Analysis of Epidemiological Factors and SNP rs3804100 of TLR2 for COVID-19 in a Cohort of Professionals Who Worked in the First Pandemic Wave in Belém-PA, Brazil
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Ethical Considerations
2.2. Settings and Participants
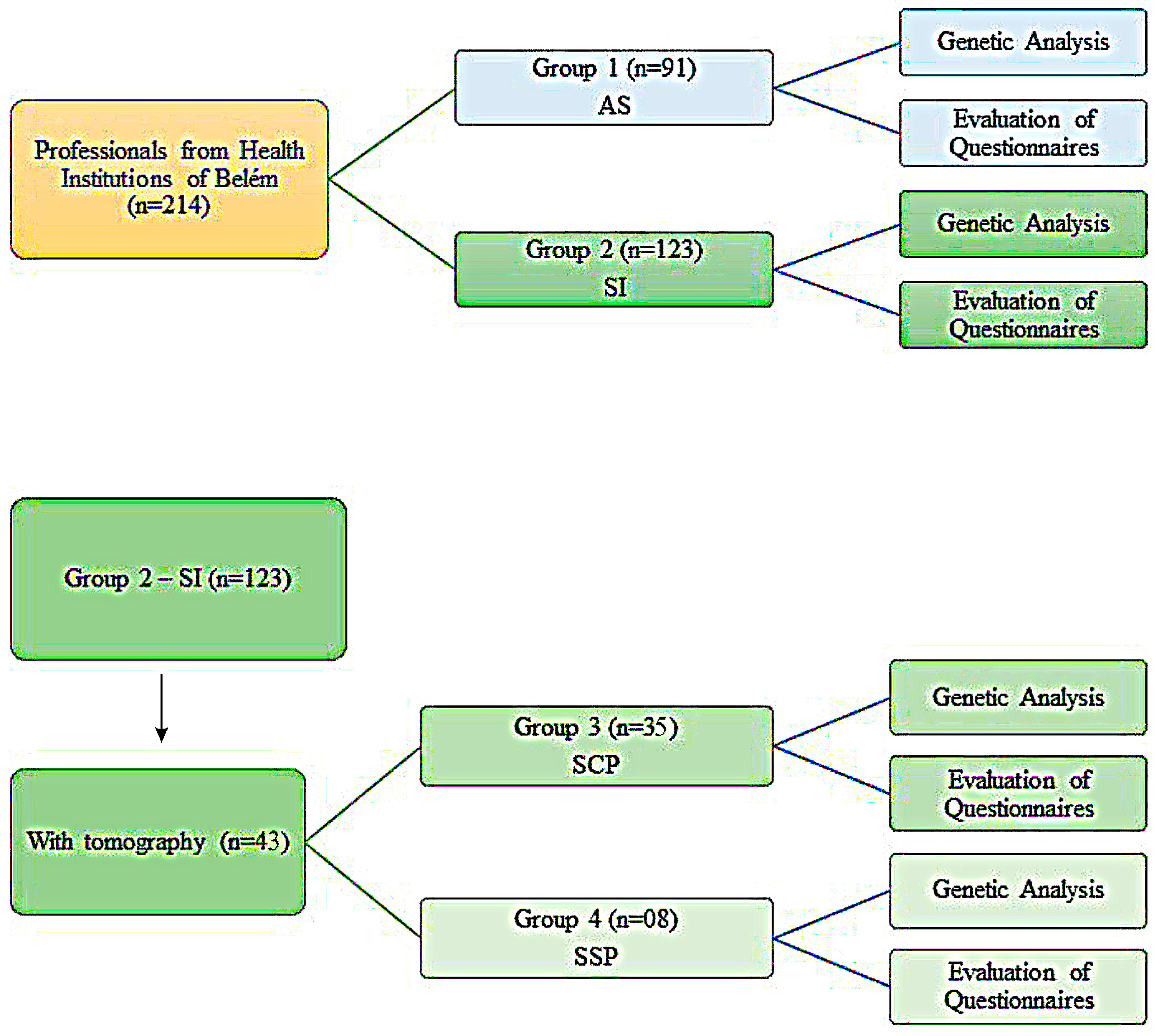
2.3. Variables and Division of Cohort in Groups of This Study
2.4. Sample Collection, DNA Isolation, and Amplification of the Samples by Polymerase Chain Reaction (PCR)
2.5. Running Samples in Capillary Electrophoresis
2.6. Presentation of Data and Statistical Analysis of Results
3. Results
3.1. Power of Sample Size, Normality of Variables, and Hardy-Weinberg Equilibrium (HWE)
3.2. Baseline Characteristics Associated with COVID-19 Symptomatology among Individuals in the Belém Professional Cohort
3.3. Baseline Characteristics Associated with the Severity of COVID-19 among Individuals in the Cohort of Professionals from Belém
3.4. Genotyping Data for TLR2 SNP rs3804100 Related to Symptomatology and Severity of COVID-19
3.5. Analysis of the Characteristics of the Non-Synonymous Mutation rs3804100
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Wang, W.; Zhao, X.; Zai, J.; Zhao, Q.; Li, Y.; Chaillon, A. Transmission Dynamics and Evolutionary History of 2019-nCoV. J. Med. Virol. 2020, 92, 501–511. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report, 72; World Health Organization: Geneva, Switzerland, 2020.
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 14 March 2023).
- Zeiser, F.A.; Donida, B.; da Costa, C.A.; de Oliveira Ramos, G.; Scherer, J.N.; Barcellos, N.T.; Alegretti, A.P.; Ikeda, M.L.R.; Müller, A.P.W.C.; Bohn, H.C.; et al. First and Second COVID-19 Waves in Brazil: A Cross-Sectional Study of Patients’ Characteristics Related to Hospitalization and in-Hospital Mortality. Lancet Reg. Health Am. 2022, 6, 100107. [Google Scholar] [CrossRef]
- Zimmermann, I.R.; Sanchez, M.N.; Frio, G.S.; Alves, L.C.; Pereira, C.C.d.A.; Lima, R.T.d.S.; Machado, C.; Santos, L.M.P.; Silva, E.N. da Trends in COVID-19 Case-Fatality Rates in Brazilian Public Hospitals: A Longitudinal Cohort of 398,063 Hospital Admissions from 1st March to 3rd October 2020. PLoS ONE 2021, 16, e0254633. [Google Scholar] [CrossRef]
- de Albuquerque Costa, W.; de Campos Carvalho, N.; Santana, V.R.; Coelho, P.A.B.; Moreira, A.M.; do Nascimento, M.S. Políticas Públicas e as lições preliminares da COVID-19 na Atenção Primária à Saúde da Ceilândia-DF. Comun. Ciências Saúde 2022, 33, 129–142. [Google Scholar] [CrossRef]
- To, K.K.-W.; Sridhar, S.; Chiu, K.H.-Y.; Hung, D.L.-L.; Li, X.; Hung, I.F.-N.; Tam, A.R.; Chung, T.W.-H.; Chan, J.F.-W.; Zhang, A.J.-X.; et al. Lessons Learned 1 Year after SARS-CoV-2 Emergence Leading to COVID-19 Pandemic. Emerg. Microbes Infect. 2021, 10, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Filho, J.d.A.B.; Sawamura, M.V.Y.; Costa, A.N.; Cerri, G.G.; Nomura, C.H. Pneumonia por COVID-19: Qual o papel da imagem no diagnóstico? J. Bras. Pneumol. 2020, 46, e20200114. [Google Scholar] [CrossRef] [PubMed]
- Kanne, J.P.; Little, B.P.; Chung, J.H.; Elicker, B.M.; Ketai, L.H. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology 2020, 296, E113–E114. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Kay, F.U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; Henry, T.S.; Kanne, J.P.; Kligerman, S.; Ko, J.P.; et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA—Secondary Publication. J. Thorac. Imaging 2020, 35, 219–227. [Google Scholar] [CrossRef]
- BRASIL. Boletim Epidemiológico No 109—Boletim COE Coronavírus—Português (Brasil); Boletim Epidemiológico 109–COE COVID-26 de abril de 2022; Ministério da Saúde, Centro de Operações de Emergência em Saúde Pública/Doença Pelo Coronavírus: Brasília, Brazil, 2022. [Google Scholar]
- World Health Organization. COVID 19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum: Towards a Research Roadmap. Glob. Res. Collab. Infect. Dis. Prep. 2020, 11, e02007. [Google Scholar]
- Hou, Y.; Zhao, J.; Martin, W.; Kallianpur, A.; Chung, M.K.; Jehi, L.; Sharifi, N.; Erzurum, S.; Eng, C.; Cheng, F. New Insights into Genetic Susceptibility of COVID-19: An ACE2 and TMPRSS2 Polymorphism Analysis. BMC Med. 2020, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Rokni, M.; Ghasemi, V.; Tavakoli, Z. Immune Responses and Pathogenesis of SARS-CoV-2 during an Outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 2020, 30, e2107. [Google Scholar] [CrossRef] [PubMed]
- Nemazee, D.; Gavin, A.; Hoebe, K.; Beutler, B. Toll-like Receptors and Antibody Responses. Nature 2006, 441, E4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 Senses the SARS-CoV-2 Envelope Protein to Produce Inflammatory Cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef]
- Root-Bernstein, R. Innate Receptor Activation Patterns Involving TLR and NLR Synergisms in COVID-19, ALI/ARDS and Sepsis Cytokine Storms: A Review and Model Making Novel Predictions and Therapeutic Suggestions. Int. J. Mol. Sci. 2021, 22, 2108. [Google Scholar] [CrossRef]
- Caetano, A.R. SNP Markers: Basic Concepts, Applications in Animal Breeding and Management and Perspectives for the Future. R. Bras. Zootec. 2009, 38, 64–71. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Lima, M.B.M.; Lima, K.V.B.; Lima, L.N.G.C. The Relationship of TLR2 Polymorphisms with Infectious Diseases. Annu. Res. Rev. Biol. 2021, 13, 57–73. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Santana, D.S.; de Oliveira, L.G.; Monteiro, E.O.L.; Lima, L.N.G.C. The Relationship between 896A/G (Rs4986790) Polymorphism of TLR4 and Infectious Diseases: A Meta-Analysis. Front. Genet. 2022, 13, 1045725. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Silva, C.S.; da Silva Vieira, M.C.; dos Santos, P.A.S.; Frota, C.C.; Lima, K.V.B.; Lima, L.N.G.C. The Relationship between TLR3 Rs3775291 Polymorphism and Infectious Diseases: A Meta-Analysis of Case-Control Studies. Genes 2023, 14, 1311. [Google Scholar] [CrossRef]
- Albiger, B.; Dahlberg, S.; Henriques-Normark, B.; Normark, S. Role of the Innate Immune System in Host Defence against Bacterial Infections: Focus on the Toll-like Receptors. J. Intern. Med. 2007, 261, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lafuse, W.P.; Zwilling, B.S. Regulation of Toll-like Receptor 2 Expression by Macrophages Following Mycobacterium Avium Infection. J. Immunol. 2000, 165, 6308–6313. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Schorey, J.S. The β-Glucan Receptor Dectin-1 Functions Together with TLR2 to Mediate Macrophage Activation by Mycobacteria. Blood 2006, 108, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.-L.; Abel, L. Human Genetics of Infectious Diseases: A Unified Theory. EMBO J. 2007, 26, 915–922. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Helsinki Declaration. World Medical Association’s Declaration of Helsinki (with Later Amendments): Ethical Principles for Medical Research Involving Human Subjects; World Medical Association Helsinki: Helsinki, Finland, 1964. [Google Scholar]
- Brasil, M.S. Resolução 466/12 Do Conselho Nacional de Saúde. In Sobre Diretrizes e Normas Regulamentadoras de Pesquisa Envolvendo Seres Humanos; Conselho Nacional de Saúde: Brasília, Brazil, 2012. [Google Scholar]
- Quigley, A.L.; Stone, H.; Nguyen, P.Y.; Chughtai, A.A.; MacIntyre, C.R. Estimating the Burden of COVID-19 on the Australian Healthcare Workers and Health System during the First Six Months of the Pandemic. Int. J. Nurs. Stud. 2021, 114, 103811. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19: Occupational Health and Safety for Health Workers: Interim Guidance, 2 February 2021; World Health Organization: Geneva, Switzerland, 2021.
- Carvajal, D.L.M.; Borges, M.A.S.B.; Zara, A.L.d.S.A.; Pereira, M.S.; da Cunha, L.P.; Turchi, M.D. Perfil Clínico Epidemiológico E Desfechos da Primeira Onda de COVID-19 em Anápolis, Goiás. Braz. J. Infect. Dis. 2022, 26, 102048. [Google Scholar] [CrossRef]
- López-Juárez, P.; Serrano-Oviedo, L.; Pérez-Ortiz, J.M.; García-Jabalera, I.; Bejarano-Ramírez, N.; Gómez-Romero, F.J.; Muñoz-Rodríguez, J.R.; Redondo-Calvo, F.J. Comparative study of the COVID-19 admissions between first and second wave in a cohort of 1,235 patients. Rev. Esp. Quim. 2021, 34, 387–389. [Google Scholar] [CrossRef]
- Choi, H.; Qi, X.; Yoon, S.H.; Park, S.J.; Lee, K.H.; Kim, J.Y.; Lee, Y.K.; Ko, H.; Kim, K.H.; Park, C.M.; et al. Extension of Coronavirus Disease 2019 on Chest CT and Implications for Chest Radiographic Interpretation. Radiol. Cardiothorac. Imaging 2020, 2, e200107. [Google Scholar] [CrossRef]
- UNA-SUS Organização Mundial de Saúde Declara Pandemia Do Novo Coronavírus. Available online: https://www.unasus.gov.br/noticia/organizacao-mundial-de-saude-declara-pandemia-de-coronavirus (accessed on 6 September 2022).
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Farrell, C.M.; Feldgarden, M.; Fine, A.M.; Funk, K. Database Resources of the National Center for Biotechnology Information in 2023. Nucleic Acids Res. 2023, 51, D29. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an Enhanced Web Interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Rapp, B.A.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2000, 28, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Lisby, G. Application of Nucleic Acid Amplification in Clinical Microbiology. Mol. Biotechnol. 1999, 12, 75–100. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Chen, B.; Cole, J.W.; Grond-Ginsbach, C. Departure from Hardy Weinberg Equilibrium and Genotyping Error. Front. Genet. 2017, 8, 167. [Google Scholar] [CrossRef]
- Schneider, S.; Roessli, D.; Excoffier, L. Arlequin: A Software for Population Genetics Data Analysis. User Man. Ver. 2000, 2, 2496–2497. [Google Scholar]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Ruch, P.; Teodoro, D.; Consortium, U. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the Worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Doss, C.G.P.; Rajith, B.; Garwasis, N.; Mathew, P.R.; Raju, A.S.; Apoorva, K.; William, D.; Sadhana, N.R.; Himani, T.; Dike, I. Screening of Mutations Affecting Protein Stability and Dynamics of FGFR1—A Simulation Analysis. Appl. Transl. Genom. 2012, 1, 37–43. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7.20.1–7.20.41. [Google Scholar] [CrossRef]
- Capriotti, E.; Fariselli, P.; Casadio, R. I-Mutant2. 0: Predicting Stability Changes upon Mutation from the Protein Sequence or Structure. Nucleic Acids Res. 2005, 33, W306–W310. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, J.; Mahdavi Poor, B.; Asgharzadeh, V.; Pourostadi, M.; Samadi Kafil, H.; Vegari, A.; Tayebi-Khosroshahi, H.; Asgharzadeh, M. Risk Factors for COVID-19. Infez. Med. 2020, 28, 469–474. [Google Scholar] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Wang, M.; Yang, N.; Luo, X.; Li, W.; Chen, X.; Liu, Y.; Ren, M.; Zhang, X.; Wang, L.; et al. Chest Computed Tomography for the Diagnosis of Patients with Coronavirus Disease 2019 (COVID-19): A Rapid Review and Meta-Analysis. Ann. Transl. Med. 2020, 8, 622. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 296, E115–E117. [Google Scholar] [CrossRef]
- Lai, C.-C.; Liu, Y.H.; Wang, C.-Y.; Wang, Y.-H.; Hsueh, S.-C.; Yen, M.-Y.; Ko, W.-C.; Hsueh, P.-R. Asymptomatic Carrier State, Acute Respiratory Disease, and Pneumonia Due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Facts and Myths. J. Microbiol. Immunol. Infect. 2020, 53, 404–412. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 Novel Coronavirus (SARS-CoV-2) Based on Current Evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef]
- International Labour Office. Women at Work: Trends 2016; ILO: Geneva, Switzerland, 2016; ISBN 92-2-130796-4. [Google Scholar]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pahan, K. Is COVID-19 Gender-Sensitive? J. Neuroimmune Pharmacol. 2021, 16, 38–47. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Patients Infected with SARS-CoV-2: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, Multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Tan, H.-X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and Circulating Follicular Helper T Cell Responses in Recovered Patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Misumi, I.; Starmer, J.; Uchimura, T.; Beck, M.A.; Magnuson, T.; Whitmire, J.K. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Rep. 2019, 27, 514–524.e5. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, X.; Ye, S.; Lian, H.; Wang, H.; Ye, J. Obesity and COVID-19: Mechanistic Insights from Adipose Tissue. J. Clin. Endocrinol. Metab. 2022, 107, 1799–1811. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk Factors for Severity and Mortality in Adult COVID-19 Inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Dong, X.; Wang, B.; Liao, M.; Lin, J.; Yan, Y.; Akdis, C.A.; Gao, Y. Distinct Characteristics of COVID-19 Patients with Initial rRT-PCR-positive and rRT-PCR-negative Results for SARS-CoV-2. Allergy 2020, 75, 1809–1812. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical Characteristics of 140 Patients Infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Gaietto, K.; Freeman, M.C.; DiCicco, L.A.; Rauenswinter, S.; Squire, J.R.; Aldewereld, Z.; Iagnemma, J.; Campfield, B.T.; Wolfson, D.; Kazmerski, T.M.; et al. Asthma as a Risk Factor for Hospitalization in Children with COVID-19: A Nested Case-control Study. Pediatr. Allergy Immunol. 2022, 33, e13696. [Google Scholar] [CrossRef] [PubMed]
- Bloom, C.I.; Cullinan, P.; Wedzicha, J.A. Asthma Phenotypes and COVID-19 Risk: A Population-Based Observational Study. Am. J. Respir. Crit. Care Med. 2022, 205, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Caminati, M.; Lombardi, C.; Micheletto, C.; Roca, E.; Bigni, B.; Furci, F.; Girelli, D.; Senna, G.; Crisafulli, E. Asthmatic Patients in COVID-19 Outbreak: Few Cases despite Many Cases. J. Allergy Clin. Immunol. 2020, 146, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Gémes, K.; Talbäck, M.; Modig, K.; Ahlbom, A.; Berglund, A.; Feychting, M.; Matthews, A.A. Burden and Prevalence of Prognostic Factors for Severe COVID-19 in Sweden. Eur. J. Epidemiol. 2020, 35, 401–409. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Warwick, A.N.; Hysi, P.G.; Kastner, A.; Dick, A.; Khaw, P.T.; Tufail, A.; Foster, P.J.; Khaw, K.-T. Associations with Covid-19 Hospitalisation amongst 406,793 Adults: The UK Biobank Prospective Cohort Study; Epidemiology: London, UK, 2020. [Google Scholar]
- Ren, J.; Pang, W.; Luo, Y.; Cheng, D.; Qiu, K.; Rao, Y.; Zheng, Y.; Dong, Y.; Peng, J.; Hu, Y.; et al. Impact of Allergic Rhinitis and Asthma on COVID-19 Infection, Hospitalization, and Mortality. J. Allergy Clin. Immunol. Pract. 2022, 10, 124–133. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Foster, K.J.; Jauregui, E.; Moore, D.; Adnan, D.; Andy-Nweye, A.B.; Khan, S.; Bishehsari, F. Asthma Prolongs Intubation in COVID-19. J. Allergy Clin. Immunol. Pract. 2020, 8, 2388–2391. [Google Scholar] [CrossRef]
- Adir, Y.; Saliba, W.; Beurnier, A.; Humbert, M. Asthma and COVID-19: An Update. Eur. Respir. Rev. 2021, 30, 210152. [Google Scholar] [CrossRef]
- Sharma, P.; Behl, T.; Sharma, N.; Singh, S.; Grewal, A.S.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Bungau, S. COVID-19 and Diabetes: Association Intensify Risk Factors for Morbidity and Mortality. Biomed. Pharmacother. 2022, 151, 113089. [Google Scholar] [CrossRef]
- Koyama, A.K.; Imperatore, G.; Rolka, D.B.; Lundeen, E.; Rutkowski, R.E.; Jackson, S.L.; He, S.; Kuklina, E.V.; Park, S.; Pavkov, M.E. Risk of Cardiovascular Disease After COVID-19 Diagnosis Among Adults With and Without Diabetes. J. Am. Heart Assoc. 2023, 12, e029696. [Google Scholar] [CrossRef]
- Domingues, C.P.F.; Rebelo, J.S.; Dionisio, F.; Botelho, A.; Nogueira, T. The Social Distancing Imposed to Contain COVID-19 Can Affect Our Microbiome: A Double-Edged Sword in Human Health. mSphere 2020, 5, e00716-20. [Google Scholar] [CrossRef] [PubMed]
- Bazzoli, A.; Probst, T.M. COVID-19 Moral Disengagement and Prevention Behaviors: The Impact of Perceived Workplace COVID-19 Safety Climate and Employee Job Insecurity. Saf. Sci. 2022, 150, 105703. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.; Conti, E.; Saccone, S.; Federico, C. Beyond Virology: Environmental Constraints of the First Wave of COVID-19 Cases in Italy. Env. Sci. Pollut. Res. 2021, 28, 31996–32004. [Google Scholar] [CrossRef] [PubMed]
- Birra, D.; Benucci, M.; Landolfi, L.; Merchionda, A.; Loi, G.; Amato, P.; Licata, G.; Quartuccio, L.; Triggiani, M.; Moscato, P. COVID 19: A Clue from Innate Immunity. Immunol. Res. 2020, 68, 161–168. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Rodrigues, Y.C.; Lima, K.V.B.; Lima, L.N.G.C. Innate Immunity to SARS-CoV-2 Infection: A Review. Epidemiol. Infect. 2022, 150, e142. [Google Scholar] [CrossRef]
- Celardo, I.; Pace, L.; Cifaldi, L.; Gaudio, C.; Barnaba, V. The Immune System View of the Coronavirus SARS-CoV-2. Biol. Direct 2020, 15, 30. [Google Scholar] [CrossRef]
- Merx, S.; Neumaier, M.; Wagner, H.; Kirschning, C.J.; Ahmad-Nejad, P. Characterization and Investigation of Single Nucleotide Polymorphisms and a Novel TLR2 Mutation in the Human TLR2 Gene. Hum. Mol. Genet. 2007, 16, 1225–1232. [Google Scholar] [CrossRef]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Van Der Sluis, R.M.; Cham, L.B.; Gris-Oliver, A.; Gammelgaard, K.R.; Pedersen, J.G.; Idorn, M.; Ahmadov, U.; Hernandez, S.S.; Cémalovic, E.; Godsk, S.H.; et al. TLR2 and TLR7 Mediate Distinct Immunopathological and Antiviral Plasmacytoid Dendritic Cell Responses to SARS-CoV-2 Infection. EMBO J. 2022, 41, e109622. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.; Savani, R.C.; Zaki, H. SARS-CoV-2 Spike Protein Induces Inflammation via TLR2-Dependent Activation of the NF-κB Pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Thuong, N.T.T.; Hawn, T.R.; Thwaites, G.E.; Chau, T.T.H.; Lan, N.T.N.; Quy, H.T.; Hieu, N.T.; Aderem, A.; Hien, T.T.; Farrar, J.J.; et al. A Polymorphism in Human TLR2 Is Associated with Increased Susceptibility to Tuberculous Meningitis. Genes. Immun. 2007, 8, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Texereau, J.; Chiche, J.-D.; Taylor, W.; Choukroun, G.; Comba, B.; Mira, J.-P. The Importance of Toll-Like Receptor 2 Polymorphisms in Severe Infections. Clin. Infect. Dis. 2005, 41, S408–S415. [Google Scholar] [CrossRef] [PubMed]
- Butty, V.; Campbell, C.; Mathis, D.; Benoist, C. DPT-1 Study Group Impact of Diabetes Susceptibility Loci on Progression from Pre-Diabetes to Diabetes in at-Risk Individuals of the Diabetes Prevention Trial-Type 1 (DPT-1). Diabetes 2008, 57, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Abrahão Silva, M.J.; Cordeiro Dos Santos, E.; Rodrigues, Y.C.; Batista Lima, K.V.; Costa Lima, L.N.G. Analysis of the Frequency of the Toll-like 2 Gene Polymorphism in Leprosy. Annu. Res. Rev. Biol. 2022, 37, 1–10. [Google Scholar] [CrossRef]
- Qiu, X.; Dong, Y.; Cao, Y.; Luo, Y. Correlation between TLR2, TLR3, TLR4, and TLR9 Polymorphisms and Susceptibility to and Prognosis of Severe Hepatitis among the Newborns. J. Clin. Lab. Anal. 2018, 32, e22292. [Google Scholar] [CrossRef]
- Quan, C.; Ping, J.; Lu, H.; Zhou, G.; Lu, Y. 3DSNP 2.0: Update and Expansion of the Noncoding Genomic Variant Annotation Database. Nucleic Acids Res. 2021, 50, D950–D955. [Google Scholar] [CrossRef]
- Semlali, A.; Parine, N.R.; Al-Numair, N.S.; Almutairi, M.; Hawsawi, Y.M.; Amri, A.A.; Aljebreen, A.M.; Arafah, M.; Almadi, M.A.; Azzam, N.A.; et al. Potential Role of Toll-like Receptor 2 Expression and Polymorphisms in Colon Cancer Susceptibility in the Saudi Arabian Population. Onco Targets Ther. 2018, 11, 8127–8141. [Google Scholar] [CrossRef]
- Manry, J.; Quintana-Murci, L. A Genome-Wide Perspective of Human Diversity and Its Implications in Infectious Disease. Cold Spring Harb. Perspect. Med. 2013, 3, a012450. [Google Scholar] [CrossRef]
- Salamaikina, S.; Karnaushkina, M.; Korchagin, V.; Litvinova, M.; Mironov, K.; Akimkin, V. TLRs Gene Polymorphisms Associated with Pneumonia before and during COVID-19 Pandemic. Diagnostics 2022, 13, 121. [Google Scholar] [CrossRef]
- Doetschman, T. Influence of Genetic Background on Genetically Engineered Mouse Phenotypes. Methods Mol. Biol. 2009, 530, 423–433. [Google Scholar] [CrossRef]
- van der Made, C.I.; Netea, M.G.; van der Veerdonk, F.L.; Hoischen, A. Clinical Implications of Host Genetic Variation and Susceptibility to Severe or Critical COVID-19. Genome Med. 2022, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, R.H.; Khater, W.S.; El-Kholy, A.; Eldin, S.M.; Gad, D.M.; Bahgat, S.; Negm, E.E.; El Kholy, J.A.; Mowafy, S.; Mahmoud, E. Compliance of Healthcare Workers to the Proper Use of Personal Protective Equipment during the First Wave of COVID-19 Pandemic. J. Infect. Public. Health 2021, 14, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.R.P.N.D.; Almeida, G.A.R.P.N.D.; Carvalho, M.R.C.T.D.; Marcolino, A.B.D.L. Aspectos Relacionados à Saúde Mental Dos Profissionais de Saúde Durante a Pandemia Do COVID-19: Uma Revisão Integrativa Da Literatura/Mental Health Aspects of Health Professionals during the Covid-19 Pandemic: An Integrative Literature Review. Braz. J. Health Rev. 2020, 3, 19481–19491. [Google Scholar] [CrossRef]
- Miguel-Puga, J.A.; Cooper-Bribiesca, D.; Avelar-Garnica, F.J.; Sanchez-Hurtado, L.A.; Colin-Martínez, T.; Espinosa-Poblano, E.; Anda-Garay, J.C.; González-Díaz, J.I.; Segura-Santos, O.B.; Vital-Arriaga, L.C.; et al. Burnout, Depersonalization, and Anxiety Contribute to Post-traumatic Stress in Frontline Health Workers at COVID-19 Patient Care, a Follow-up Study. Brain Behav. 2021, 11, e02007. [Google Scholar] [CrossRef]

| Variable n (%) | AS (n = 91) n (%) | SI (n = 123) n (%) | p-Value |
|---|---|---|---|
| Age group | |||
| 19–34 years | 40 (44%) | 63 (51.2%) | p > 0.05 |
| 35–50 years | 43 (47.2%) | 49 (39.9%) | |
| >50 years | 8 (8.8%) | 11 (8.9%) | |
| Sex | |||
| Female | 67 (73.6%) | 81 (65.9%) | p > 0.05 |
| Male | 24 (26.4%) | 42 (34.1%) | |
| Presence of pre-existing comorbidities | |||
| No comorbidities | 75 (82.4%) | 79 (64.2%) | p = 0.0034 a |
| With comorbidities | 16 (17.6%) | 44 (35.8%) | |
| Quantity of comorbidities | |||
| 1 comorbidity | 16 (100%) | 32 (72.73%) | p = 0.020 b |
| ≥2 comorbidities | 0 (0%) | 12 (27.27%) | |
| Types of comorbidities | |||
| Asthma | 4 (25%) | 11 (18.33%) | p > 0.05 |
| Cardiopathies | 0 (0%) | 4 (6.67%) | p > 0.05 |
| Diabetes mellitus | 0 (0%) | 7 (11.67%) | p = 0.021 c |
| Systemic Arterial Hypertension (SAH) | 5 (31.25%) | 10 (16.67%) | p > 0.05 |
| Overweight and obesity | 5 (31.25%) | 24 (40%) | p = 0.003 d |
| Autoimmune disease | 2 (12.5%) | 0 (0%) | p > 0.05 |
| Kidney disease | 0 (0%) | 1 (1.66%) | p > 0.05 |
| Pulmonary fibrosis | 0 (0%) | 1 (1.66%) | p > 0.05 |
| Glaucoma | 0 (0%) | 2 (3.33%) | p > 0.05 |
| Profession category | |||
| Administrative | 23 (25.3%) | 37 (30.1%) | p > 0.05 |
| Healthcare professional | 56 (61.5%) | 65 (52.8%) | |
| General Services | 12 (13.2%) | 21 (17.1%) | |
| Variable n (%) | Kinship | AS (n = 91) n (%) | SI (n = 123) n (%) | p-Value |
|---|---|---|---|---|
| Getting sick | ||||
| Relatives who did not get sick | Blood Relatives | 38 (41.76%) | 40 (32.52%) | p > 0.05 |
| Relatives who became ill | 53 (58.24%) | 83 (67.48%) | ||
| Living with the research participant | 24 (45.28%) | 58 (69.88%) | p = 0.016 a | |
| Not living with the research participant | 29 (54.72%) | 25 (30.12%) | ||
| Getting sick in the same household | ||||
| Relatives who did not get sick | Non-blood relatives | 64 (70.3%) | 73 (59.3%) | p > 0.05 |
| Relatives who became ill | 27 (29.7%) | 50 (40.7%) | ||
| Variable n (%) | AS (n = 91) n (%) | SCP (n = 35) n (%) | SSP (n = 08) n (%) | p-Value (AS vs. SCP) | p-Value (AS vs. SSP) | p-Value (SCP vs. SSP) |
|---|---|---|---|---|---|---|
| Age group | ||||||
| 19–34 years | 40 (44%) | 12 (34.3%) | 4 (50%) | p > 0.05 | p > 0.05 | p > 0.05 |
| 35–50 years | 43 (47.3%) | 18 (51.4%) | 4 (50%) | |||
| >50 years | 8 (8.8%) | 5 (14.3%) | 0 | |||
| Sex | ||||||
| Female | 67 (73.6%) | 21 (60%) | 6 (75%) | p > 0.05 | p > 0.05 | p > 0.05 |
| Male | 24 (26.4%) | 14 (40%) | 2 (25%) | |||
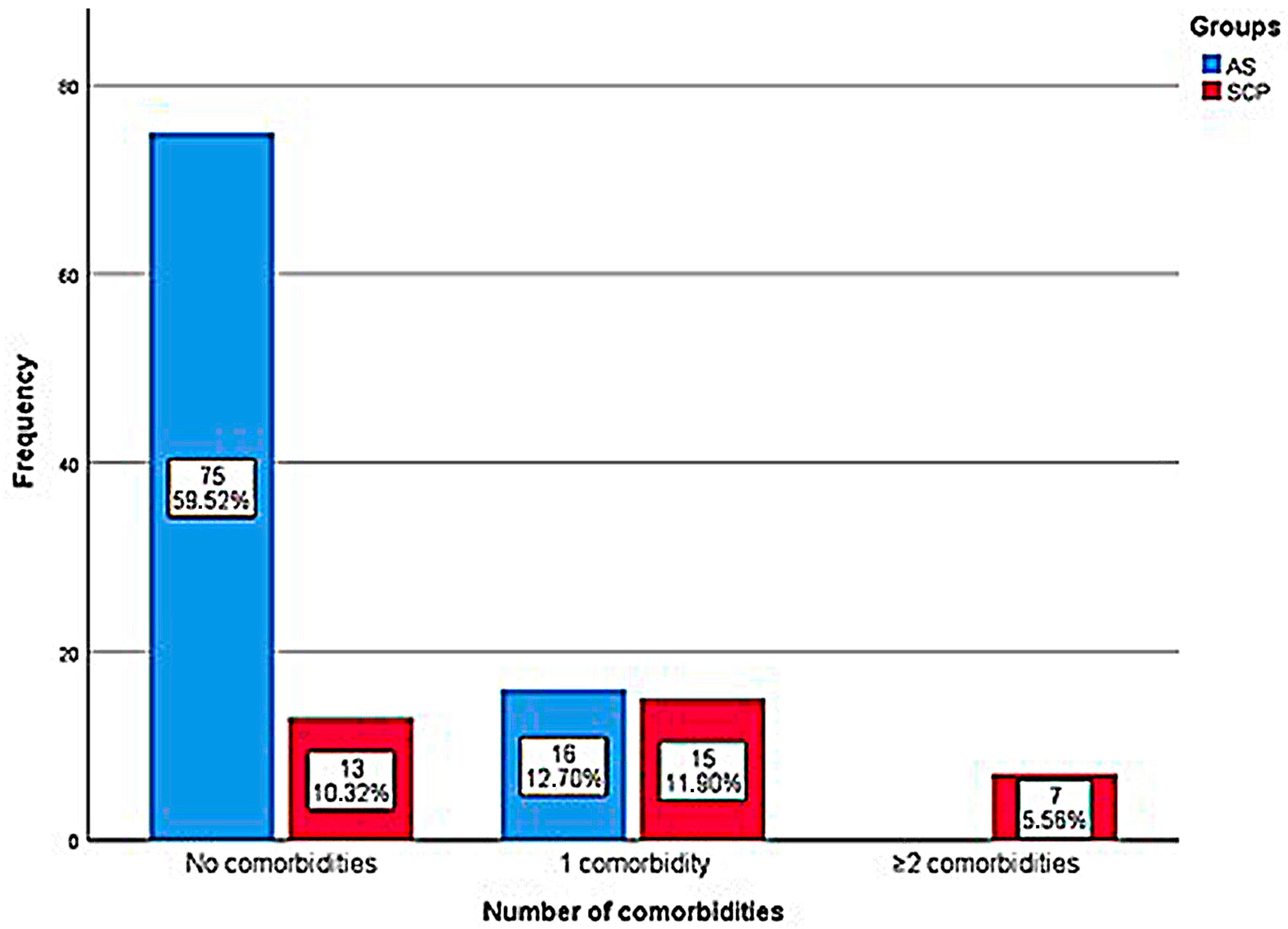
| Presence of pre-existing comorbidities | ||||||
| No comorbidities | 75 (82.4%) | 13 (37.1%) | 7 (87.5%) | p < 0.001 a | p > 0.05 | p = 0.016 b |
| With comorbidities | 16 (17.6%) | 22 (62.9%) | 1 (12.5%) | |||
| Quantity of comorbidities | ||||||
| 1 comorbidity | 16 (100%) | 15 (68.2%) | 0 | p = 0.012 c | p > 0.05 * | p > 0.05 |
| ≥2 comorbidities | 0 | 7 (31.8%) | 1 (100%) | |||
| Types of comorbidities | ||||||
| Asthma | 4 (25%) | 6 (18.75%) | 1 (50%) | p = 0.018 d | p > 0.05 * | p > 0.05 |
| Cardiopathies | 0 | 2 (6.25%) | 0 | p = 0.022 e | - | p > 0.05 |
| Diabetes mellitus | 0 | 4 (12.5%) | 0 | p = 0.001 f | - | p > 0.05 * |
| Systemic arterial hypertension | 5 (31.25%) | 6 (18.75%) | 0 | p = 0.038 g | p > 0.05 * | p > 0.05 * |
| Overweight and obesity | 5 (31.25%) | 12 (37.5%) | 1 (50%) | p < 0.001 h | p > 0.05 * | p > 0.05 |
| Autoimmune disease | 2 (12.5%) | 0 | 0 | p > 0.05 * | p > 0.05 * | - |
| Pulmonary fibrosis | 0 | 1 (3.125%) | 0 | p > 0.05 * | - | p > 0.05 * |
| Glaucoma | 0 | 1 (3.125%) | 0 | p > 0.05 * | - | p > 0.05 * |
| Profession category | ||||||
| Administrative | 23 (25.3%) | 6 (17.1%) | 2 (25%) | p > 0.05 | p > 0.05 | p > 0.05 |
| Healthcare professional | 56 (61.5%) | 25 (71.4%) | 6 (75%) | |||
| General Services | 12 (13.2%) | 4 (11.4%) | 0 | |||
| Variable n (%) | Kinship | AS (n = 91) n (%) | SCP (n = 35) n (%) | SSP (n = 08) n (%) | p-Value (AS vs. SCP) | p-Value (AS vs. SSP) | p-Value (SCP vs. SSP) |
|---|---|---|---|---|---|---|---|
| Getting Sick | |||||||
| Relatives who did not get sick | Blood relatives | 38 (41.76%) | 7 (20%) | 1 (12.5%) | p = 0.022 a | p > 0.05 | p > 0.05 |
| Relatives who became ill | 53 (58.24%) | 28 (80%) | 7 (87.5%) | ||||
| Living with the research participant | 24 (45.28%) | 18 (64.29%) | 6 (85.71%) | p > 0.05 | p = 0.04 b | p > 0.05 | |
| Not living with the research participant | 29 (54.72%) | 10 (35.71%) | 1 (14.29%) | ||||
| Getting sick in the same household | |||||||
| Relatives who did not get sick | Non-blood relatives | 64 (70.3%) | 20 (57.1%) | 5 (62.5%) | p > 0.05 | p> 0.05 | p > 0.05 |
| Relatives who did not get sick | 27 (29.7%) | 15 (42.9%) | 3 (37.5%) | ||||
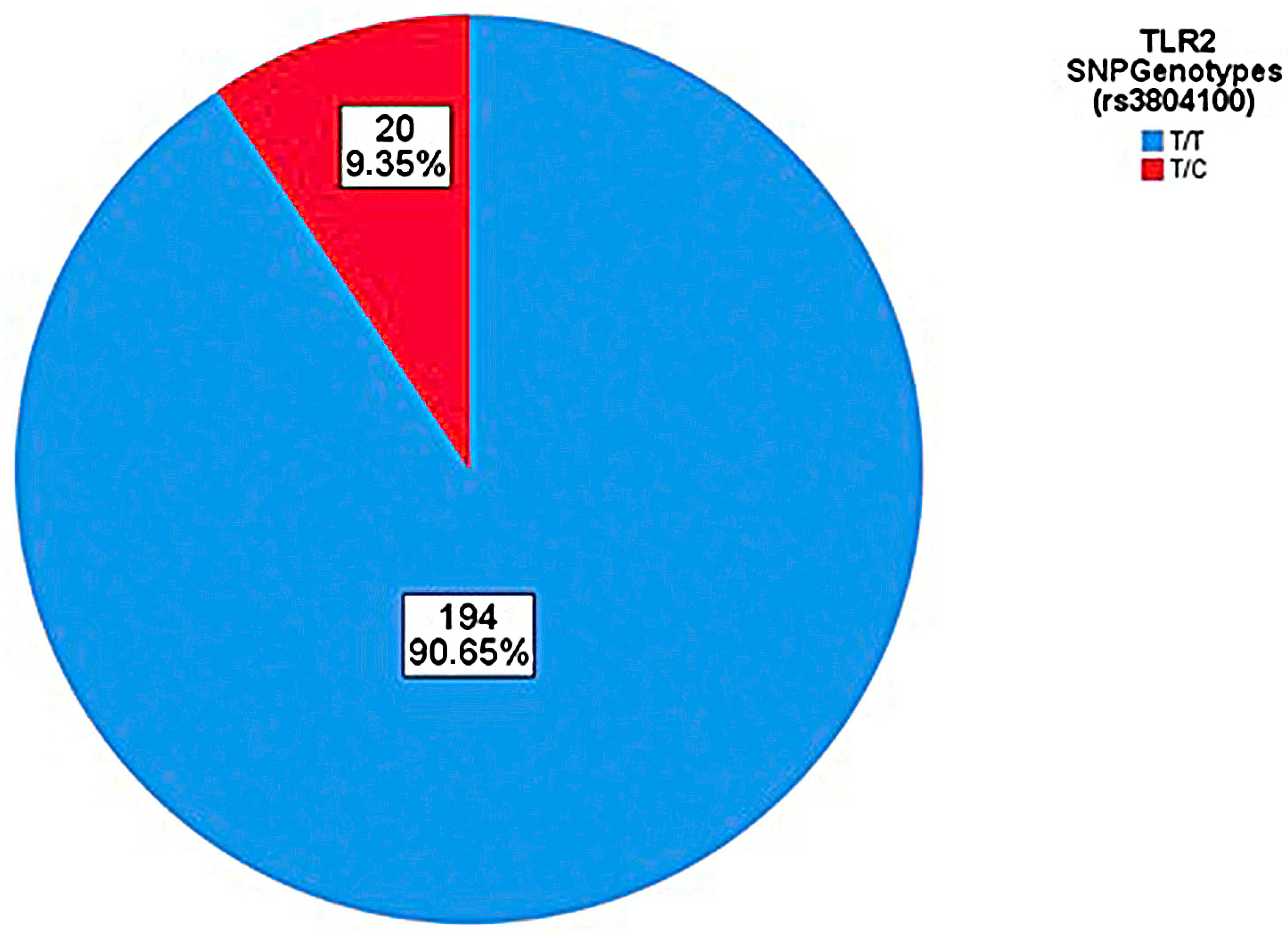
| Genotyping n (%) | AS (n = 91) n (%) | SI (n = 123) n (%) | p-Value |
|---|---|---|---|
| T/T | 83 (91.2%) | 111 (90.2%) | p > 0.05 |
| T/C | 8 (8.8%) | 12 (9.8%) | |
| T (wild allele) | 174 (95.60%) | 234 (95.12%) | p > 0.05 |
| C | 8 (4.4%) | 12 (4.88%) |
| Genotyping n (%) | AS (n = 91) n (%) | SCP (n = 35) n (%) | SSP (n = 08) n (%) | p-Value (AS vs. SCP) | p-Value (AS vs. SSP) | p-Value (SCP vs. SSP) |
|---|---|---|---|---|---|---|
| T/T | 83 (91.2%) | 31 (88.6%) | 8 (100%) | p > 0.05 | p > 0.05 | p > 0.05 |
| T/C | 8 (8.8%) | 4 (11.4%) | 0 | |||
| T (wild allele) | 174 (95.6%) | 66 (94.3%) | 16 (100%) | p > 0.05 | p > 0.05 | p > 0.05 |
| C | 8 (4.4%) | 4 (5.7%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.J.A.; Silva, C.S.; Marinho, R.L.; Cabral, J.G.; Gurrão, E.P.d.C.; dos Santos, P.A.S.; Casseb, S.M.M.; Lima, K.V.B.; Lima, L.N.G.C. Analysis of Epidemiological Factors and SNP rs3804100 of TLR2 for COVID-19 in a Cohort of Professionals Who Worked in the First Pandemic Wave in Belém-PA, Brazil. Genes 2023, 14, 1907. https://doi.org/10.3390/genes14101907
Silva MJA, Silva CS, Marinho RL, Cabral JG, Gurrão EPdC, dos Santos PAS, Casseb SMM, Lima KVB, Lima LNGC. Analysis of Epidemiological Factors and SNP rs3804100 of TLR2 for COVID-19 in a Cohort of Professionals Who Worked in the First Pandemic Wave in Belém-PA, Brazil. Genes. 2023; 14(10):1907. https://doi.org/10.3390/genes14101907
Chicago/Turabian StyleSilva, Marcos Jessé Abrahão, Caroliny Soares Silva, Rebecca Lobato Marinho, Jeanne Gonçalves Cabral, Ellen Polyana da Costa Gurrão, Pabllo Antonny Silva dos Santos, Samir Mansour Moraes Casseb, Karla Valéria Batista Lima, and Luana Nepomuceno Gondim Costa Lima. 2023. "Analysis of Epidemiological Factors and SNP rs3804100 of TLR2 for COVID-19 in a Cohort of Professionals Who Worked in the First Pandemic Wave in Belém-PA, Brazil" Genes 14, no. 10: 1907. https://doi.org/10.3390/genes14101907
APA StyleSilva, M. J. A., Silva, C. S., Marinho, R. L., Cabral, J. G., Gurrão, E. P. d. C., dos Santos, P. A. S., Casseb, S. M. M., Lima, K. V. B., & Lima, L. N. G. C. (2023). Analysis of Epidemiological Factors and SNP rs3804100 of TLR2 for COVID-19 in a Cohort of Professionals Who Worked in the First Pandemic Wave in Belém-PA, Brazil. Genes, 14(10), 1907. https://doi.org/10.3390/genes14101907







