Abstract
Aim: Smith–Magenis syndrome (SMS) is a rare genetic neurodevelopmental disorder caused by a 17p11.2 deletion or pathogenic variant in the RAI1 gene. SMS is associated with developmental delay, intellectual disability (ID), and major sleep and behavioral disturbances. To explore how genetic variants may affect intellectual functioning and behavior, we compared intellectual and behavioral phenotypes between individuals with a 17p11.2 deletion and pathogenic RAI1 variant. Method: We reviewed available clinical records from individuals (aged 0–45 years) with SMS, ascertained through a Dutch multidisciplinary SMS specialty clinic. Results: We included a total of 66 individuals (n = 47, 71.2% with a 17p11.2 deletion and n = 19, 28.8% with a pathogenic RAI1 variant) for whom data were available on intellectual functioning, severity of ID (n = 53), and behavioral problems assessed with the Child Behavior Checklist (CBCL, n = 39). Median full-scale IQ scores were lower (56.0 vs. 73.5, p = 0.001) and the proportion of individuals with more severe ID was higher (p = 0.01) in the 17p11.2 deletion group. Median total CBCL 6–18 scores (73.5 vs. 66.0, p = 0.02) and scores on the sub-scales somatic complaints (68.0 vs. 57.0, p = 0.001), withdrawn/depressed behavior (69.5 vs. 55.0, p = 0.02), and internalizing behavior (66.0 vs. 55.0, p = 0.002) were higher in the RAI1 group. Conclusion: The results of this study suggest that 17p11.2 deletions are associated with a lower level of intellectual functioning and less internalizing of problems compared to pathogenic RAI1 variants. The findings of this study may contribute to personalized-management strategies in individuals with SMS.
1. Introduction
Smith–Magenis syndrome (SMS) is a rare genetic neurodevelopmental disorder, estimated to be present in 1:15,000–25,000 births [1,2]. SMS is caused by a 17p11.2 deletion or a pathogenic variant in the retinoic acid induced 1 gene (RAI1), which is located within the 17p11.2 chromosomal region [3,4], and has been shown to be responsible for most SMS features [5]. Other genes may play a role in the variability and severity of the phenotype [5]. The syndrome is associated with several physical and other manifestations, including developmental delay, intellectual disability (ID), sleep disturbances, obesity, and behavioral problems, although the expression may differ from individual to individual [6,7]. Clinical phenotyping summaries on SMS can be found in GeneReviews® [8].
Previous studies have reported that the majority of individuals with SMS have ID, most often moderate or mild ID [9,10,11,12], which is characterized by significant limitations in intellectual (i.e., full-scale IQ scores < 70), adaptive, and everyday executive functioning [13,14]. In addition, many individuals with SMS are reported to exhibit problematic externalizing (e.g., aggression and self-injury) and internalizing (e.g., anxiety, depression, and somatic complaints) behavior that pose a substantial burden to patients and their families, and negatively effects their quality of life [12].
However, research examining the effects of the disease-causing genetic variants on intellectual and behavioral phenotypes in SMS is scarce, and previous studies have typically been performed on individuals with a 17p11.2 deletion. For example, a report on 48 individuals with a 17p11.2 deletion suggested that individuals with large deletions (>3.7 Mb) were more likely to have lower full-scale IQ (FSIQ) scores and lower levels of adaptive behavior functioning compared to those with small (<3.7 Mb) or common 17p11.2 deletions (3.7 Mb) [15]. Knowledge about intellectual functioning in individuals with a pathogenic RAI1 variant has been limited to a few individuals reported in the literature [10,16,17]. Strikingly, these individuals had relatively high FSIQ scores compared to what has been reported in individuals with a 17p11.2 deletion.
Similarly, there is a paucity of literature on behavioral problems in individuals with a pathogenic RAI1 variant, and thus little is known about to what extent the available knowledge of SMS is applicable to those with such a genetic mutation [8]. In a previous study on 31 children and adults with a 17p11.2 deletion and 10 with a pathogenic RAI1 variant, attention seeking and self-injurious behavior were reported in the majority of patients, based on parental report [18]. No differences between the two groups were observed in these behaviors. In another study on 105 individuals with SMS that collected patient data by report (e.g., parent surveys, educational evaluations, and specialist reports), those with a pathogenic RAI1 variant (n = 10) were reported to show more polyembolokoilamania (insertion of hands or objects into mouths or other body openings), skin picking, self-hugging, overeating issues, and obsessive-compulsive tendencies, compared to those with a 17p11.2 deletion [19].
In this study, we aimed to address the knowledge gap on the relationship between genetic variants and intellectual functioning and behavioral problems in SMS, by systematically comparing phenotypes between individuals with a 17p11.2 deletion and pathogenic RAI1 variant.
2. Methods
2.1. Study Design and Setting
This retrospective cohort study was based on a comprehensive review of patient records in an outpatient sample of individuals with SMS [20]. The setting was a national multidisciplinary clinic for children, adolescents, and adults with SMS at ’s Heeren Loo, a large healthcare organization for individuals with intellectual disabilities in The Netherlands. In this clinic, ID physicians, behavioral specialists, speech therapists, dieticians, and occupational therapists provide clinical practice recommendations to parents, caregivers, and healthcare professionals on SMS-associated morbidities.
Patients were referred by their pediatrician, clinical geneticist, general practitioner, or ID physician. The study was approved by the Institutional Review Board of Amsterdam UMC in The Netherlands (#W20_098).
2.2. Characterization of Individuals with SMS
We ascertained individuals with molecularly confirmed SMS who were referred to our clinic between 2002 and 2020. We systematically collected relevant and anonymized clinical data on each individual, including information on demographic characteristics (age at most recent assessment, age at genetic diagnosis, and sex), the results of genetic testing reports (including FISH, microarray, and WES data) to ascertain genetic profiles (referring to a 17p11.2 deletion or pathogenic RAI1 variant, as well as deletion size and variant type, respectively), and intellectual and behavioral phenotypes. Individuals with no data on intellectual functioning and/or behavioral problems were not included in the study.
2.3. Full-Scale IQ and Intellectual Disability
Available FSIQ scores were collected from official psychometric test reports in patient records. The presence or absence, and the severity, of ID, were determined based on all information on intellectual functioning and adaptive behavior (covering conceptual, social, and practical domains) in lifetime clinical records, school reports, and collateral history from family members, in addition to FSIQ scores, and according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [21].
We dichotomized the ID data in borderline/mild ID and moderate/severe ID to be able to perform statistical analysis, given low proportions of individuals with borderline ID (total cohort), and moderate and severe ID in individuals with a pathogenic RAI1 variant.
2.4. Behavioral Questionnaires
Data regarding behavioral problems were recorded through review of available Child Behavior Checklist (CBCL) data: the CBCL 1.5–5 for children aged 1.5 to 5 years and the CBCL 6–18 for children and adolescents aged 6 to 18 years [22,23]. These questionnaires, containing 99 and 113 items, respectively, and three-point Likert-scales, were completed by parents or primary caregivers of the individual with SMS. CBCL questionnaires contain empirically based (CBCL 1.5–5) or syndrome sub-scales (CBCL 6–18), internalizing and externalizing behavior sub-scales, and total scores. For analyses, we used age- and sex-adjusted T-scores. Scores of 70 or higher on one of the empirically based/syndrome sub-scales, and 64 or higher on the internalizing, externalizing, or total problems scales, were classified as ‘clinical’, indicating psychopathology [22,23].
2.5. Statistical Analyses
We used Fisher’s exact tests for categorical and Mann–Whitney U tests and Spearman’s rank correlations for continuous data, given the relatively small sample sizes and asymmetric data distribution. Judgement as to whether continuous variables were normally distributed was based on an integral assessment of the information from descriptive statistics and normality plots. We used the Benjamini–Hochberg procedure to correct the analyses for multiple comparisons. In addition to between-group comparisons (17p11.2 deletion vs. RAI1 group) in CBCL scores, we compared the proportion of patients with scores in the clinical range between both groups. We calculated Spearman’s rank correlations between FSIQ scores and scores on the CBCL 6–18 for those domains that were statistically significantly different between both groups (17p11.2 deletion vs. RAI1 group), in order to get an impression as to what extent these differences could reflect an IQ, rather than a true genotype, effect. All analyses were two-tailed, with statistical significance defined as p < 0.05, and performed in IBM SPSS software (Statistics 22; SPSS, Inc., Chicago, IL, USA).
3. Results
Demographic data are presented in Table 1. For a total of 66 individuals (aged 2–45 years), data on intellectual (n = 53, 80.3%) and/or behavioral phenotypes (n = 39, 59.1%) were available. Forty-seven individuals (71.2%) had a 17p11.2 deletion: twenty-one (44.7%) a 3.7 (≥3.3, ≤3.8) Mb deletion, three (6.4%) a small deletion (1.1, 1.4, and 2.1 Mb, respectively), and three (6.4%) a large deletion (all 4.8 Mb). For 20 (42.6%) individuals, the deletion size was unknown. Nineteen individuals (28.8%) had a pathogenic RAI1 variant: fifteen (78.9%) frameshift, three (15.8%) nonsense, and one (5.3%) unknown. Details on the RAI1 mutations per study participant (i.e., nucleotide change, protein change, and type of mutation) are provided in Supplementary Table S1. There were no significant between-group differences in age at last assessment, children <18 years, or sex. Median age at genetic SMS diagnosis was significantly higher in the group of individuals with a pathogenic RAI1 variant.

Table 1.
Demographics of 66 individuals with Smith–Magenis syndrome. Bold font indicates statistical significance. a Mann–Whitney U tests for continuous data and Fisher’s exact tests for categorical data. IQR = interquartile range, y = years.
3.1. Full-Scale IQ and Intellectual Disability
FSIQ data were available for 41 individuals (Figure 1). Although the proportion of individuals who had IQ data available was lower in those with a 17p11.2 deletion (n = 27, 57.4%) than in individuals with a pathogenic RAI1 variant (n = 14, 73.7%), this difference was not statistically different (p = 0.40).
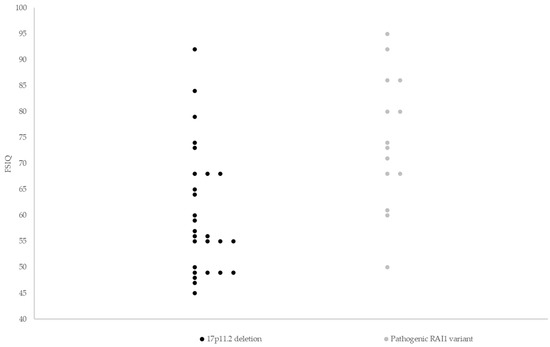
Figure 1.
Full-scale IQ scores of 41 individuals with Smith–Magenis syndrome. Median FSIQ scores were lower in the group of individuals with a 17p11.2 deletion (56.0, range 45–92) compared to individuals with a pathogenic RAI1 variant (73.5, range 50–95, p = 0.001). Horizontal lines indicate median FSIQ scores. FSIQ = full-scale intelligence quotient.
Median FSIQ scores were lower in those with a 17p11.2 deletion (56.0, range 45–92) compared to individuals with a pathogenic RAI1 variant (73.5, range 50–95, p = 0.001). The proportion of moderate/severe ID was higher in individuals with 17p11.2 deletion (22 out of 34; 64.7%) than in those with a pathogenic RAI1 variant (5 out of 19; 26.3%, p = 0.01, Figure 2).
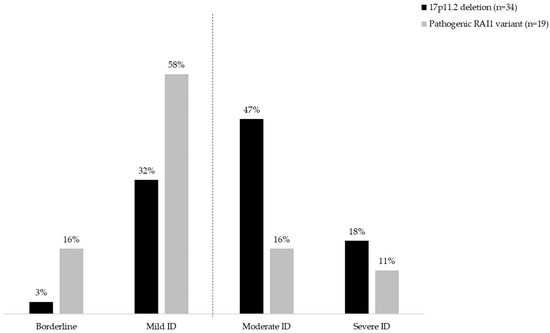
Figure 2.
ID severity in 53 individuals with Smith–Magenis syndrome. The dotted line represents a line to divide the individuals with borderline/mild from those with moderate/severe ID. The proportion of individuals with moderate/severe ID was higher in the 17p11.2 deletion group (22 out of 34; 64.7%) than in the group with a pathogenic RAI1 variant (5 out of 19; 26.3%, p = 0.01). ID = intellectual disability.
3.2. Behavioral Questionnaires
Visual representations of CBCL scores on an individual level are provided in Table 2 (CBCL 6–18) and Supplementary Table S2 (CBCL 1.5–5). CBCL 6–18 data were available for 24 individuals, including 10 with a pathogenic RAI1 variant (Table 3). CBCL 1.5–5 data were available for 17 individuals, 2 with a pathogenic RAI1 variant (Supplementary Table S3). Median total CBCL 6–18 scores and scores on the sub-scales withdrawn/depressed behavior, somatic complaints, and internalizing behavior, were higher in the RAI1 group than in the 17p11.2 deletion group. Results for somatic complaints and internalizing behavior remained significant after Benjamini–Hochberg correction for multiple testing. A higher proportion of patients in the RAI1 group compared to the 17p11.2 deletion group had scores in the clinical range on the CBCL 6–18 sub-scales of withdrawn/depressed behavior (50% vs. 0%, p = 0.006), somatic complaints (40% vs. 0%, p = 0.02), and internalizing behavior (70% vs. 7%, p = 0.002). Results for withdrawn/depressed behavior and internalizing behavior remained significant after Benjamini–Hochberg correction for multiple testing.

Table 2.
Heatmap depicting CBCL 6–18 scores in 24 individuals with Smith–Magenis syndrome.

Table 3.
Age- and sex-adjusted T-scores on the CBCL 6–18 in 24 individuals with Smith–Magenis syndrome.
3.3. Relationship between FSIQ Scores and CBCL 6–18 Scores
Eighteen individuals (n = 8 with a pathogenic RAI1 variant) had FSIQ and CBCL 6–18 data. For this sub-sample, there were strong statistically significant positive correlations between FSIQ scores and the CBCL 6–18 sub-score somatic complaints (rs = 0.68, p = 0.002), and moderate statistically significant positive correlations between FSIQ scores and internalizing behavior (rs = 0.59, p = 0.01), which may suggest an effect of IQ on CBCL 6–18 scores. However, no such correlations were found in the sub-group analyses: somatic complaints (17p11.2 deletion, rs < 0.01, p = 0.99 vs. pathogenic RAI1 variant rs = 0.13, p = 0.77) and internalizing behavior (17p11.2 deletion, rs = −0,16, p = 0.67 vs. pathogenic RAI1 variant rs = 0.32, p = 0.45). Correlations between FSIQ scores and withdrawn/depressed behavior were not statistically significant (rs = 0.37, p = 0.13).
4. Discussion
To the best of our knowledge, this study is the first to systematically compare FSIQ scores and behavioral questionnaires in a sample of individuals with a 17p11.2 deletion and a pathogenic RAI1 variant. The study adds to the scarce body of literature on genotype–phenotype correlations in SMS.
In line with previous reports in smaller uncontrolled samples [10,15,16,17], median FSIQ scores were higher in the group of individuals with a pathogenic RAI1 variant, in comparison with those with a 17p11.2 deletion. However, the range in FSIQ scores in both groups was wide, i.e., 45–92 in the 17p11.2 group and 50–95 in the RAI1 group. This suggests that variation in FSIQ scores may be significant regardless of genetic subtype. This variability has also been reported in studies that included individuals with a 17p11.2 deletion only [12,15]. We notice that, in our study, median FSIQ scores (56, range 45–92) in individuals with a 17p11.2 deletion were somewhat higher than reported in a previous study in children and adults (48, range 41–81) [10]. Possible explanations for this finding include differences between the intelligence scales used and in who was or was not selected for taking a specific intelligence test. Another explanation may be that individuals assessed in earlier studies may have had a more severe SMS phenotype on average, due to the fact that genetic testing for SMS (i.e., FISH) was less available clinically and required an index of suspicion in the early years, potentially resulting in a selection bias towards a more severe phenotype. Previously reported FSIQ scores in a total of 10 children and adults with a pathogenic RAI1 variant ranged from 57 to 89 [10,16,17], with a median of 67.5, in line with findings in this study.
The proportion of individuals with more severe ID was lower in the RAI1 group than in the 17p11.2 deletion group. In the RAI1 group, about three quarters had borderline to mild ID. In individuals with a 17p11.2 deletion, the majority had mild to moderate ID (about 50% moderate ID), similar to what has been reported earlier [10,12]. A challenge in comparison with earlier research, in addition to the paucity of reports in individuals with a RAI1 variant [16], is that historically, ID severity was mainly based on intellectual functioning. Classification of ID has changed over time, with adaptive functioning (collection of conceptual, social, and practical skills) have become now equally important [24].
Remarkably, while externalizing behaviors are common in both genetic subtypes (based on this and previous studies) [7,12], the results of this study suggest that 17p11.2 deletions are associated with less internalizing problems, i.e., behavioral problems directed toward oneself such as withdrawal and depression, and somatic complaints, as compared to pathogenic RAI1 variants. Only one (7%) individual with a 17p11.2 deletion had clinical internalizing behavior scores in the clinical range, compared to >41 in previous studies [7,12]. Clinical externalizing behavior in individuals with a 17p11.2 deletion (50%) is lower compared to previous studies (82%) [7,12]. Although we do not have an explanation for this discrepancy, it may suggest relative under-reporting of internalizing behaviors in individuals with a 17p11.2 deletion in our CBCL 6–18 data (in contrast to those of the CBCL 1.5–5), which may also have driven the differences between internalizing behavior problems in individuals with a 17p11.2 deletion compared to those with a pathogenic RAI1 variant. How to understand the high proportion of individuals with a pathogenic RAI1 variant and scores in the clinical range on the domain internalizing behaviors? Individuals with a pathogenic RAI1 variant were diagnosed at a later age compared to individuals with a 17p11.2 deletion. In addition, to direct genetic effects, this may have impacted the natural course of and accessibility to services. SMS requires multidisciplinary management, including psycho-education, parental guidance, and specific treatments [25]. If these are not provided, a less favorable course with respect to mental health could be hypothesized. In addition, it is conceivable that individuals with a pathogenic RAI1 variant suffer more from asynchronies between their cognitive and emotional development, with emotional maturity delayed beyond intellectual functioning, which may lead to internalizing behaviors [8]. Individuals with a pathogenic RAI1 variant typically are emotionally ‘young’ compared to their cognitive level of functioning [5,26]. Although one might think that higher levels of intellectual functioning would go along with better verbal and mentalizing (reflective) skills, which may lead to less internalizing behavioral problems [27], we did not find a correlation of FSIQ scores with scores on the CBCL either in the 17p11.2 deletion or RAI1 group. Future research is needed to better understand these probably much more complex relationships.
5. Implications and Future Directions
Our findings, collectively, have implications for psycho-education and management of individuals with SMS, and their relatives. Historic SMS literature tended to focus on externalizing behaviors, such as self-injurious and aggressive behavior, and on hyperactivity, attention problems, and stereotypic behavior [6]. In line with recent reports [7,12], this study also shows internalizing behaviors in SMS. We recommend that internalizing behavior should also be considered, especially in those with a pathogenic RAI1 variant. In choosing the right school, adults and teachers should not only focus on the cognitive abilities of the child, which may be relatively high, in particular in those with a pathogenic RAI1 variant, but should also pay attention to social, communicative, and practical skills and emotional development. Research into the potential role of the aforementioned asynchrony in cognitive and emotional functioning in individuals with SMS, needs consideration. We argue that future studies on SMS should preferably stratify by genetic variant, or at least provide clarity on the genetic information of the study participants, to enable interpretation of the study results. Well-powered prospective studies will be needed to optimize personalized approaches that take differences into account regarding cognitive, emotional, and adaptive functioning, and the risk of developing both externalizing and internalizing behaviors.
6. Strengths and Limitations
This study has the largest number of individuals with a pathogenic RAI1 variant to date, which enabled systematic comparisons between individuals with 17p11.2 deletions and pathogenic RAI1 variants. A limitation is the retrospective nature of the study. Although age differences were taken into account by using T-scores, and although there was no statistically significant between-group difference in age, we cannot rule out an effect of age on the study findings. In addition, the age range was large, especially for studying behavior, including both children and adults. When interpreting the FSIQ findings, account must be taken that, for some individuals with SMS, no scores were available, due to the fact that their cognitive impairments made it impossible to complete an intelligence test with a FSIQ score. We included scores for individuals with significant discrepancies between (non-) verbal and performance IQ scores (17p11.2 deletion n = 3, pathogenic RAI1 variant n = 2) to maximize use of available data, even though FSIQ scores based on such discrepant IQ profiles may not be clinically reliable on an individual level. Another limitation is that quantitative data on FSIQ and adaptive functioning were not available or could not be determined in young children. In addition, in this study, information on adaptive functioning was not based on one standardized questionnaire. Finally, the fact that only a very few individuals were known to have a small or large 17p11.2 deletion, hampered our ability to test for any effect of deletion size.
7. Conclusions
This study identified differences in the intellectual and behavioral phenotypes of SMS and suggests that pathogenic RAI1 variants are associated with relatively higher FSIQ scores and more internalizing behaviors than 17p11.2 deletions. Prospective longitudinal studies are required to validate and refine these findings to better inform the clinical implications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081514/s1, Table S1: RAI1 mutations in 19 patients with a pathogenic RAI1 variant. Table S2: Heatmap depicting CBCL 1.5–5 T-scores in 17 individuals with Smith–Magenis syndrome. Table S3: CBCL 1.5–5 scores in 17 individuals with Smith–Magenis syndrome.
Author Contributions
Conceptualization, C.C.L. and E.B.; Methodology, C.C.L. and E.B.; Formal Analysis, C.C.L. and E.B.; Investigation, C.C.L. and E.B.; Data Curation, C.C.L.; Writing—Original Draft Preparation, C.C.L.; Writing—Review & Editing, C.C.L., A.M.v.E., J.R.Z., M.-J.v.d.B. and E.B.; Visualization, C.C.L.; Supervision, E.B.; Project Administration, C.C.L.; Funding Acquisition, C.C.L., A.M.v.E., M.-J.v.d.B. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported financially by the Dutch National Institutes of Health (ZonMw; #845006105). The funding body had no role in the study design, collection, analysis and interpretation of data, nor any role in writing or submitting this paper for publication.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of Amsterdam UMC in The Netherlands (#W20_098).
Informed Consent Statement
The institutional Review Board of Amsterdam UMC waived the need for written consent to use pseudonymized clinical data.
Data Availability Statement
The data are not publicly available due to ethical restrictions and privacy concerns. Any data requests can be directed to the corresponding author.
Acknowledgments
The authors thank the participants and their families for their participation, Stichting Smith Magenis Syndroom Nederland for their support, and the students and colleagues at ‘s Heeren Loo for their contributions to this study.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Elsea, S.H.; Girirajan, S.S. Smith-Magenis Syndrome. Eur. J. Hum. Genet. 2008, 16, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, F.; Guzzetta, V.; Montes de Oca-Luna, R.M.; Magenis, R.E.; Smith, A.C.M.; Richter, S.F.; Kondo, I.; Dobyns, W.B.; Patel, P.I.; Lupski, J.R. Molecular Analysis of the Smith-Magenis Syndrome: A Possible Contiguous-Gene Syndrome Associated with del(17)(p11.2). Am. J. Hum. Genet. 1991, 49, 1207–1218. [Google Scholar] [PubMed]
- Smith, A.C.M.; McGavran, L.; Waldstein, G. Deletion of the 17 Short Arm in Two Patients with Facial Clefts. Am. J. Hum. Genet. 1982, 34, A410. [Google Scholar]
- Slager, R.E.; Newton, T.L.; Vlangos, C.N.; Finucane, B.; Elsea, S.H. Mutations in RAI1 Associated with Smith–Magenis Syndrome. Nat. Genet. 2003, 33, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Finucane, B.; Haas-Givler, B. Smith-Magenis Syndrome: Genetic Basis and Clinical Implications. J. Ment. Health Res. Intellect. Disabil. 2009, 2, 134–148. [Google Scholar] [CrossRef]
- Rinaldi, B.; Villa, R.; Sironi, A.; Garavelli, L.; Finelli, P.; Bedeschi, M.F. Smith-Magenis Syndrome—Clinical Review, Biological Background and Related Disorders. Genes 2022, 13, 335. [Google Scholar] [CrossRef]
- Garayzábal, E.; Hidalgo, I.; Miranda de Souza, A.L.D.; Da Silva, N.C.; Giacheti, C.M.; Pinato, L. Sleep Disturbances and Behavior in Smith-Magenis Syndrome. Res. Dev. Disabil. 2022, 128, 104286. [Google Scholar] [CrossRef]
- Smith, A.C.M.; Boyd, K.E.; Brennan, C.; Charles, J.; Elsea, S.H.; Finucane, B.M.; Foster, R.; Gropman, A.; Girirajan, S.; Haas-Givler, B. Smith-Magenis Syndrome; GeneReviews®: Seattle, WA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1310/ (accessed on 28 June 2023).
- Nag, H.E.; Nærland, T. Age-Related Changes in Behavioural and Emotional Problems in Smith–Magenis Syndrome Measured with the Developmental Behavior Checklist. J. Intellect. Disabil. 2020, 25, 429–440. [Google Scholar] [CrossRef]
- Osório, A.; Cruz, R.; Sampaio, A.; Garayzábal, E.; Carracedo, A.; Fernández-Prieto, M. Cognitive Functioning in Children and Adults with Smith-Magenis Syndrome. Eur. J. Med. Genet. 2012, 55, 394–399. [Google Scholar] [CrossRef]
- Udwin, O.; Webber, C.; Horn, I. Abilities and Attainment in Smith-Magenis Syndrome. Dev. Med. Child Neurol. 2001, 43, 823–828. [Google Scholar] [CrossRef][Green Version]
- Rive Le Gouard, N.; Jacquinet, A.; Ruaud, L.; Deleersnyder, H.; Ageorges, F.; Gallard, J.; Lacombe, D.; Odent, S.; Mikaty, M.; Manouvrier-Hanu, S.; et al. Smith-Magenis Syndrome: Clinical and Behavioral Characteristics in a Large Retrospective Cohort. Clin. Genet. 2021, 99, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wilde, L.; Oliver, C. Brief Report: Contrasting Profiles of Everyday Executive Functioning in Smith–Magenis Syndrome and Down Syndrome. J. Autism Dev. Disord. 2017, 47, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.C.; Wolters, P.L.; Smith, A.C. Adaptive and Maladaptive Behavior in Children with Smith-Magenis Syndrome. J. Autism Dev. Disord. 2006, 36, 541–552. [Google Scholar] [CrossRef]
- Madduri, N.; Peters, S.U.; Voigt, R.G.; Llorente, A.M.; Lupski, J.R.; Potocki, L. Cognitive and Adaptive Behavior Profiles in Smith-Magenis Syndrome. J. Dev. Behav. Pediatr. 2006, 27, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Vilboux, T.; Ciccone, C.; Blancato, J.K.; Cox, G.F.; Deshpande, C.; Introne, W.J.; Gahl, W.A.; Smith, A.C.; Huizing, M. Molecular analysis of the Retinoic Acid Induced 1 gene (RAI1) in patients with suspected Smith-Magenis syndrome without the 17p11.2 deletion. PLoS ONE 2011, 6, e22861. [Google Scholar] [CrossRef] [PubMed]
- Girirajan, S.; Elsas, L.J.; Devriendt, K.; Elsea, S.H. RAI1 Variations in Smith–Magenis Syndrome Patients Without 17p11. 2 deletions. J. Med. Genet. 2005, 42, 820–828. [Google Scholar] [CrossRef]
- Girirajan, S.; Vlangos, C.N.; Szomju, B.B.; Edelman, E.; Trevors, C.D.; Dupuis, L.; Nezarati, M.; Bunyan, D.J.; Elsea, S.H. Genotype–Phenotype Correlation in Smith-Magenis Syndrome: Evidence That Multiple Genes in 17p11.2 Contribute to the Clinical Spectrum. Genet. Med. 2006, 8, 417–427. [Google Scholar] [CrossRef]
- Edelman, E.A.; Girirajan, S.; Finucane, B.; Patel, P.; Lupski, J.; Smith, A.; Elsea, S.H. Gender, Genotype, and Phenotype Differences in Smith–Magenis Syndrome: A Meta-Analysis of 105 cases. Clin. Genet. 2007, 71, 540–550. [Google Scholar] [CrossRef]
- Boot, E.; Linders, C.C.; Tromp, S.H.; van den Boogaard, M.J.; van Eeghen, A.M. Possible Underreporting of Pathogenic Variants in RAI1 Causing Smith–Magenis Syndrome. Am. J. Med. Genet. A 2021, 185, 3167–3169. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Preschool Forms & Profiles; Burlington, VT University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT, USA, 2000. [Google Scholar]
- Achenbach, T.M.; Rescorla, L. Manual for the ASEBA School-Age Forms & Profiles; Burlington, VT University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Schalock, R.; Luckasson, R.; Tassé, M. Twenty Questions and Answers Regarding the 12th Edition of the AAIDD Manual: Intellectual Disability: Definition, Diagnosis, Classification, and Systems of Supports; American Association on Intellectual and Developmental Disabilities: Silver Spring, MD, USA, 2021; Available online: https://www.aaidd.org/docs/default-source/intellectualdisability/12th-ed-twenty-questions-faq.pdf?sfvrsn=a6403421_8 (accessed on 28 June 2023).
- Poisson, A.; Nicolas, A.; Cochat, P.; Sanlaville, D.; Rigard, C.; de Leersnyder, H.; Franco, P.; Portes, V.D.; Edery, P.; Demily, C. Behavioral Disturbance and Treatment Strategies in Smith-Magenis Syndrome. Orphanet J. Rare Dis. 2015, 10, 111. [Google Scholar] [CrossRef]
- Nag, H.E.; Hoxmark, L.B.; Nærland, T. Parental Experiences with Behavioural Problems in Smith–Magenis Syndrome: The Need for Syndrome-Specific Competence. J. Intellect. Disabil. 2019, 23, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Luyten, P.; Campbell, C.; Allison, E.; Fonagy, P. The Mentalizing Approach to Psychopathology: State of the Art and Future Directions. Annu. Rev. Clin. Psychol. 2020, 16, 297–325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).