Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review
Abstract
1. Introduction
2. Description of MSC-EVs
2.1. Classification, Labeling, Formation, and Delivery of MSC-EVs
2.2. MSCs and MSC-EVs
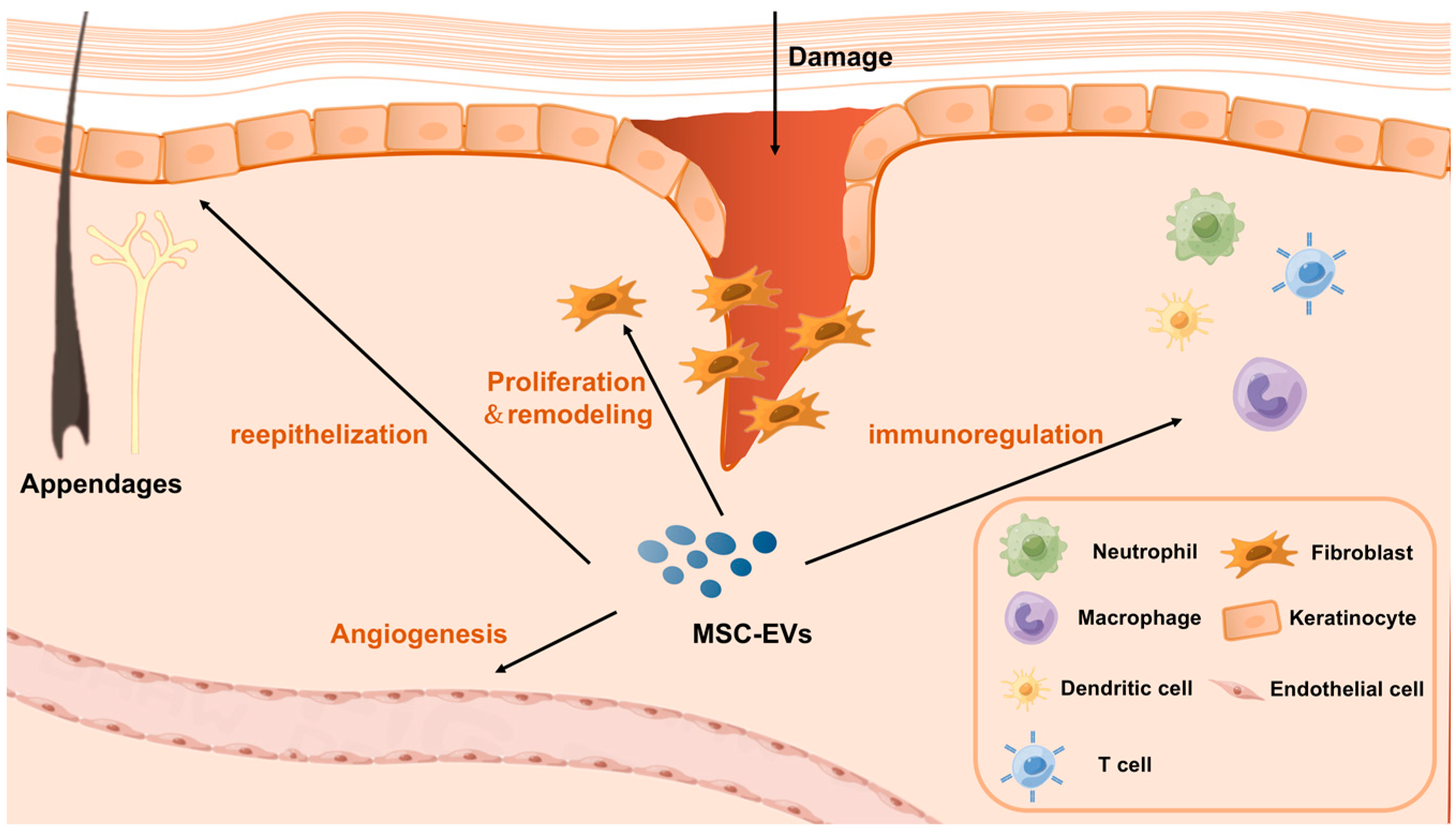
3. Mechanism of MSC-EVs in Promoting Wound Healing and Skin Regeneration
3.1. MSC-EVs Regulate Neutrophil Changes
3.2. MSC-EVs Regulate Macrophage Changes
3.3. MSC-EVs Regulate T Cell Changes
3.4. MSC-EVs Regulate Fibroblast, Keratinocyte, and Endothelial Cell Changes
3.5. MSC-EVs Promote Scar-Free Repair of Skin Wounds
3.6. Application of MSC-EVs in Animal Studies
4. Challenges in Applying MSC-EVs to Promote Wound Healing and Skin Regeneration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salibian, A.A.; Widgerow, A.D.; Abrouk, M.; Evans, G.R. Stem Cells in Plastic Surgery: A Review of Current Clinical and Translational Applications. Arch. Plast. Surg. 2013, 40, 666–675. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.; Gorskaja, J.; Kulagina, N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Kourembanas, S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Massa, M.; Croce, S.; Campanelli, R.; Abbà, C.; Lenta, E.; Valsecchi, C.; Avanzini, M.A. Clinical Applications of Mesenchymal Stem/Stromal Cell Derived Extracellular Vesicles: Therapeutic Potential of an Acellular Product. Diagnostics 2020, 10, 999. [Google Scholar] [CrossRef]
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease out-comes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424. [Google Scholar] [CrossRef]
- Caplan, A.; Dennis, J. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Koliaraki, V.; Prados, A.; Armaka, M.; Kollias, G. The mesenchymal context in inflammation, immunity and cancer. Nat. Immunol. 2020, 21, 974–982. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Li, F.; You, M.; Zhong, L.; Li, W.; Zhang, B.; Chen, Q. The biological changes of umbilical cord mesenchymal stem cells in inflammatory environment induced by different cytokines. Mol. Cell. Biochem. 2018, 446, 171–184. [Google Scholar] [CrossRef]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Zöller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, R.; Kamga, P.T.; Tanasi, I.; Krampera, M. Extracellular Vesicle-Dependent Communication Between Mesenchymal Stromal Cells and Immune Effector Cells. Front. Cell Dev. Biol. 2020, 8, 596079. [Google Scholar] [CrossRef]
- Ramos, T.L.; Sánchez-Abarca, L.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. CCS 2016, 14, 2. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.; Ji, H.-L. Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses. Stem Cells Transl. Med. 2019, 8, 344–354. [Google Scholar] [CrossRef]
- Hur, Y.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in stem cell biology. Stem Cells 2020, 38, 469–476. [Google Scholar] [CrossRef]
- O’brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Vardi, A. Extracellular vesicles—New players in cell–cell communication in aquatic environments. Curr. Opin. Microbiol. 2018, 43, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.; Thum, T. Exosomes: New players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, N.H.; Perez, R.L.; Debus, J.; Huber, P.E. Mesenchymal stem cells—A new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015, 366, 133–140. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fang, H.; Shao, S.; Dang, E.; Zhang, J.; Qiao, P.; Yang, A.; Wang, G. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019, 33, 13241–13253. [Google Scholar] [CrossRef]
- Martin, C.; Muir, I. The role of lymphocytes in wound healing. Br. J. Plast. Surg. 1990, 43, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.; Zhang, B.; Teo, J.; Lai, R.; Choo, A.; Lam, K.; Lim, S. Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy 2022, 24, 711–719. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef] [PubMed]
- Gregorius, J.; Wang, C.; Stambouli, O.; Hussner, T.; Qi, Y.; Tertel, T.; Börger, V.; Yusuf, A.M.; Hagemann, N.; Yin, D.; et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res. Cardiol. 2021, 116, 40. [Google Scholar] [CrossRef]
- Wang, L.; Dorn, P.; Zeinali, S.; Froment, L.; Berezowska, S.; Kocher, G.J.; Alves, M.P.; Brügger, M.; Esteves, B.I.O.; Blank, F.; et al. CD90+CD146+ identifies a pulmonary mesenchymal cell subtype with both immune modulatory and perivascular-like function in postnatal human lung. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L813–L830. [Google Scholar] [CrossRef] [PubMed]
- Sicco, C.L.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.; Kourembanas, S. Mesen-chymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Cho, K.-S.; Kang, S.A.; Kim, S.-D.; Mun, S.-J.; Yu, H.S.; Roh, H.-J. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res. 2019, 39, 101500. [Google Scholar] [CrossRef]
- Hyvärinen, K.; Holopainen, M.; Skirdenko, V.; Ruhanen, H.; Lehenkari, P.; Korhonen, M.; Käkelä, R.; Laitinen, S.; Kerkelä, E. Mesenchymal Stromal Cells and Their Extracellular Vesicles Enhance the Anti-Inflammatory Phenotype of Regulatory Macrophages by Downregulating the Production of Interleukin (IL)-23 and IL-22. Front. Immunol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Guo, L.; Lai, P.; Wang, Y.; Huang, T.; Chen, X.; Geng, S.; Huang, X.; Luo, C.; Wu, S.; Ling, W.; et al. Extracellular vesicles derived from mesenchymal stem cells prevent skin fibrosis in the cGVHD mouse model by suppressing the activation of macrophages and B cells immune response. Int. Immunopharmacol. 2020, 84, 106541. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived from Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yu, L.; Cheng, Z.; Peng, Y.; Cao, Z.; Chen, B.; Duan, Y.; Wang, Y. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J. Nanobiotechnology 2022, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A., Jr.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–677.e6. [Google Scholar] [CrossRef]
- Nosbaum, A.; Prevel, N.; Truong, H.-A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef]
- Cao, W.; Cao, K.; Cao, J.; Wang, Y.; Shi, Y. Mesenchymal stem cells and adaptive immune responses. Immunol. Lett. 2015, 168, 147–153. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.; Bayes-Genis, A.; Borràs, F. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: Implications for nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef]
- Guo, L.; Lai, P.; Wang, Y.; Huang, T.; Chen, X.; Luo, C.; Geng, S.; Huang, X.; Wu, S.; Ling, W.; et al. Extracellular vesicles from mesenchymal stem cells prevent contact hypersensitivity through the suppression of Tc1 and Th1 cells and expansion of regulatory T cells. Int. Immunopharmacol. 2019, 74, 105663. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, F.F.; Andrade-Oliveira, V.; de Almeida, D.C.; da Silva, T.B.; de Souza Breda, C.N.; Cruz, M.C.; Faquim-Mauro, E.; Cenedeze, M.A.; Hiyane, M.I.; Pacheco-Silva, A.; et al. Extracellular Vesicles isolated from Mesenchymal Stromal Cells Modulate CD4+ T Lymphocytes Toward a Regulatory Profile. Cells 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Hao, Y.; Wang, F.; Hou, W.; Chen, H.; Luo, Y. Mesenchymal stromal exosome–functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Sci. Adv. 2021, 7, eabf7207. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-C.; Zheng, C.-X.; Sui, B.-D.; Liu, W.-J.; Jin, Y. Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, L.; Zou, H.; He, Y.; Pan, Y.; Ye, L.; Huang, Y.; Fan, W.; Zhang, J.; Ma, Y.; et al. Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnol. 2023, 21, 176. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chenguang, Z. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321, Correction on Sci. Rep. 2021, 11, 3245. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. Histochem. J. 2017, 48, 121–132. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Shi, X.; Shi, X.; Zhang, W.; Wu, G.; Wang, X.; Su, L.; Hu, D. Exosomal MicroRNAs Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing by Promoting the Proliferation and Migration of Fibroblasts. Stem Cells Int. 2018, 2018, 5420463. [Google Scholar] [CrossRef]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef]
- Shen, C.; Tao, C.; Zhang, A.; Li, X.; Guo, Y.; Wei, H.; Yin, Q.; Li, Q.; Jin, P. Exosomal microRNA-93-3p secreted by bone marrow mesenchymal stem cells downregulates apoptotic peptidase activating factor 1 to promote wound healing. Bioengineered 2022, 13, 27–37. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Gemmati, D. Clinical implications of gene polymorphisms in venous leg ulcer: A model in tissue injury and reparative process. Thromb. Haemost. 2007, 98, 131–137. [Google Scholar] [PubMed]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef]
- Elsharkawi, M.; Ghoneim, B.; Westby, D.; Jones, D.; Tawfick, W.; Walsh, S.R. Adipose-derived stem cells in patients with venous ulcers: Systematic review. Vascular 2022, 17085381221098279. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Faculty Opinions recommendation of Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2012, 27, 451–483. [Google Scholar] [CrossRef]
- Spiekman, M.; Przybyt, E.; Plantinga, J.A.; Gibbs, S.; van der Lei, B.; Harmsen, M.C. Adipose Tissue–Derived Stromal Cells Inhibit TGF-β1–Induced Differentiation of Human Dermal Fibroblasts and Keloid Scar–Derived Fibroblasts in a Paracrine Fashion. Plast. Reconstr. Surg. 2014, 134, 699–712. [Google Scholar] [CrossRef]
- Son, D.; Harijan, A. Overview of surgical scar prevention and management. J. Korean Med. Sci. 2014, 29, 751–757. [Google Scholar] [CrossRef]
- Li, C.; Wei, S.; Xu, Q.; Sun, Y.; Ning, X.; Wang, Z. Application of ADSCs and their Exosomes in Scar Prevention. Stem Cell Rev. Rep. 2021, 18, 952–967. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993, Correction on Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- An, S.; Anwar, K.; Ashraf, M.; Lee, H.; Jung, R.; Koganti, R.; Ghassemi, M.; Djalilian, A.R. Wound-Healing Effects of Mesenchymal Stromal Cell Secretome in the Cornea and the Role of Exosomes. Pharmaceutics 2023, 15, 1486. [Google Scholar] [CrossRef]
- Ong, H.S.; Riau, A.K.; Yam, G.H.; Yusoff, N.; Han, E.J.Y.; Goh, T.W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Ex-osomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Guan, L.; Liu, Z.; Yan, F.; Fang, S.; Zhang, X.; Gao, C. Using extracellular vesicles derived from human umbilical cord mesenchymal stem cells for a topical coating promotes oral mucositis healing in rats. Ann. Transl. Med. 2021, 10, 290. [Google Scholar] [CrossRef]
- Levy, D.; Abadchi, S.N.; Shababi, N.; Ravari, M.R.; Pirolli, N.H.; Bergeron, C.; Obiorah, A.; Mokhtari-Esbuie, F.; Gheshlaghi, S.; Abraham, J.M.; et al. Induced Pluripotent Stem Cell-Derived Extracellular Vesicles Promote Wound Repair in a Diabetic Mouse Model via an Anti-Inflammatory Immunomodulatory Mechanism. Adv. Heal. Mater. 2023, e2300879. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Board-Davies, E.; Brown, H.; Clayton, A.; Davis, T.; Karatas, B.; Burston, J.; Tabi, Z.; Falcon-Perez, J.M.; Paisey, S.; et al. Oral Progenitor Cell Line-Derived Small Extracellular Vesicles as a Treatment for Preferential Wound Healing Outcome. Stem Cells Transl. Med. 2022, 11, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Li, X.; Zhang, J.-J.; Yin, Y.; Tian, Y.; Gan, D.; Wu, R.; Wang, J.; Tian, B.-M.; Chen, F.-M.; et al. Biotin–Avidin System-Based Delivery Enhances the Therapeutic Performance of MSC-Derived Exosomes. ACS Nano 2023, 17, 8530–8550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ling, Y.; Sun, Y.; Xiao, F.; Wang, L. Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review. Genes 2023, 14, 1516. https://doi.org/10.3390/genes14081516
Zhang W, Ling Y, Sun Y, Xiao F, Wang L. Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review. Genes. 2023; 14(8):1516. https://doi.org/10.3390/genes14081516
Chicago/Turabian StyleZhang, Weiyuan, Yang Ling, Yang Sun, Fengjun Xiao, and Lisheng Wang. 2023. "Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review" Genes 14, no. 8: 1516. https://doi.org/10.3390/genes14081516
APA StyleZhang, W., Ling, Y., Sun, Y., Xiao, F., & Wang, L. (2023). Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review. Genes, 14(8), 1516. https://doi.org/10.3390/genes14081516




