.; Warren, L.; Aponte, J.; Fraser, D.; King, K.; Johansson, K.; Barnes, A.; MacPhee, C.; Davies, R.; et al. Pharmacogenetic meta-analysis of baseline risk factors, pharmacodynamic, efficacy and tolerability endpoints from two large global cardiovascular outcomes trials for darapladib. PLoS ONE 2017, 12, e0182115. [Google Scholar] [CrossRef] [PubMed]
- Åkerblom, A.; Eriksson, N.; Wallentin, L.; Siegbahn, A.; Barratt, B.J.; Becker, R.C.; Budaj, A.; Himmelmann, A.; Husted, S.; Storey, R.F.; et al. Polymorphism of the cystatin C gene in patients with acute coronary syndromes: Results from the PLATelet inhibition and patient Outcomes study. Am. Heart J. 2014, 168, 96–102.e2. [Google Scholar] [CrossRef] [PubMed]
- Varenhorst, C.; Eriksson, N.; Johansson, A.A.S.A.; Barratt, B.J.; Hagström, E.; Åkerblom, A.; Syvänen, A.-C.; Becker, R.C.; James, S.K.; Katus, H.A.; et al. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur. Heart J. 2015, 36, 1901–1912. [Google Scholar] [CrossRef]
- Klarin, D.; Zhu, Q.M.; Emdin, C.A.; Chaffin, M.; Horner, S.; McMillan, B.J.; Leed, A.; Weale, M.E.; Spencer, C.C.; Aguet, F.; et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat. Genet. 2017, 49, 1392–1397. [Google Scholar] [CrossRef]
- van der Harst, P.; Verweij, N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res. 2018, 122, 433–443. [Google Scholar] [CrossRef]
- Mehta, N.N. A Genome-Wide Association Study in Europeans and South Asians Identifies 5 New Loci for Coronary Artery Disease. Circ. Cardiovasc. Genet. 2011, 4, 465–466. [Google Scholar] [CrossRef]
- Zhong, W.-P.; Wu, H.; Chen, J.-Y.; Li, X.-X.; Lin, H.-M.; Zhang, B.; Zhang, Z.-W.; Ma, D.-L.; Sun, S.; Li, H.-P.; et al. Genomewide Association Study Identifies Novel Genetic Loci That Modify Antiplatelet Effects and Pharmacokinetics of Clopidogrel. Clin. Pharmacol. Ther. 2017, 101, 791–802. [Google Scholar] [CrossRef]
- Saw, J.; Yang, M.-L.; Trinder, M.; Tcheandjieu, C.; Xu, C.; Starovoytov, A.; Birt, I.; Mathis, M.R.; Hunker, K.L.; Schmidt, E.M.; et al. Chromosome 1q21.2 and additional loci influence risk of spontaneous coronary artery dissection and myocardial infarction. Nat. Commun. 2020, 11, 4432. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Ito, K.; Terao, C.; Akiyama, M.; Horikoshi, M.; Momozawa, Y.; Matsunaga, H.; Ieki, H.; Ozaki, K.; Onouchi, Y.; et al. Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat. Genet. 2020, 52, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hager, J.; Kamatani, Y.; Cazier, J.-B.; Youhanna, S.; Ghassibe-Sabbagh, M.; Platt, D.E.; Abchee, A.B.; Romanos, J.; Khazen, G.; Othman, R.; et al. Genome-Wide Association Study in a Lebanese Cohort Confirms PHACTR1 as a Major Determinant of Coronary Artery Stenosis. PLoS ONE 2012, 7, e38663. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; The Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Consortium; Wang, L.; Chen, S.; He, L.; Yang, X.; Shi, Y.; Cheng, J.; Zhang, L.; Gu, C.C.; et al. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat. Genet. 2012, 44, 890–894. [Google Scholar] [CrossRef]
- Nelson, C.P.; Goel, A.; Butterworth, A.S.; Kanoni, S.; Webb, T.R.; Marouli, E.; Zeng, L.; Ntalla, I.; Lai, F.Y.; Hopewell, J.C.; et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat. Genet. 2017, 49, 1385–1391. [Google Scholar] [CrossRef]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar]
- Dichgans, M.; Rainer, M.; König, I.R. Shared Genetic Susceptibility to Ischemic Stroke and Coronary Artery Disease: A Ge-nome-Wide Analysis of Common Variants. In Stroke; Lippincott Williams and Wilkins: Ambler, PA, USA, 2013. [Google Scholar]
- Temprano-Sagrera, G.; Sitlani, C.M.; Bone, W.P.; Martin-Bornez, M.; Voight, B.F.; Morrison, A.C.; Damrauer, S.M.; de Vries, P.S.; Smith, N.L.; Sabater-Lleal, M.; et al. Multi-phenotype analyses of hemostatic traits with cardiovascular events reveal novel genetic associations. J. Thromb. Haemost. 2022, 20, 1331–1349. [Google Scholar] [CrossRef]
- Vujkovic, M.; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; Zhu, X.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef]
- Zhou, W.; Nielsen, J.B.; Fritsche, L.G.; Dey, R.; Gabrielsen, M.E.; Wolford, B.N.; LeFaive, J.; VandeHaar, P.; Gagliano, S.A.; Gifford, A.; et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 2018, 50, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Ito, K.; Akiyama, M.; Takahashi, A.; Koyama, S.; Nomura, S.; Ieki, H.; Ozaki, K.; Onouchi, Y.; Sakaue, S.; et al. Transethnic Meta-Analysis of Genome-Wide Asso-ciation Studies Identifies Three New Loci and Characterizes Population-Specific Differences for Coronary Artery Disease. Circ. Genom. Precis. Med. 2020, 13, e002670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, H.; Zhu, Q.; Zhang, B.; Yan, H.; Li, H.; Meng, J.; Lai, W.; Li, L.; Yu, D.; et al. A genome-wide association study on lipoprotein (a) levels and coronary artery disease severity in a Chinese population. J. Lipid Res. 2019, 60, 1440–1448. [Google Scholar] [CrossRef]
- Schunkert, H.; König, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–338. [Google Scholar] [CrossRef]
- Van Zuydam, N.R.; Stiby, A.; Abdalla, M.; Austin, E.; Dahlström, E.H.; McLachlan, S.; Vlachopoulou, E.; Ahlqvist, E.; Di Liao, C.; Sandholm, N.; et al. Genome-Wide Association Study of Peripheral Artery Disease. Circ. Genom. Precis. Med. 2021, 14, e002862. [Google Scholar] [CrossRef]
- Ishigaki, K.; Akiyama, M.; Kanai, M.; Takahashi, A.; Kawakami, E.; Sugishita, H.; Sakaue, S.; Matoba, N.; Low, S.-K.; Okada, Y.; et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat. Genet. 2020, 52, 669–679. [Google Scholar] [CrossRef]
- Fall, T.; Gustafsson, S.; Orho-Melander, M.; Ingelsson, E. Genome-wide association study of coronary artery disease among individuals with diabetes: The UK Biobank. Diabetologia 2018, 61, 2174–2179. [Google Scholar] [CrossRef]
- Ward-Caviness, C.K.; Neas, L.M.; Blach, C.; Haynes, C.S.; LaRocque-Abramson, K.; Grass, E.; Dowdy, E.; Devlin, R.B.; Diaz-Sanchez, D.; Cascio, W.E.; et al. Genetic variants in the bone mor-phogenic protein gene family modify the association between residential exposure to traffic and peripheral arterial disease. PLoS ONE 2016, 11, e0152670. [Google Scholar] [CrossRef]
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef]
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the Human Cytochrome P450 Enzymes Involved in the Two Oxidative Steps in the Bioactivation of Clopidogrel to Its Pharmacologically Active Metabolite. Drug Metab. Dispos. 2010, 38, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Bura, A.; Villard, E.; Azizi, M.; Remones, V.; Goyenvalle, C.; Aiach, M.; Lechat, P.; Gaussem, P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 2006, 108, 2244–2247. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasukochi, Y.; Kato, K.; Oguri, M.; Horibe, H.; Fujimaki, T.; Takeuchi, I.; Sakuma, J. Identification of 26 novel loci that confer susceptibility to early-onset coronary artery disease in a Japanese population. Biomed. Rep. 2018, 9, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Xu, H.; Geng, Q.; Mak, W.H.; Ling, F.; Su, Z.; Yang, F.; Zhang, T.; Chen, J.; et al. New genetic variants associated with major adverse cardiovascular events in patients with acute coronary syndromes and treated with clopidogrel and aspirin. Pharmacogenomics J. 2021, 21, 664–672. [Google Scholar] [CrossRef]
- Shaul, O. How introns enhance gene expression. Int. J. Biochem. Cell Biol. 2017, 91, 145–155. [Google Scholar]
- Pai, A.A.; Pritchard, J.K.; Gilad, Y. The Genetic and Mechanistic Basis for Variation in Gene Regulation. PLoS Genet. 2015, 11, e1004857. [Google Scholar] [CrossRef] [PubMed]
- Scherba, J.C.; Halushka, M.K.; Andersen, N.D.; Maleszewski, J.J.; Landstrom, A.P.; Bursac, N.; Glass, C. BRG1 is a biomarker of hypertrophic cardiomyopathy in human heart specimens. Sci. Rep. 2022, 12, 7996. [Google Scholar] [CrossRef] [PubMed]
- Kelloniemi, A.; Szabo, Z.; Serpi, R.; Näpänkangas, J.; Ohukainen, P.; Tenhunen, O.; Kaikkonen, L.; Koivisto, E.; Bagyura, Z.; Kerkelä, R.; et al. The Early-Onset Myocardial Infarction Associated PHACTR1 Gene Regulates Skeletal and Cardiac Alpha-Actin Gene Expression. PLoS ONE 2015, 10, e0130502. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Program, V.M.V.; Lynch, J.; Aragam, K.; Chaffin, M.; Assimes, T.L.; Huang, J.; Lee, K.M.; Shao, Q.; Huffman, J.E.; et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat. Med. 2019, 25, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Henry, A.; Roselli, C.; Lin, H.; Sveinbjörnsson, G.; Fatemifar, G.; Hedman, Å.K.; Wilk, J.B.; Morley, M.P.; Chaffin, M.D.; et al. Genome-wide association, and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat. Commun. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Mastrangelo, M.A.; Ture, S.K.; Smith, C.O.; Loelius, S.G.; Berg, R.A.; Shi, X.; Burke, R.M.; Spinelli, S.L.; Cameron, S.J.; et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat. Commun. 2020, 11, 3479. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; Bergmeijer, T.O.; Gong, L.; Reny, J.; Lewis, J.P.; Mitchell, B.D.; Alexopoulos, D.; Aradi, D.; Altman, R.B.; ICPC Investigators; et al. Genomewide Association Study of Platelet Reactivity and Cardiovascular Response in Patients Treated with Clopidogrel: A Study by the International Clopidogrel Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 2020, 108, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Levy-Sakin, M.; Pastor, S.; Mostovoy, Y.; Li, L.; Leung, A.K.Y.; McCaffrey, J.; Young, E.; Lam, E.T.; Hastie, A.R.; Wong, K.H.Y.; et al. Genome maps across 26 human populations reveal population-specific patterns of structural variation. Nat. Commun. 2019, 10, 1025. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Caribbean Hispanics residing in Puerto Rico Both genders (males/females) Age ≥ 21 Receiving clopidogrel (75 mg/day) for these therapeutic indications: ACS, CAD, and PAD # No clinically active hepatic abnormality The ability to understand the requirements of the study The ability to comply with the study procedures and protocols A female patient is eligible to enter the study if she is of child-bearing potential and not pregnant or nursing, or not of child-bearing potential | Non-Hispanic patients Currently enrolled in another active research protocol Other therapeutic indications # BUN > 30 and creatinine > 2.0 mg/dL Hematocrit (Hct) ≤ 25% Nasogastric or enteral feedings Acute illness (e.g., sepsis, infection, anemia) HIV/AIDS, hepatitis B patients Alcoholism and drug abuse Patients with any cognitive and mental health impairment Sickle cell patients Active malignancy Patients taking another antiplatelet |
| Variable | Mean | SD | SEM | Min. | Max. | Median |
|---|---|---|---|---|---|---|
| Age (years) | 68.01 | 10.95 | 0.51 | 27.00 | 94.00 | 69.00 |
| BMI (kg/m2) | 28.40 | 5.71 | 0.27 | 11.48 | 52.67 | 27.67 |
| Variable | n | % | ||||
| Gender (males) | 282 | 55.29 | ||||
| Diabetes mellitus | 280 | 54.79 | ||||
| Hypertension | 428 | 83.92 | ||||
| Dyslipidemias | 367 | 71.96 | ||||
| Smoking | 69 | 13.53 | ||||
| MACE ¶ | 42 | 13.77 | ||||
| MIs ¶ | 19 | 6.23 | ||||
| Stent thrombosis ¶ | 15 | 4.92 | ||||
| Deaths ¶ | 4 | 1.31 | ||||
| Bleeding events * | 83 | 16.24 | ||||
| Aspirin use | 323 | 63.32 | ||||
| Statin use | 404 | 79.21 | ||||
| CCBs | 137 | 26.86 | ||||
| PPIs | 102 | 19.96 | ||||
| LVEF ≤ 30% | 42 | 8.23 | ||||
| ACS and stable CAD | 387 | 75.88 | ||||
| Coronary artery stenting # | 191 | 37.45 | ||||
| PAD | 123 | 24.12 | ||||
| Chr | POS | SNP | Relation | Reference |
|---|---|---|---|---|
| 19 | 45412079 | rs7412 | ACS | [23] |
| 6 | 46677098 | rs76863441 | ACS | [23] |
| 1 | 109817590 | rs12740374 | ACS | [23] |
| 20 | 23616469 | rs35610040 | ACS | [24] |
| 7 | 99421085 | rs62471956 | ACS | [25] |
| 6 | 161010118 | rs10455872 | ACS | [23] |
| 15 | 44408401 | rs2733201 | ACS | [23] |
| 15 | 44293137 | rs11638352 | ACS | [23] |
| 6 | 161111700 | rs186696265 | ACS | [23] |
| 7 | 99286639 | rs188845491 | ACS | [25] |
| 7 | 98932759 | rs147642358 | ACS | [25] |
| 7 | 100103523 | rs140607780 | ACS | [25] |
| 6 | 160751531 | rs9295128 | ACS | [23] |
| 7 | 99543627 | rs140104968 | ACS | [25] |
| 14 | 84804488 | rs117714106 | ACS | [23] |
| 7 | 99841354 | rs117038461 | ACS | [25] |
| 15 | 44564692 | rs144972973 | ACS | [23] |
| 1 | 172995643 | rs201052613 | ACS | [23] |
| 12 | 125307053 | rs11057830 | ACS | [23] |
| 9 | 107396924 | rs189889864 | ACS | [23] |
| 6 | 161013013 | rs140570886 | CAD | [26,27] |
| 6 | 12903957 | rs9349379 | CAD | [26,27,28,29,30,31,32,33,34,35] |
| 1 | 109821511 | rs602633 | CAD | [27,36,37,38] |
| 19 | 11202306 | rs6511720 | CAD | [23,27,34,37] |
| 19 | 11188153 | rs55791371 | CAD | [27,31,36] |
| 15 | 79141784 | rs7173743 | CAD | [27,31,36] |
| 21 | 35593827 | rs28451064 | CAD | [26,27,30,34,35,37,39] |
| 2 | 203873743 | rs6728861 | CAD | [27] |
| 2 | 203968973 | rs72934535 | CAD | [27,37,39] |
| 2 | 203893999 | rs115654617 | CAD | [27] |
| 6 | 160911596 | rs147555597 | CAD | [27] |
| 6 | 134209837 | rs2327429 | CAD | [27,31,40] |
| 6 | 161018174 | rs7770628 | CAD | [41] |
| 12 | 111884608 | rs3184504 | CAD | [27,35,36,37,42] |
| 11 | 103660567 | rs974819 | CAD | [27,28] |
| 6 | 134159622 | rs1966248 | CAD | [27] |
| 1 | 222829550 | rs35158675 | CAD | [27,31] |
| 1 | 56966350 | rs17114046 | CAD | [27,28,39] |
| 1 | 222837939 | rs17465982 | CAD | [27] |
| 12 | 111932800 | rs7137828 | CAD | [27,37] |
| 6 | 160985526 | rs118039278 | PAD | [26,43] |
| 9 | 22103183 | rs1537372 | PAD | [26,43] |
| 15 | 78915864 | rs10851907 | PAD | [26,43] |
| 1 | 169519049 | rs6025 | PAD | [26,43] |
| 7 | 19049388 | rs2107595 | PAD | [26,43,44] |
| 1 | 109817192 | rs7528419 | PAD | [26,43] |
| 12 | 112871372 | rs11066301 | PAD | [26,43] |
| 7 | 22786532 | rs4722172 | PAD | [26,43] |
| 10 | 114758349 | rs7903146 | PAD | [26,43] |
| 9 | 136149229 | rs505922 | PAD | [26,43] |
| 10 | 69996292 | rs4746743 | Clopidogrel | This study |
| 10 | 69998055 | rs1900005 | Clopidogrel | This study |
| 10 | 69999026 | rs12098677 | Clopidogrel | This study |
| 21 | 39485558 | rs9980291 | Clopidogrel | This study |
| 10 | 70004551 | rs1900003 | Clopidogrel | This study |
| 10 | 70004552 | rs1900002 | Clopidogrel | This study |
| 10 | 69996455 | rs4745950 | Clopidogrel | This study |
| 4 | 185205210 | rs3796692 | Clopidogrel | This study |
| 17 | 21225519 | rs4021557 | Clopidogrel | This study |
| 10 | 69991853 | rs7916697 | Clopidogrel | This study |
| rs Number | Chr | POS | cM | IBS | YRI | NA |
|---|---|---|---|---|---|---|
| rs7528419 | 1 | 109817192 | 138.712247 | 0.064 | 0.09053 | 0.84549 |
| rs17465982 | 1 | 222837939 | 246.478996 | 0.0074 | 0.67445 | 0.3182 |
| rs9349379 | 6 | 12903957 | 28.8898785 | 0.0172 | 0.41137 | 0.57144 |
| rs10455872 | 6 | 161010118 | 177.557204 | 0.5659 | 0.41854 | 0.01556 |
| rs4722172 | 7 | 22786532 | 40.0928349 | 0.0329 | 0.39048 | 0.57665 |
| rs11066301 | 12 | 112871372 | 130.305862 | 0.1981 | 0.00386 | 0.79809 |
| rs11057830 | 12 | 125307053 | 149.857655 | 0.1827 | 0.16692 | 0.65043 |
| rs10851907 | 15 | 78915864 | 102.193041 | 0.0029 | 0.45321 | 0.54385 |
| rs55791371 | 19 | 11188153 | 31.8736735 | 0.0027 | 0.08713 | 0.91016 |
| rs28451064 | 21 | 35593827 | 36.2865085 | 0.0789 | 0.51911 | 0.40204 |
| rs Number | Chr | POS | cM | IBS | YRI | NA |
|---|---|---|---|---|---|---|
| rs62394277 | 5 | 169924749 | 185.487416 | 0.23 | 0.39 | 0.37 |
| rs12033818 | 1 | 106368717 | 134.610925 | 0.05 | 0.01 | 0.94 |
| rs10960270 | 9 | 11818115 | 25.717268 | 0.19 | 0.24 | 0.56 |
| rs2471660 | 12 | 75534099 | 89.727097 | 0.00 | 0.79 | 0.21 |
| rs12627722 | 21 | 17918366 | 7.52177123 | 0.15 | 0.70 | 0.15 |
| rs11653073 | 17 | 55687365 | 83.7730559 | 0.00 | 0.41 | 0.59 |
| rs17025629 | 3 | 88354232 | 109.781656 | 0.67 | 0.22 | 0.11 |
| rs7578180 | 2 | 158772058 | 176.901137 | 0.10 | 0.56 | 0.34 |
| rs75910375 | 17 | 11281457 | 29.8450381 | 0.01 | 0.49 | 0.50 |
| rs74936620 | 1 | 55832969 | 81.3389065 | 0.12 | 0.24 | 0.64 |
| rs77240763 | 5 | 145752501 | 153.573126 | 0.31 | 0.31 | 0.38 |
| rs75970478 | 3 | 138843097 | 150.937494 | 0.11 | 0.62 | 0.26 |
| rs12956301 | 18 | 71834423 | 105.245876 | 0.00 | 0.80 | 0.20 |
| rs10172437 | 2 | 174701949 | 193.753831 | 0.01 | 0.60 | 0.39 |
| rs56919396 | 14 | 95350520 | 95.3439121 | 0.36 | 0.16 | 0.48 |
| rs2567778 | 13 | 103742001 | 103.442192 | 0.36 | 0.52 | 0.12 |
| rs17128958 | 14 | 93708224 | 91.6536648 | 0.42 | 0.14 | 0.44 |
| rs576400715 | 2 | 89571430 | 120.624 | 0.05 | 0.30 | 0.65 |
| rs17089367 | 13 | 73011013 | 70.4386922 | 0.30 | 0.60 | 0.10 |
| rs10948131 | 6 | 44291641 | 69.8263172 | 0.00 | 0.57 | 0.43 |
| rs114354246 | 3 | 137295824 | 149.759253 | 0.15 | 0.61 | 0.24 |
| rs7128963 | 11 | 33599446 | 50.7219531 | 0.06 | 0.29 | 0.66 |
| rs6691697 | 1 | 42534375 | 70.0178338 | 0.07 | 0.32 | 0.61 |
| rs12060034 | 1 | 198224252 | 215.353224 | 0.28 | 0.21 | 0.51 |
| rs12205759 | 6 | 122752471 | 127.395537 | 0.04 | 0.43 | 0.54 |
| rs1611636 | 6 | 29836703 | 50.3216201 | 0.04 | 0.32 | 0.64 |
| rs10512946 | 3 | 134916937 | 148.665958 | 0.15 | 0.61 | 0.23 |
| rs72866243 | 18 | 2094235 | 5.55281054 | 0.45 | 0.44 | 0.11 |
| rs114208611 | 11 | 11100541 | 21.4527621 | 0.00 | 0.40 | 0.60 |
| rs13136539 | 4 | 174513213 | 183.196957 | 0.47 | 0.26 | 0.27 |
| rs77282632 | 3 | 121384210 | 133.9296 | 0.43 | 0.30 | 0.27 |
| rs730489 | 6 | 151399891 | 162.916228 | 0.29 | 0.48 | 0.23 |
| rs528255 | 8 | 12878637 | 33.8526902 | 0.10 | 0.51 | 0.39 |
| rs72834798 | 17 | 38217299 | 64.1399895 | 0.01 | 0.56 | 0.43 |
| rs16883890 | 5 | 9950908 | 22.6794291 | 0.12 | 0.08 | 0.80 |
| rs11996214 | 8 | 135211378 | 159.947948 | 0.01 | 0.60 | 0.39 |
| rs4782152 | 16 | 9326011 | 22.425679 | 0.01 | 0.13 | 0.86 |
| rs13432204 | 2 | 107262432 | 128.94 | 0.05 | 0.20 | 0.74 |
| rs16974292 | 16 | 84652644 | 114.995507 | 0.03 | 0.30 | 0.67 |
| rs116953645 | 19 | 46613411 | 72.3867019 | 0.01 | 0.31 | 0.67 |
| rs2422672 | 20 | 1990286 | 7.8190193 | 0.10 | 0.70 | 0.20 |
| rs17188743 | 6 | 30385111 | 50.6059471 | 0.05 | 0.32 | 0.63 |
| rs973295 | 14 | 94372768 | 92.5873731 | 0.39 | 0.15 | 0.46 |
| rs1889877 | 6 | 69729678 | 84.7215414 | 0.01 | 0.48 | 0.51 |
| rs10114476 | 9 | 96631459 | 113.576597 | 0.15 | 0.71 | 0.14 |
| rs113629052 | 9 | 95358140 | 112.692987 | 0.14 | 0.71 | 0.15 |
| rs62568088 | 9 | 6277534 | 15.8022578 | 0.12 | 0.32 | 0.56 |
| rs4631137 | 5 | 52420445 | 63.8860104 | 0.04 | 0.56 | 0.40 |
| rs2165183 | 2 | 4830190 | 9.36070063 | 0.11 | 0.67 | 0.21 |
| rs675465 | 4 | 20543817 | 36.4528078 | 0.33 | 0.27 | 0.40 |
| rs76887708 | 5 | 98125372 | 109.316657 | 0.00 | 0.76 | 0.24 |
| rs8043824 | 16 | 79847142 | 101.522556 | 0.01 | 0.31 | 0.68 |
| rs17123256 | 14 | 87745773 | 81.7304956 | 0.45 | 0.36 | 0.19 |
| rs115079994 | 22 | 45016212 | 55.6235323 | 0.01 | 0.07 | 0.92 |
| rs113899132 | 5 | 156284101 | 166.222937 | 0.17 | 0.31 | 0.52 |
| rs73142595 | 20 | 54320992 | 85.8174786 | 0.21 | 0.22 | 0.57 |
| rs7700639 | 5 | 6606807 | 16.6711274 | 0.20 | 0.01 | 0.79 |
| rs7714907 | 5 | 142145386 | 149.584982 | 0.28 | 0.37 | 0.35 |
| rs114959453 | 6 | 24423918 | 47.4334264 | 0.03 | 0.35 | 0.62 |
| rs9812688 | 3 | 86878962 | 109.260905 | 0.67 | 0.22 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monero-Paredes, M.; Feliu-Maldonado, R.; Carrasquillo-Carrion, K.; Gonzalez, P.; Rogozin, I.B.; Roche-Lima, A.; Duconge, J. Non-Random Enrichment of Single-Nucleotide Polymorphisms Associated with Clopidogrel Resistance within Risk Loci Linked to the Severity of Underlying Cardiovascular Diseases: The Role of Admixture. Genes 2023, 14, 1813. https://doi.org/10.3390/genes14091813
Monero-Paredes M, Feliu-Maldonado R, Carrasquillo-Carrion K, Gonzalez P, Rogozin IB, Roche-Lima A, Duconge J. Non-Random Enrichment of Single-Nucleotide Polymorphisms Associated with Clopidogrel Resistance within Risk Loci Linked to the Severity of Underlying Cardiovascular Diseases: The Role of Admixture. Genes. 2023; 14(9):1813. https://doi.org/10.3390/genes14091813
Chicago/Turabian StyleMonero-Paredes, Mariangeli, Roberto Feliu-Maldonado, Kelvin Carrasquillo-Carrion, Pablo Gonzalez, Igor B. Rogozin, Abiel Roche-Lima, and Jorge Duconge. 2023. "Non-Random Enrichment of Single-Nucleotide Polymorphisms Associated with Clopidogrel Resistance within Risk Loci Linked to the Severity of Underlying Cardiovascular Diseases: The Role of Admixture" Genes 14, no. 9: 1813. https://doi.org/10.3390/genes14091813
APA StyleMonero-Paredes, M., Feliu-Maldonado, R., Carrasquillo-Carrion, K., Gonzalez, P., Rogozin, I. B., Roche-Lima, A., & Duconge, J. (2023). Non-Random Enrichment of Single-Nucleotide Polymorphisms Associated with Clopidogrel Resistance within Risk Loci Linked to the Severity of Underlying Cardiovascular Diseases: The Role of Admixture. Genes, 14(9), 1813. https://doi.org/10.3390/genes14091813