yaaJ, the tRNA-Specific Adenosine Deaminase, Is Dispensable in Bacillus subtilis
Abstract
1. Introduction
2. Materials and Methods
- Construction of B. subtilis strains of the yaaJ and tRNAArg(CCG)
- tRNA isolation
- Mass spectrometry
- Sporulation assay
- Transformation efficiency assay
- Northern blot analysis of B. subtilis tRNAArg(I/ACG) and tRNAArg(CCG)
- Reverse-transcription polymerase chain reaction (RT-PCR) and DNA sequencing
- β−Galactosidase assay
- Frameshift assay
- Quantitative real-time PCR (qPCR)
3. Results
- B. subtilis yaaJ encodes a tRNA adenosine deaminase and is dispensable but beneficial for growth and competency
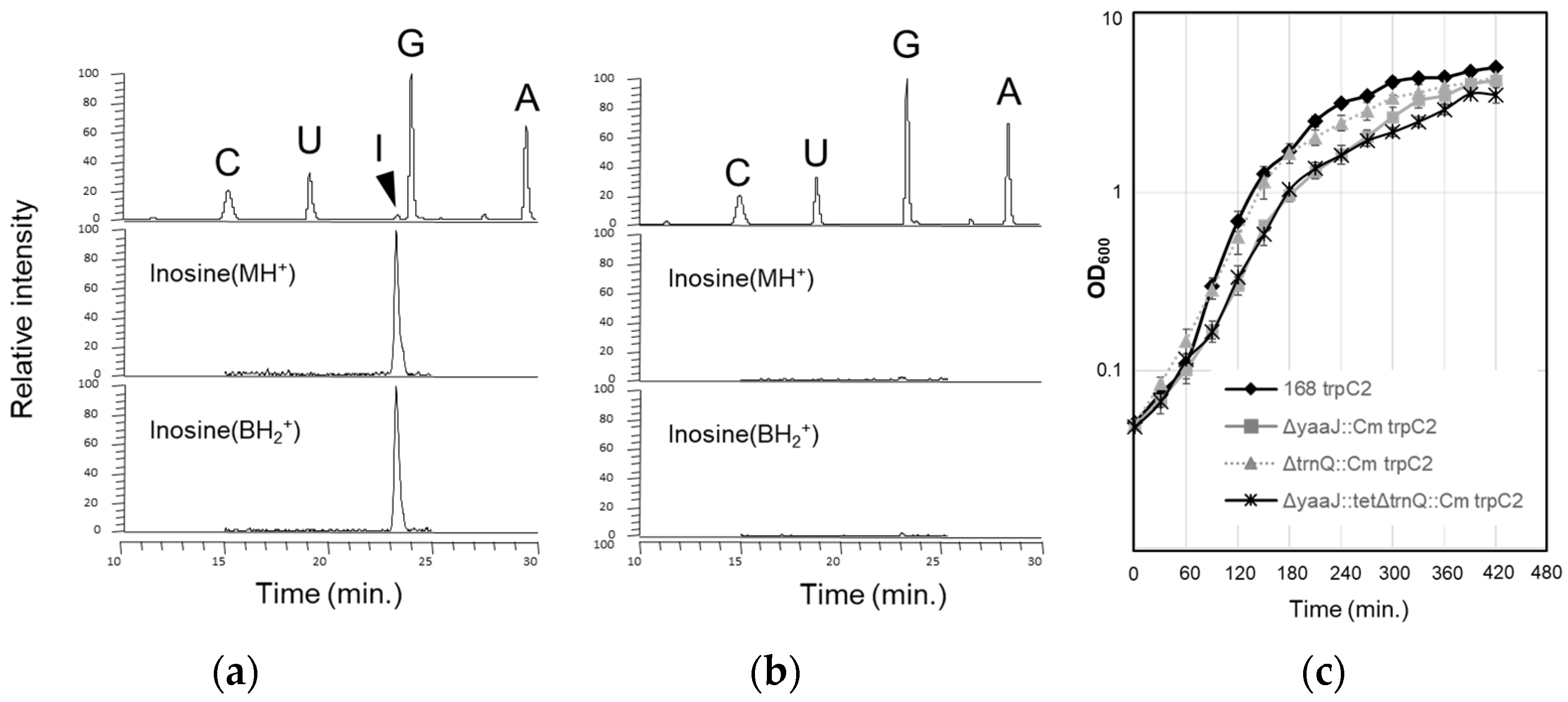
- Translational activity without I34 of B. subtilis tRNAArg
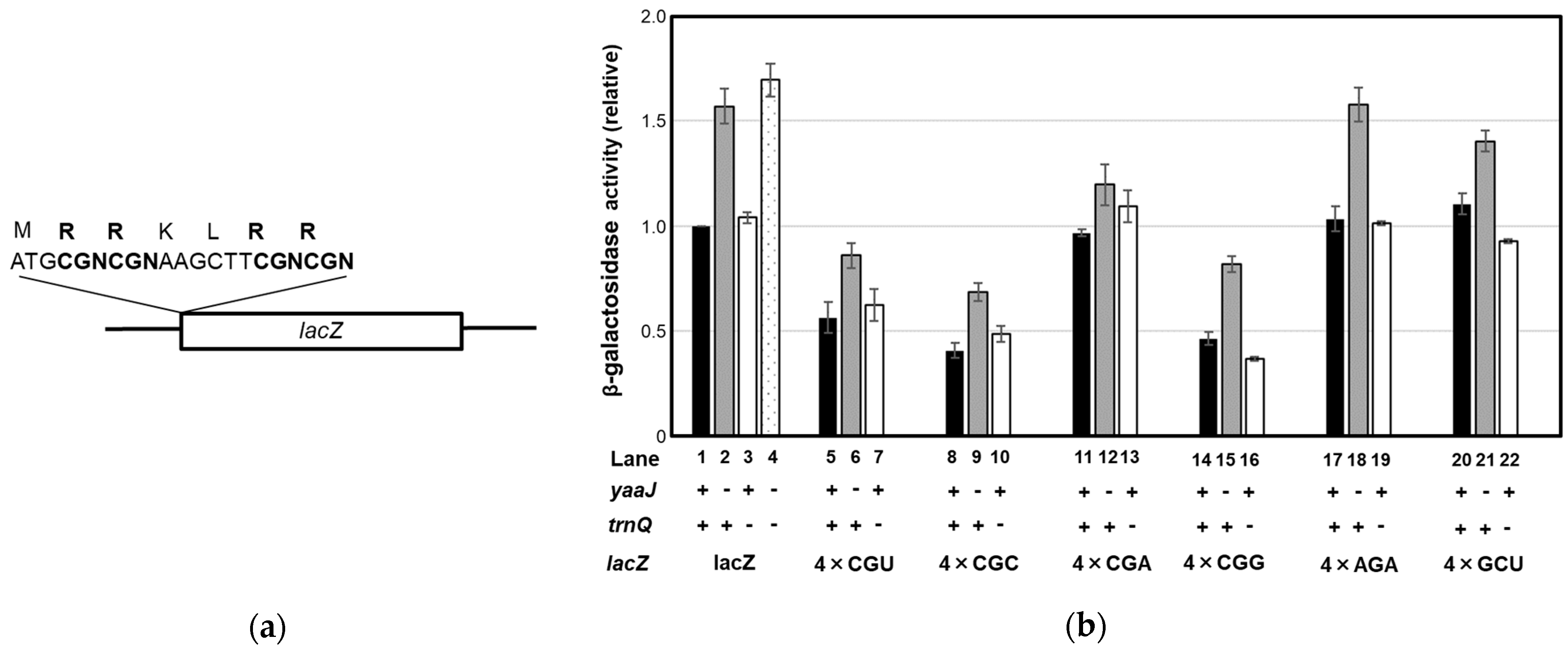
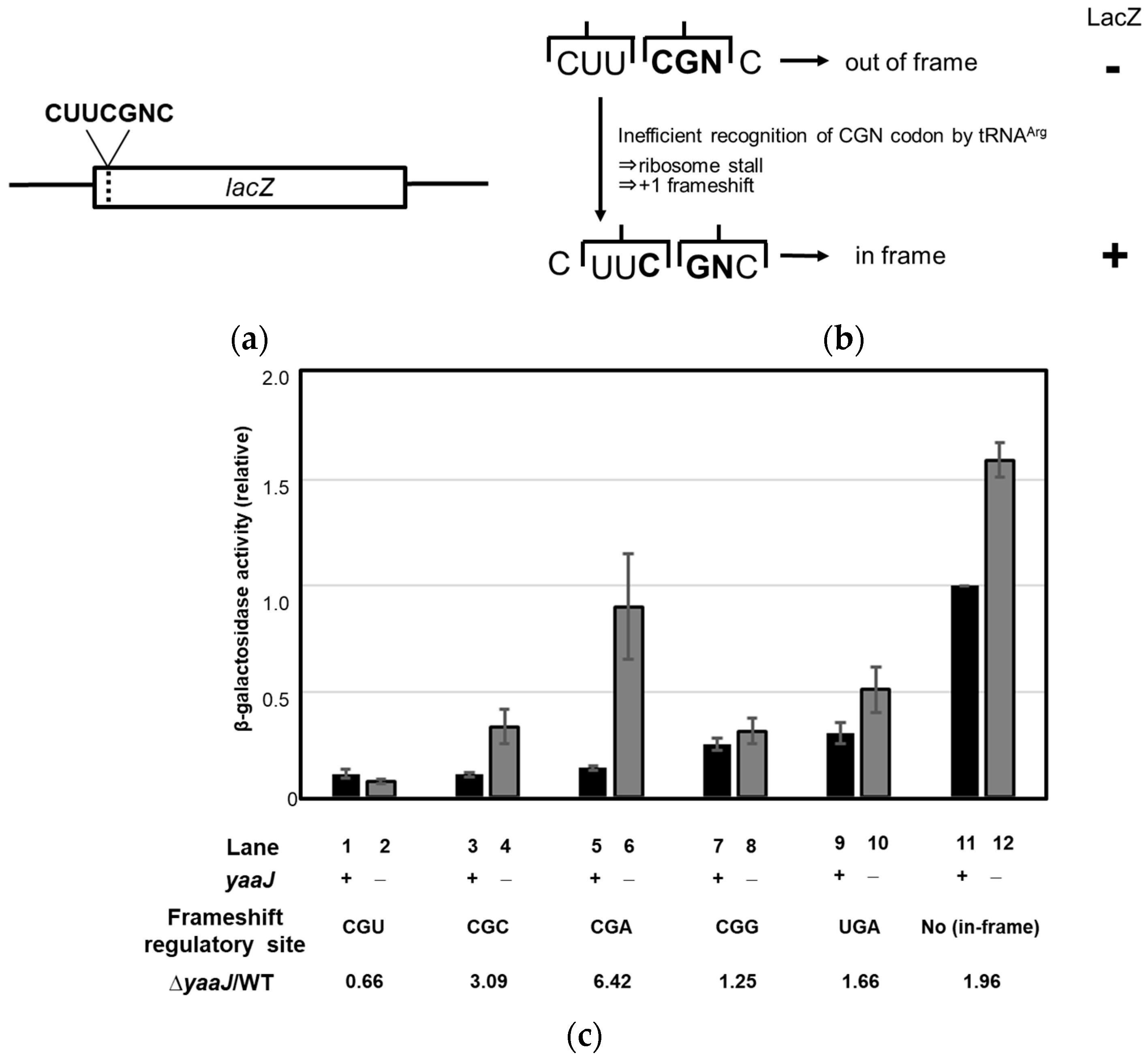
- Lack of I34 increases the rate of frameshifting at the CGA and CGC codons
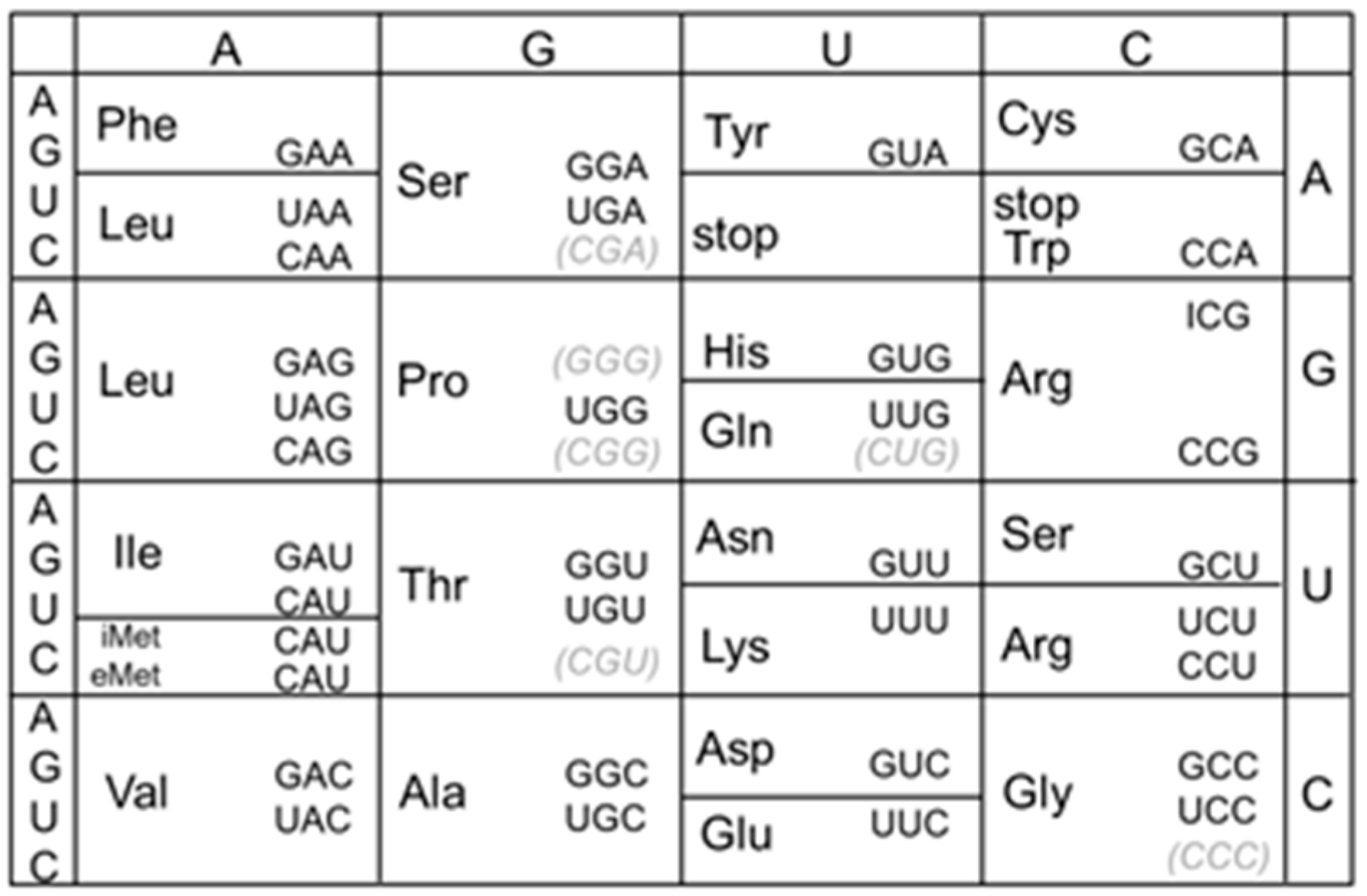
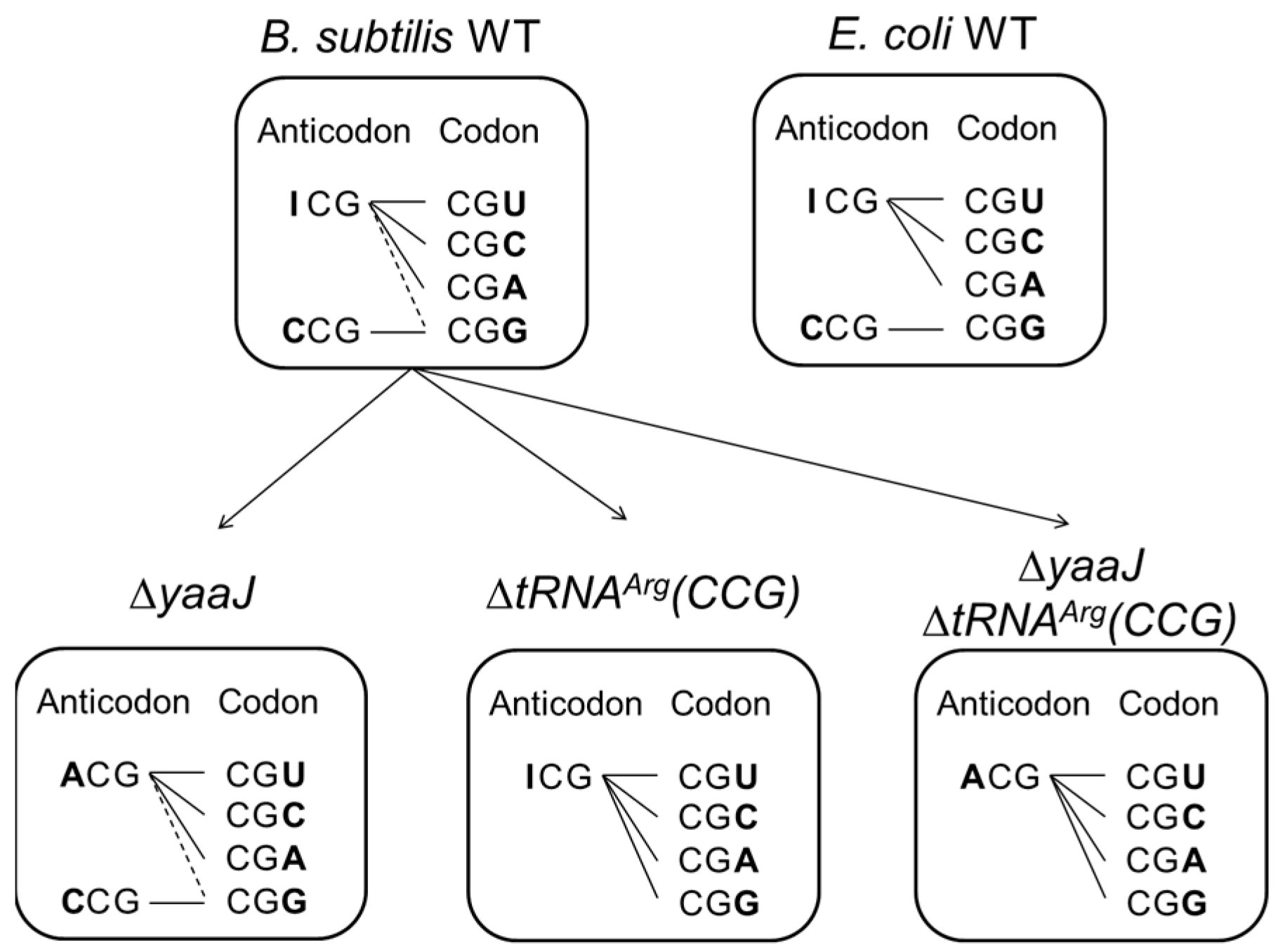
- tRNAArg(CCG) is dispensable, and both tRNAArg(ICG) and tRNAArg(ACG) can each decode all four CGN codons in B. subtilis
- Decoding of the CGN codon family is not accomplished by C34
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crick, F.H. Codon—Anticodon pairing: The wobble hypothesis. J. Mol. Biol. 1966, 19, 548–555. [Google Scholar] [CrossRef]
- Grosjean, H.; Westhof, E. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res. 2016, 44, 8020–8040. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, I.; Pohl, C.; Mittelstaet, J.; Konevega, A.L.; Rodnina, M.V. Evolutionary optimization of speed and accuracy of decoding on the ribosome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2979–2986. [Google Scholar] [CrossRef]
- Yusupova, G.Z.; Yusupov, M.M.; Cate, J.H.; Noller, H.F. The path of messenger RNA through the ribosome. Cell 2001, 106, 233–241. [Google Scholar] [CrossRef]
- Björk, G.R. Biosynthesis and Function of Modified Nucleosides. In tRNA, Structure, Biosynthesis, and Function; Söll, D.R., RajBhandary, U.L., Eds.; American Society for Microbiology: Washington, DC, USA, 1995; pp. 165–205. [Google Scholar] [CrossRef]
- Suzuki, T. Biosynthesis and function of tRNA wobble modifications. In Fine-Tuning of RNA Functions by Modification and Editing; Grosjean, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 12, pp. 23–69. [Google Scholar] [CrossRef]
- Agris, P.F.; Eruysalb, E.R.; Narendranb, A.; Väre, V.Y.P.; Vangavetia, S.; Ranganathan, S.V. Celebrating wobble decoding: Half a century and still much is new. RNA Biol. 2018, 15, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell. Biol. 2021, 22, 375–392. [Google Scholar] [CrossRef]
- Gerber, A.P.; Keller, W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 1999, 286, 1146–1149. [Google Scholar] [CrossRef]
- Liao, W.; Nie, W.; Ahmad, I.; Chen, G.; Zhu, B. The occurrence, characteristics, and adaptation of A-to-I RNA editing in bacteria: A review. Front. Microbiol. 2023, 14, 1143929. [Google Scholar] [CrossRef]
- Jühling, F.; Mörl, M.; Hartmann, R.K.; Sprinzl, M.; Stadler, P.F.; Pütz, J. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009, 37, D159–D162. [Google Scholar] [CrossRef] [PubMed]
- Su, A.A.; Randau, L. A-to-I and C-to-U editing within transfer RNAs. Biochemistry 2011, 76, 932–937. [Google Scholar] [CrossRef]
- Yokoyama, S.; Nishimura, S. Modified Nucleotides and Codon Recognition. In tRNA, Structure, Biosynthesis, and Function; Söll, D.R., RajBhandary, U.L., Eds.; American Society for Microbiology: Washington, DC, USA, 1995; pp. 207–224. [Google Scholar] [CrossRef]
- Curran, J.F. Decoding with the A:I wobble pair is inefficient. Nucleic Acids Res. 1995, 23, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.V.T.; Ramakrishnan, V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat. Struct. Mol. Biol. 2004, 11, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.M.; Alexander, R.W. Bacterial wobble modifications of NNA-decoding tRNAs. IUBMB Life 2019, 71, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Pineyro, D.; Filonava, L.; Stracker, T.H.; Batlle, E.; Ribas de Pouplana, L. A-to-I editing on tRNAs: Biochemical, biological and evolutionary implications. FEBS Lett. 2014, 588, 4279–4286. [Google Scholar] [CrossRef]
- Abe, T.; Inokuchi, H.; Yamada, Y.; Muto, A.; Iwasaki, Y.; Ikemura, T. tRNADB-CE: tRNA gene database well-timed in the era of big sequence data. Front. Genet. 2014, 5, 114. [Google Scholar] [CrossRef]
- Hunter, W.N.; Brown, T.; Anand, N.N.; Kennard, O. Structure of an adenine-cytosine base pair in DNA and its implications for mismatch repair. Nature 1986, 320, 552–555. [Google Scholar] [CrossRef]
- Boren, T.; Elias, P.; Samuelsson, T.; Claesson, C.; Barciszewska, M.; Gehrke, C.W.; Kuo, K.C.; Lustig, F. Undiscriminating codon reading with adenosine in the wobble position. J. Mol. Biol. 1993, 230, 739–749. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kojima, A.; Bessho, Y.; Hori, H.; Ohama, T.; Osawa, S. Translation of synonymous codons in family boxes by Mycoplasma capricolum tRNAs with unmodified uridine or adenosine at the first anticodon position. J. Mol. Biol. 1995, 251, 486–492. [Google Scholar] [CrossRef]
- Lim, V. Analysis of action of the wobble adenine on codon reading within the ribosome. J. Mol. Biol. 1995, 252, 277–282. [Google Scholar] [CrossRef]
- Takai, K.; Takaku, H.; Yokoyama, S. In vitro codon-reading specificities of unmodified tRNA molecules with different anticodons on the sequence background of Escherichia coli tRNASer. Biochem. Biophys. Res. Commun. 1999, 257, 662–667. [Google Scholar] [CrossRef]
- Takai, K. Classification of the possible pairs between the first anticodon and the third codon positions based on a simple model assuming two geometries with which the pairing effectively potentiates the decoding complex. J. Theor. Biol. 2006, 242, 564–580. [Google Scholar] [CrossRef]
- Aldinger, C.A.; Leisinger, A.K.; Gaston, K.W.; Limbach, P.A.; Igloi, G.L. The absence of A-to-I editing in the anticodon of plant cytoplasmic tRNA(Arg) ACG demands a relaxation of the wobble decoding rules. RNA Biol. 2012, 9, 1239–1246. [Google Scholar] [CrossRef]
- Cantara, W.A.; Bilbille, Y.; Kim, J.; Kaiser, R.; Leszczynska, G.; Malkiewicz, A.; Agris, P.F. Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNA(Arg1,2). J. Mol. Biol. 2012, 416, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Lyngdoh, R.H. Role of wobble base pair geometry for codon degeneracy: Purine-type bases at the anticodon wobble position. J. Mol. Model. 2012, 18, 3805–3820. [Google Scholar] [CrossRef] [PubMed]
- Westhof, E. Isostericity and tautomerism of base pairs in nucleic acids. FEBS Lett. 2014, 588, 2464–2469. [Google Scholar] [CrossRef]
- Westhof, E.; Yusupov, M.; Yusupova, G. Recognition of Watson-Crick base pairs: Constraints and limits due to geometric selection and tautomerism. F1000Prime Rep. 2014, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Osawa, S. tRNA Sequences and Variations in the Genetic Code. In tRNA; Söll, D.R., RajBhandary, U.L., Eds.; American Society for Microbiology: Washington, DC, USA, 1995; pp. 225–250. [Google Scholar] [CrossRef]
- Yokobori, S.; Kitamura, A.; Grosjean, H.; Bessho, Y. Life without tRNAArg-adenosine deaminase TadA: Evolutionary consequences of decoding the four CGN codons as arginine in Mycoplasmas and other Mollicutes. Nucleic Acids Res. 2013, 41, 6531–6543. [Google Scholar] [CrossRef] [PubMed]
- Igloi, G.L.; Aldinger, C.A. Where have all the inosines gone? Conflicting evidence for A-to-I editing of the anticodon of higher eukaryotic tRNAACGArg questions the dogma of a universal wobble-mediated decoding of CGN codons. IUBMB Life 2016, 68, 419–422. [Google Scholar] [CrossRef][Green Version]
- Yamazaki, Y.; Niki, H.; Kato, J. Profiling of Escherichia coli chromosome database. Methods Mol. Biol. 2008, 416, 385–389. [Google Scholar] [CrossRef]
- Wolf, J.; Gerber, A.P.; Keller, W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J. 2002, 21, 3841–3851. [Google Scholar] [CrossRef]
- Ogasawara, N. Systematic function analysis of Bacillus subtilis genes. Res. Microbiol. 2000, 151, 129–134. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ehrlich, S.D.; Albertini, A.; Amati, G.; Andersen, K.K.; Arnaud, M.; Asai, K.; Ashikaga, S.; Aymerich, S.; Bessieres, P.; et al. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 2003, 100, 4678–4683. [Google Scholar] [CrossRef]
- Koo, B.M.; Kritikos, G.; Farelli, J.D.; Todor, H.; Tong, K.; Kimsey, K.; Wapinski, I.; Galardini, M.; Cabal, A.; Peters, J.M.; et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 2016, 12, 13. [Google Scholar] [CrossRef]
- Soma, A.; Ikeuchi, Y.; Kanemasa, S.; Kobayashi, K.; Ogasawara, N.; Ote, T.; Kato, J.; Watanabe, K.; Sekine, Y.; Suzuki, T. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell 2003, 12, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Suzuki, T.; Kapushoc, S.T.; Rubio, M.A.; Ghazvini, J.; Watanabe, K.; Simpson, L. Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: Implication for tRNA sorting mechanism. EMBO J. 2003, 22, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Shigi, N.; Kato, J.; Nishimura, A.; Suzuki, T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell 2006, 21, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Leighton, T.J.; Doi, R.H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J. Biol. Chem. 1971, 25, 3189–3195. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Spizizen, J. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 1961, 81, 741–746. [Google Scholar] [CrossRef]
- Soma, A.; Onodera, A.; Sugahara, J.; Kanai, A.; Yachie, N.; Tomita, M.; Kawamura, F.; Sekine, Y. Permuted tRNA genes expressed via a circular RNA intermediate in Cyanidioschyzon merolae. Science 2007, 318, 450–453. [Google Scholar] [CrossRef]
- Casadaban, M.J.; Martinez-Arias, A.; Shapira, S.K.; Chou, J. β−galactosidase gene fusions for analyzing gene expression in Escherichia coli and yeast. Methods Enzymol. 1983, 100, 293–308. [Google Scholar] [CrossRef]
- Morimoto, T.; Loh, P.C.; Hirai, T.; Asai, K.; Kobayashi, K.; Moriya, S.; Ogasawara, N. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology 2002, 148, 3539–3552. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1992. [Google Scholar]
- Curran, J.F.; Yarus, M. Base substitutions in the tRNA anticodon arm do not degrade the accuracy of reading frame maintenance. Proc. Natl. Acad. Sci. USA 1988, 83, 6538–6542. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.F. Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res. 1993, 21, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Errington, J. Bacillus subtilis sporulation: Regulation of gene expression and control of morphogenesis. Microbiol. Rev. 1993, 57, 1–33. [Google Scholar] [CrossRef]
- Stragier, P.; Losick, R. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 1996, 30, 297–341. [Google Scholar] [CrossRef]
- Schultz, D.; Wolynes, P.G.; Ben Jacob, E.; Onuchic, J.N. Deciding fate in adverse times: Sporulation and competence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2009, 106, 21027–21034. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Mekhedov, S.L.; Puigbo, P.; Smirnov, S.; Wolf, Y.I.; Rigden, D.J. Genomic determinants of sporulation in Bacilli and Clostridia: Towards the minimal set of sporulation-specific genes. Environ. Microbiol. 2012, 14, 2870–2890. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N. Competence and Sporulation in Bacillus subtilis. In Fundamentals of Bacterial Physiology and Metabolism; Springer, Singapore: Tannery Lane, Singapore, 2021; pp. 653–670. [Google Scholar] [CrossRef]
- Ogasawara, N. Markedly unbiased codon usage in Bacillus subtilis. Gene 1985, 40, 145–150. [Google Scholar] [CrossRef]
- Kanaya, S.; Yamada, Y.; Kudo, Y.; Ikemura, T. Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: Gene expression level and species-specific diversity of codon usage based on multivariate analysis. Gene 1999, 238, 143–155. [Google Scholar] [CrossRef]
- Kong, J.H.; Dubnau, D. The regulation of competence transcription factor synthesis constitutes a critical control point in the regulation of competence in Bacillus subtilis. J. Bacteriol. 1994, 176, 5753–5761. [Google Scholar]
- Iost, I.; Dreyfus, M. The stability of Escherichia coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 1995, 14, 3252–3261. [Google Scholar] [CrossRef]
- Sharp, J.S.; Bechhofer, D.H. Effect of translational signals on mRNA decay in Bacillus subtilis. J. Bacteriol. 2003, 185, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Duviau, M.P.; Chen, F.; Emile, A.; Cocaign-Bousquet, M.; Girbal, L.; Nouaille, S. When translation elongation is impaired, the mRNA is uniformly destabilized by the RNA degradosome, while the concentration of mRNA is altered along the molecule. Nucleic Acids Res. 2023, 51, 2877–2890. [Google Scholar] [CrossRef]
- Oba, T.; Andachi, Y.; Muto, A.; Osawa, S. CGG: An unassigned or nonsense codon in Mycoplasma capricolum. Proc. Natl. Acad. Sci. USA 1991, 88, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, E.; Le Ret, M.; Faivre-Nitschke, E.; Estavillo, G.M.; Bergdoll, M.; Taylor, N.L.; Pogson, B.J.; Small, I.; Imbault, P.; Gualberto, J.M. Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation. Plant Cell 2009, 21, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, M.; Karcher, D.; Bock, R. Superwobbling facilitates translation with reduced tRNA sets. Nat. Struct. Mol. Biol. 2008, 15, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Payne, B.; Feyertag, F.; Alvarez-Ponce, D. The codon statistics database: A database of codon usage bias. Mol. Biol. Evol. 2022, 39, msac157. [Google Scholar] [CrossRef]
- Alkatib, S.; Scharf, L.B.; Rogalski, M.; Fleischmann, T.; Matthes, A.; Seeger, S.; Schöttler, M.A.; Ruf, S.; Bock, R. The contributions of wobbling and superwobbling to the reading of the genetic code. PLoS Genet. 2012, 8, e1003076. [Google Scholar] [CrossRef]
- Yarus, M. Crick wobble and superwobble in standard genetic code evolution. J. Mol. Evol. 2021, 89, 50–61. [Google Scholar] [CrossRef]
- Lei, L.; Burton, Z.F. “Superwobbling” and tRNA-34 wobble and tRNA-37 anticodon loop modifications in evolution and devolution of the genetic code. Life 2022, 12, 252. [Google Scholar] [CrossRef]
- Karijolich, J.; Yu, Y.T. Modifying the genetic code: Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 2011, 474, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Matsugi, J.; Ishikura, H.; Murao, K. Bacillus subtilis tRNA(Pro) with the anticodon mo5UGG can recognize the codon CCC. Biochim. Biophys. Acta 2005, 1728, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ogle, J.M.; Ramakrishnan, V. Structural insights into translational fidelity. Annu. Rev. Biochem. 2005, 74, 129–177. [Google Scholar] [CrossRef]
- Demeshkina, N.; Jenner, L.; Westhof, E.; Yusupov, M.; Yusupova, G. A new understanding of the decoding principle on the ribosome. Nature 2012, 484, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.; Demeshkina, N.; Khusainov, I.; Westhof, E.; Yusupov, M.; Yusupova, G. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 2016, 7, 10457. [Google Scholar] [CrossRef]
- Rozov, A.; Demeshkina, N.; Westhof, E.; Yusupov, M.; Yusupova, G. New structural insights into translational miscoding. Trends Biochem. Sci. 2016, 41, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Hoernes, T.P.; Faserl, K.; Juen, M.A.; Kremser, J.; Gasser, C.; Fuchs, E.; Shi, X.; Siewert, A.; Lindner, H.; Kreutz, C.; et al. Translation of non-standard codon nucleotides reveals minimal requirements for codon-anticodon interactions. Nat. Commun. 2018, 9, 4865. [Google Scholar] [CrossRef]
- Ou, X.; Cao, J.; Cheng, A.; Peppelenbosch, M.P.; Pan, Q. Errors in translational decoding: tRNA wobbling or misincorporation? PLoS Genet. 2019, 15, e1008017. [Google Scholar] [CrossRef]
- Claesson, C.; Lustig, F.; Boren, T.; Simonsson, C.; Barciszewska, M.; Lagerkvist, U. Glycine codon discrimination and the nucleotide in position 32 of the anticodon loop. J. Mol. Biol. 1995, 247, 191–196. [Google Scholar] [CrossRef]
- Li, J.; Esberg, B.; Curran, J.F.; Bjork, G.R. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 1997, 271, 209–221. [Google Scholar] [CrossRef]
- Olejniczak, M.; Uhlenbeck, O.C. tRNA residues that have coevolved with their anticodon to ensure uniform and accurate codon recognition. Biochimie 2006, 88, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, D. Tryptophan transfer RNA as the UGA suppressor. J. Mol. Biol. 1971, 58, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Cochella, L.; Green, R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science 2005, 308, 1178–1180. [Google Scholar] [CrossRef]
- Schmeing, T.M.; Voorhees, R.M.; Kelley, A.C.; Ramakrishnan, V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat. Struct. Mol. Biol. 2011, 18, 432–436. [Google Scholar] [CrossRef]
- Stephen, P.; Ye, S.; Zhou, M.; Song, J.; Zhang, R.; Wang, E.D.; Giegé, R.; Lin, S.X. Structure of Escherichia coli Arginyl-tRNA synthetase in complex with tRNAArg: Pivotal role of the D-loop. J. Mol. Biol. 2018, 430, 1590–1606. [Google Scholar] [CrossRef]
- Fabret, C.; Dervyn, E.; Dalmais, B.; Guillot, A.; Marck, C.; Grosjean, H.; Noirot, P. Life without the essential bacterial tRNA Ile2-lysidine synthetase TilS: A case of tRNA gene recruitment in Bacillus subtilis. Mol. Microbiol. 2011, 80, 1062–1074. [Google Scholar] [CrossRef]
- Köhrer, C.; Mandal, D.; Gaston, K.W.; Grosjean, H.; Limbach, P.A.; Rajbhandary, U.L. Life without tRNAIle-lysidine synthetase: Translation of the isoleucine codon AUA in Bacillus subtilis lacking the canonical tRNA2Ile. Nucleic Acids Res. 2014, 42, 1904–1915. [Google Scholar] [CrossRef]
- Taniguchi, T.; Miyauchi, K.; Nakane, D.; Miyata, M.; Muto, A.; Nishimura, S.; Suzuki, T. Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res. 2013, 41, 2621–2631. [Google Scholar] [CrossRef]
- Murayama, R.; Akanuma, G.; Makino, Y.; Nanamiya, H.; Kawamura, F. Spontaneous transformation and its use for genetic mapping in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2004, 68, 1672–1680. [Google Scholar] [CrossRef][Green Version]
| Strain | Genotype (Characteristics) | Source or Reference |
|---|---|---|
| 168 | trpC2 (Wild-type) | Laboratory stock |
| KUB10 | ΔyaaJ::cat trpC (yaaJ-deletion) | This study |
| SOM1 | ΔtrnQ-Arg(CCG)::cat trpC2 (trnQ-deletion) | This study |
| SOM2 | ΔyaaJ::tet ΔtrnQ-Arg(CCG)::cat trpC2 (yaaJ, trnQ-double deletion) | This study |
| SOM3 | ΔtrnQ-Arg(CCG)::spc trpC2 (trnQ-deletion) | This study |
| Strain | Viable Bacteria Count (c.f.u. mL−) | Frequency (%) | |
|---|---|---|---|
| Total | Spore | ||
| Wild-type (trpC2) | (2.30 ± 0.63) × 108 | (2.06 ± 0.23) × 108 | 89.6 |
| KUB10 (ΔyaaJ trpC2) | (1.55 ± 0.14) × 108 | (1.09 ± 0.87) × 108 | 70.0 |
| Strain | Viable Bacteria Count (c.f.u. mL−) | Transformation Frequency (%) | |
|---|---|---|---|
| Total | Transformant | ||
| Wild-type (trpC2) | (4.36 ± 0.17) × 108 | (1.16 ± 0.30) × 105 | 0.027 |
| KUB10 (ΔyaaJ trpC2) | (4.01 ± 0.22) × 108 | (1.17 ± 0.01) × 103 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soma, A.; Kubota, A.; Tomoe, D.; Ikeuchi, Y.; Kawamura, F.; Arimoto, H.; Shiwa, Y.; Kanesaki, Y.; Nanamiya, H.; Yoshikawa, H.; et al. yaaJ, the tRNA-Specific Adenosine Deaminase, Is Dispensable in Bacillus subtilis. Genes 2023, 14, 1515. https://doi.org/10.3390/genes14081515
Soma A, Kubota A, Tomoe D, Ikeuchi Y, Kawamura F, Arimoto H, Shiwa Y, Kanesaki Y, Nanamiya H, Yoshikawa H, et al. yaaJ, the tRNA-Specific Adenosine Deaminase, Is Dispensable in Bacillus subtilis. Genes. 2023; 14(8):1515. https://doi.org/10.3390/genes14081515
Chicago/Turabian StyleSoma, Akiko, Atsushi Kubota, Daisuke Tomoe, Yoshiho Ikeuchi, Fujio Kawamura, Hijiri Arimoto, Yuh Shiwa, Yu Kanesaki, Hideaki Nanamiya, Hirofumi Yoshikawa, and et al. 2023. "yaaJ, the tRNA-Specific Adenosine Deaminase, Is Dispensable in Bacillus subtilis" Genes 14, no. 8: 1515. https://doi.org/10.3390/genes14081515
APA StyleSoma, A., Kubota, A., Tomoe, D., Ikeuchi, Y., Kawamura, F., Arimoto, H., Shiwa, Y., Kanesaki, Y., Nanamiya, H., Yoshikawa, H., Suzuki, T., & Sekine, Y. (2023). yaaJ, the tRNA-Specific Adenosine Deaminase, Is Dispensable in Bacillus subtilis. Genes, 14(8), 1515. https://doi.org/10.3390/genes14081515






