IL-21 (rs2055979 and rs2221903)/IL-21R (rs3093301) Polymorphism and High Levels of IL-21 Are Associated with Rheumatoid Arthritis in Mexican Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Assessment
2.3. Inflammatory and Serological Parameters
2.4. Genomic DNA Extraction and Genotyping
2.5. Cytokine Quantification
2.6. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Association of the IL-21/IL-21R Polymorphisms with RA
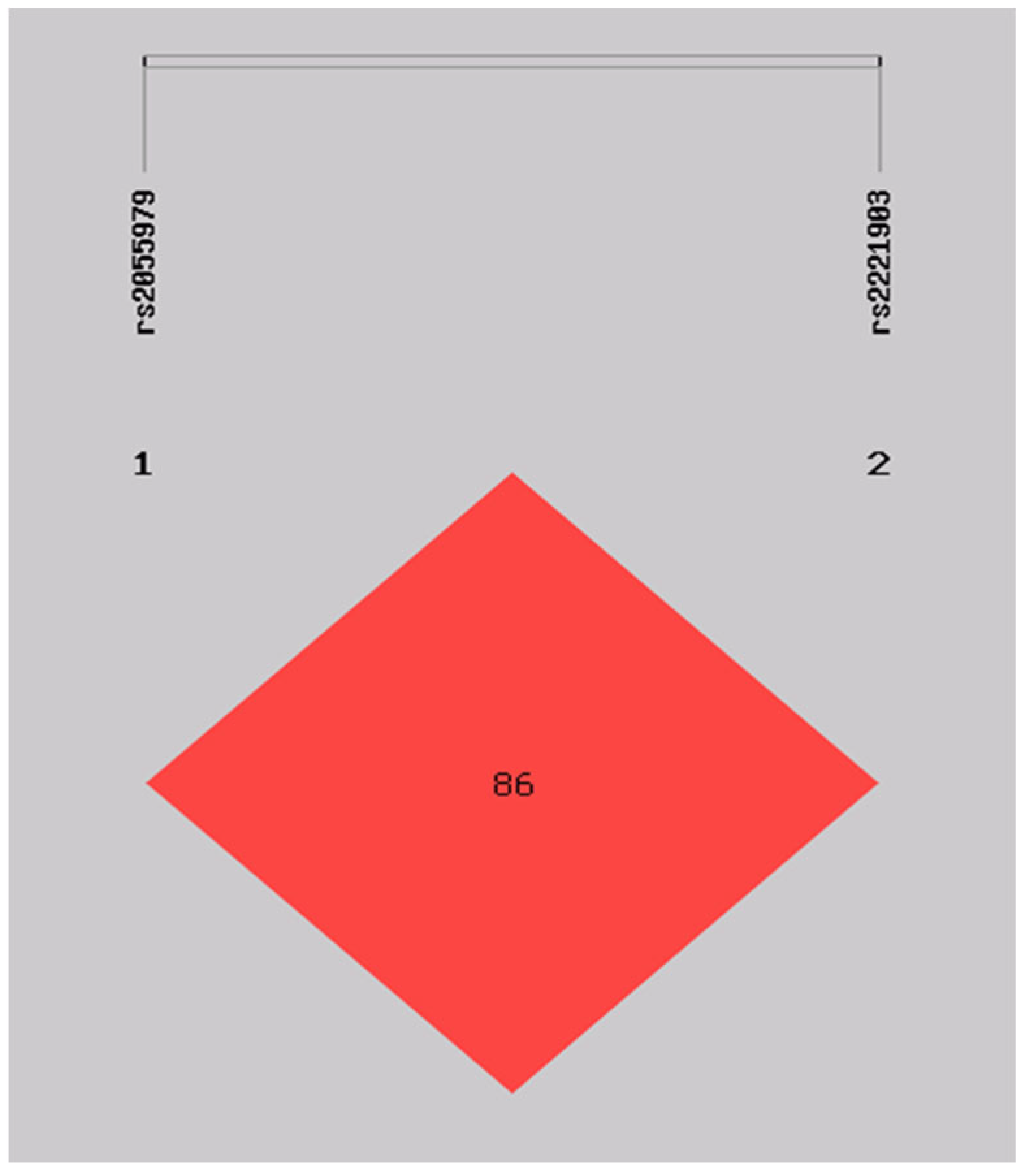
3.3. Haplotype Frequencies of IL-21 SNPs and the Association with RA
3.4. Association of IL-21 and IL-21R Polymorphisms with DAS28-ESR, Acute Phase Reactants, and Autoantibody Positivity
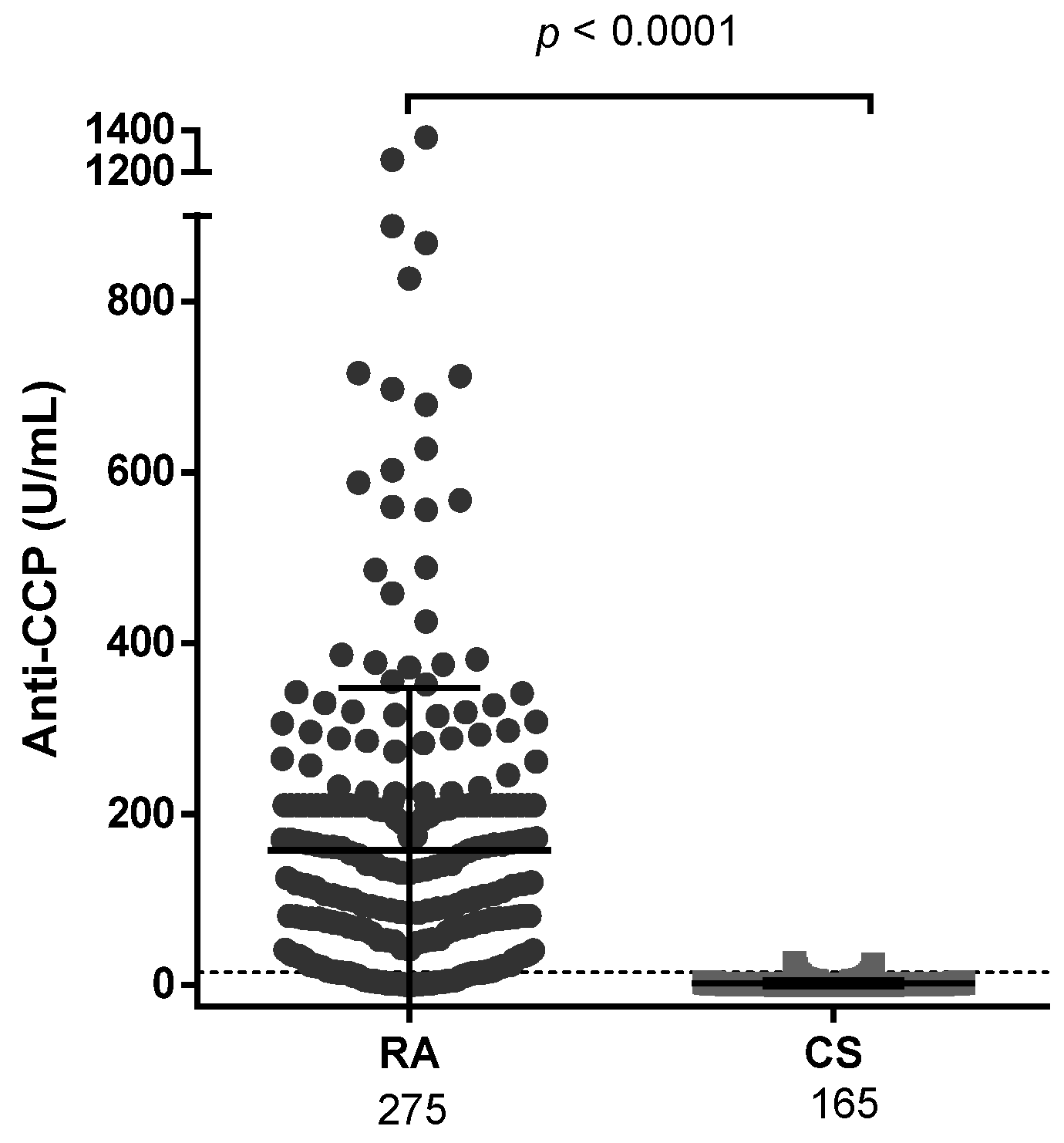
3.5. Autoantibody Levels
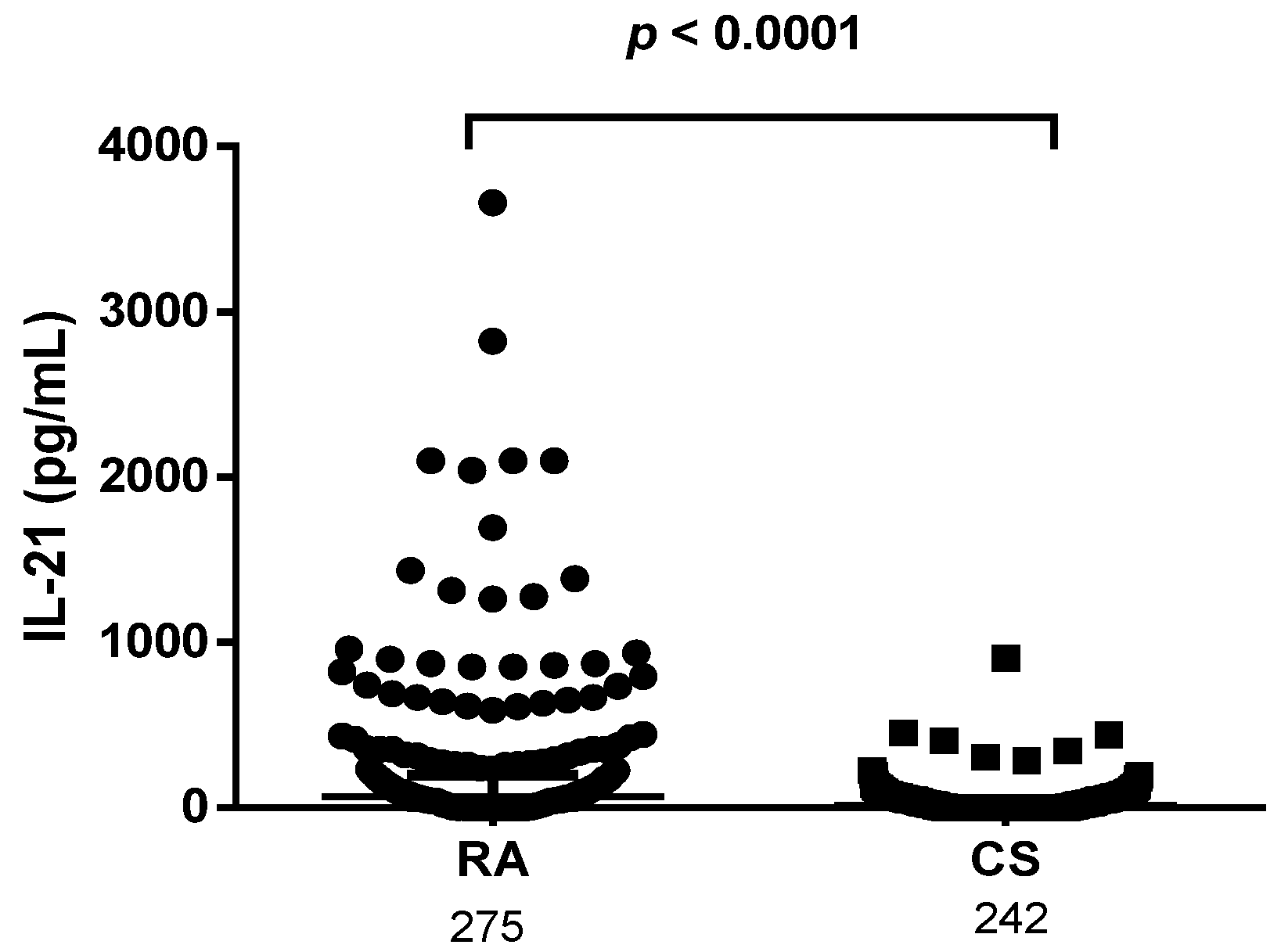
3.6. Levels of IL-21
3.7. Combined Effect of IL-21 Polymorphisms on IL-21 Serum Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.; Paradowska-Gorycka, A.; Safranow, K.; Pawlik, A. Interleukin-21 gene polymorphism rs2221903 is associated with disease activity in patients with rheumatoid arthritis. Arch. Med. Sci. AMS 2017, 13, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Perez, I.V.; Sanchez-Hernandez, P.E.; Munoz-Valle, J.F.; Martinez-Bonilla, G.E.; Garcia-Iglesias, T.; Gonzalez-Diaz, V.; Garcia-Arellano, S.; Cerpa-Cruz, S.; Polanco-Cruz, J.; Ramirez-Duenas, M.G. Cytokines (IL-15, IL-21, and IFN-γ) in rheumatoid arthritis: Association with positivity to autoantibodies (RF, anti-CCP, anti-MCV, and anti-PADI4) and clinical activity. Clin. Rheumatol. 2019, 38, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Sun, L.; Wu, D.; Jin, Y.; Li, C.; Liu, X.; Zhao, J. Autoantibodies against interleukin-21 correlate with disease activity in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, P.; Rasool, M. Multifaceted role of IL-21 in rheumatoid arthritis: Current understanding and future perspectives. J. Cell. Physiol. 2018, 233, 3918–3928. [Google Scholar] [CrossRef]
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Research 2016, 5, 224. [Google Scholar] [CrossRef]
- Xing, R.; Jin, Y.; Sun, L.; Yang, L.; Li, C.; Li, Z.; Liu, X.; Zhao, J. Interleukin-21 induces migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Clin. Exp. Immunol. 2016, 184, 147–158. [Google Scholar] [CrossRef]
- Gharibi, T.; Kazemi, T.; Aliparasti, M.R.; Farhoudi, M.; Almasi, S.; Dehghanzadeh, R.; Seyfizadeh, N.; Babaloo, Z. Investigation of IL-21 gene polymorphisms (rs2221903, rs2055979) in cases with multiple sclerosis of Azerbaijan, Northwest Iran. Am. J. Clin. Exp. Immunol. 2015, 4, 7–14. [Google Scholar]
- Lan, Y.; Luo, B.; Wang, J.L.; Jiang, Y.W.; Wei, Y.S. The association of interleukin-21 polymorphisms with interleukin-21 serum levels and risk of systemic lupus erythematosus. Gene 2014, 538, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, W.X.; Zhu, Y.F.; Muhali, F.S.; Xiao, L.; Jiang, W.J.; Shi, X.H.; Zhou, L.H.; Zhang, J.A. Polymorphisms of interleukin-21 and interleukin-21-receptor genes confer risk for autoimmune thyroid diseases. BMC Endocr. Disord. 2013, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, T.; Majidi, J.; Kazemi, T.; Dehghanzadeh, R.; Motallebnezhad, M.; Babaloo, Z. Biological effects of IL-21 on different immune cells and its role in autoimmune diseases. Immunobiology 2016, 221, 357–367. [Google Scholar] [CrossRef]
- Scherer, H.U.; Huizinga, T.W.J.; Kronke, G.; Schett, G.; Toes, R.E.M. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat. Reviews. Rheumatol. 2018, 14, 157–169. [Google Scholar] [CrossRef]
- Niu, X.; He, D.; Zhang, X.; Yue, T.; Li, N.; Zhang, J.Z.; Dong, C.; Chen, G. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum. Immunol. 2010, 71, 334–341. [Google Scholar] [CrossRef]
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef]
- Padyukov, L. Genetics of rheumatoid arthritis. Semin. Immunopathol. 2022, 44, 47–62. [Google Scholar] [CrossRef]
- Ali Abdulla, A.; Abdulaali Abed, T.; Razzaq Abdul-Ameer, W. Impact of IL-21 Gene Polymorphisms (rs2055979) and the Levels of Serum IL-21 on the Risk of Multiple Sclerosis. Arch. Razi Inst. 2022, 77, 81–86. [Google Scholar] [CrossRef]
- Hao, Y.; Xie, L.; Xia, J.; Liu, Z.; Yang, B.; Zhang, M. Plasma interleukin-21 levels and genetic variants are associated with susceptibility to rheumatoid arthritis. BMC Musculoskelet. Disord. 2021, 22, 246. [Google Scholar] [CrossRef]
- Webb, R.; Merrill, J.T.; Kelly, J.A.; Sestak, A.; Kaufman, K.M.; Langefeld, C.D.; Ziegler, J.; Kimberly, R.P.; Edberg, J.C.; Ramsey-Goldman, R.; et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- Sauna, Z.E.; Kimchi-Sarfaty, C. Understanding the contribution of synonymous mutations to human disease. Nat. Reviews. Genet. 2011, 12, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; King, C. IL-21-producing Th cells in immunity and autoimmunity. J. Immunol. 2013, 191, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Chen, Y.; Wu, H.; Zhao, M.; Lu, Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J. Autoimmun. 2019, 99, 1–14. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yang, H.Y.; Lai, J.H. Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis. Int. J. Mol. Sci. 2020, 21, 4015. [Google Scholar] [CrossRef]
- Wu, Y.; van Besouw, N.M.; Shi, Y.; Hoogduijn, M.J.; Wang, L.; Baan, C.C. The Biological Effects of IL-21 Signaling on B-Cell-Mediated Responses in Organ Transplantation. Front. Immunol. 2016, 7, 319. [Google Scholar] [CrossRef]
- Romao, V.C.; Canhao, H.; Fonseca, J.E. Old drugs, old problems: Where do we stand in prediction of rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med. 2013, 11, 17. [Google Scholar] [CrossRef]
- Szostak, B.; Machaj, F.; Rosik, J.; Pawlik, A. Using pharmacogenetics to predict methotrexate response in rheumatoid arthritis patients. Expert Opin. Drug Metab. Toxicol. 2020, 16, 617–626. [Google Scholar] [CrossRef]
- Jekic, B.; Maksimovic, N.; Damnjanovic, T. Methotrexate pharmacogenetics in the treatment of rheumatoid arthritis. Pharmacogenomics 2019, 20, 1235–1245. [Google Scholar] [CrossRef]
| Parameters | RA n = 275 | CS n = 280 | p |
|---|---|---|---|
| Gender (F/M) | 225/50 | 222/58 | |
| Age (years) * | 48.36 ± 12.65 | 36.06 ± 12.53 | |
| Disease evolution (years) * | 7.1 ± 7.9 | ||
| Serological parameters | |||
| ESR (mm/h) * | 35.15 ± 15.72 | 18.51 ± 10.87 | <0.0001 |
| CRP (U/mL) * | 19.28 ± 25.36 | 4.12 ± 4.28 | <0.0001 |
| RF (UI/mL) * | 61.42 ± 27.04 | 6.87 ± 8.28 | <0.0001 |
| Anti-CCP (U/mL) * | 157.50 ± 190.30 | 1.94 ± 3.65 | <0.0001 |
| Stages of disease | % (n) | ||
| Established > 1 year | 78.6 (216) | _ | |
| Early < 1 year | 17.8 (49) | _ | |
| Very early < 3 months | 3.6 (10) | _ | |
| Disease activity (DAS28-ESR) | % (n) | ||
| High | 20.6 (51) | _ | |
| Moderate | 44.0 (109) | _ | |
| Low | 16.9 (42) | _ | |
| Remission | 18.5 (46) | _ | |
| Risk Factors | % (n) | ||
| Smoking | 32.0 (88) | _ | |
| Extra-articular manifestations | 24.0 (66) | _ | |
| High RF positives a | 26.9 (74) | _ | |
| High anti-CCP positives a | 72.7 (200) | _ | |
| Treatment | % (n) | Treatment combination | |
| Without treatment | 13.45 (37) | ||
| With treatment | 86.55 (238) | ||
| Monotherapy | 28.99 (69) | MTX/LEF/SSZ/CQ | |
| Double therapy | 39.92 (95) | MTX-SSZ/MTX-CQ/CQ-SSZ | |
| Triple therapy | 31.09 (74) | MTX-CQ-SSZ | |
| Polymorphism | RA | CS | p | OR (95% CI) |
|---|---|---|---|---|
| N = 275 (%) | N = 280 (%) | |||
| IL-21 rs2055979 C/A | ||||
| Genotypes | ||||
| CC | 65 (23.64) | 96 (34.29) | ||
| CA | 148 (53.82) | 132 (47.14) | 0.0115 | 1.656 (1.118–2.452) |
| AA | 62 (22.55) | 52 (18.57) | 0.0216 | 1.761 (1.085–2.859) |
| Alleles | ||||
| C | 278 (51) | 324 (58) | ||
| A | 272 (49) | 236 (42) | 0.0145 | 1.343 (1.060–1.702) |
| Model | ||||
| Recessive CC + CA AA | 213 (77.45) 62 (22.54) | 228 (81.42) 52 (18.57) | 0.2466 | 0.784 (0.518–1.184) |
| Dominant AA + CA CC | 210 (76.36) 65 (23.64) | 184 (65.71) 96 (34.29) | 0.0057 | 1.686 (1.162–2.445) |
| IL-21 rs2221903 T/C | ||||
| Genotypes | ||||
| TT | 203 (73.82) | 214 (76.43) | ||
| TC | 70 (25.45) | 58 (20.71) | 0.2345 | 1.272 (0.855–1.893) |
| CC | 2 (0.73) | 8 (2.86) | 0.0728 | 0.264 (0.55–1.256) |
| Alleles | ||||
| T | 476 (86.5) | 486 (86.79) | ||
| C | 74 (13.4) | 74 (13.21) | 0.9062 | 1.021 (0.722–1.443) |
| Model | ||||
| Recessive TT + CT CC | 273 (99.27) 2 (0.71) | 272 (97.14) 8 (2.85) | 0.0592 | 4.015 (0.845–19.078) |
| Dominant CC + CT TT | 72 (26.18) 203 (73.81) | 66 (23.57) 214 (76.42) | 0.4768 | 1.150 (0.782–1.691) |
| IL-21R rs3093301 T/A | ||||
| Genotypes | ||||
| TT | 106 (38.55) | 119 (42.5) | ||
| TA | 120 (43.64) | 133 (47.5) | 0.9442 | 1.013 (0.707–1.451) |
| AA | 49 (17.82) | 28 (10) | 0.0122 | 1.965 (1.153–3.348) |
| Alleles | ||||
| T | 332 (60.36) | 371 (66.25) | ||
| A | 218 (39.64) | 189 (33.75) | 0.0418 | 1.289 (1.009–1.646) |
| Model | ||||
| Recessive TT + TA AA | 226 (41.09) 49 (8.90) | 252 (90) 28 (10) | 0.0077 | 0.512 (0.312–0.843) |
| Dominant TA + AA TT | 169 (61.45) 106 (38.54) | 161 (57.5) 119 (42.5) | 0.3427 | 1.178 (0.839–1.654) |
| IL-21 (rs2055979/rs2221903) Haplotype | RA 2n (%) | CS 2n (%) | OR (CI 95%) | p |
|---|---|---|---|---|
| AT | 271.98 (0.495) | 227.31 (0.406) | 1.395 (1.099–1.769) | 0.00614 |
| CC | 73.98 (0.135) | 65.31 (0.117) | 1.157 (0.810–1.651) | 0.42264 |
| CT | 204.01 (0.371) | 258.69 (0.462) | 0.667 (0.524–0.848) | 0.00095 |
| AC | (0.000) | 8.69 (0.016) |
| Polymorphisms | Clinical Activity and Serological Parameters | |||||
|---|---|---|---|---|---|---|
| Genotypes | DAS28-ESR Mean SD (p) | ESR Mean SD (p) | CRP Mean SD (p) | RF Mean SD (p) | CCP Mean SD (p) | IL-21 Mean SD (p) |
| rs2055979 | ||||||
| CC | 3.803 ± 1.58 | 33.21 ± 17.87 | 19.93 ± 27.03 | 56.58 ± 27.87 | 162.6 ± 176.7 | 249.4 ± 543 |
| CA | 3.915 ± 1.39 | 35.38 ± 15.61 | 19.86 ± 25.44 | 62.59 ± 27.50 | 135.7 ± 173.9 | 181.6 ± 305 |
| AA | 4.090 ±1.41 | 36.69 ± 13.37 | 17.24 ± 23.62 | 63.71 ± 24.77 | 204.2 ± 231.5 (0.0296) * | 279.9 ± 587 |
| rs2221903 | ||||||
| TT | 3.959 ± 1.39 | 35.88 ± 15.36 | 19.45 ± 27.18 | 63.84 ± 26.05 (0.0292) * | 159.9 ± 184.6 | 215.4 ± 422 |
| TC | 3.843 ± 1.59 | 33.26 ± 16.60 | 19.00 ± 19.67 | 54.29 ± 28.79 | 153.4 ± 208.9 | 197.3 ± 404 |
| CC | 3.845 ± 0.96 | 31.50 ± 23.33 | 12.55 ± 12.94 | 65.37 ± 35.34 | 66.52 ± 82.89 | 1457 ± 1928 |
| rs3093301 | ||||||
| TT | 3.889 ± 1.40 | 35.39 ± 15.44 | 17.84 ± 19.56 | 59.14 ± 25.89 | 133.9 ± 159.3 | 196.4 ± 446 |
| TA | 3.855 ± 1.41 | 35.30 ± 16.84 | 18.71 ± 26.24 | 60.77 ± 28.95 | 167.6 ± 195.8 | 220.4 ± 440 |
| AA | 4.178 ± 1.60 | 34.27 ± 13.71 | 23.80 ± 33.19 | 67.96 ± 23.95 | 184.1 ± 232.1 | 268.7 ± 454 |
| Combined Effect of IL-21 Polymorphisms | Clinical Activity and Serological Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| IL-21 rs2055979 | IL-21 rs2221903 | n | DAS28 Mean (CI) * p = 0.6852 | ESR Mean (CI) * p = 0.4138 | CRP Mean (CI) * p = 0.6852 | RF Mean (CI) * p = 0.0066 | CCP Mean (CI) * p = 0.1511 | IL-21 Mean (CI) * p = 0.7160 |
| AA | TT | 62 | 4.090 (3.7–4.4) | 36.69 (32–40) | 17.24 (11–23) | 63.71 (57–70) | 204.2 (145–263) | 279.9 (130–429) |
| CA | TC | 42 | 3.932 (3.4–4.3) | 35.77 (30–41) | 18.22 (12–23) | 51.62 * (42–60) p = 0.0128 | 151.7 (78–225) | 155.5 (77–233) |
| CA | TT | 106 | 3.908 (3.6–4.1) | 35.22 (32–38) | 20.51 (15–25) | 66.93 * (62–71) | 129.4 (101–156) | 191.9 (129–254) |
| CC | TC | 28 | 3.710 (2.9–4.4) | 29.63 (23–36) | 20.18 (11–28) | 58.29 (47–69) | 155.9 (92–219) | 259.9 (41–478) |
| CC | TT | 35 | 3.886 (3.3–4.4) | 36.66 (29–43) | 20.15 (9–30) | 54.72 (44–64) | 173.5 (107–239) | 172 (62–218) |
| CC | CC | 2 | NA # | NA # | NA # | NA # | NA # | NA # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreño-Saavedra, N.M.; Reyes-Pérez, I.V.; Machado-Sulbaran, A.C.; Martínez-Bonilla, G.E.; Ramírez-Dueñas, M.G.; Muñoz-Valle, J.F.; Olaya-Valdiviezo, V.; García-Iglesias, T.; Martínez-García, E.A.; Sánchez-Hernández, P.E. IL-21 (rs2055979 and rs2221903)/IL-21R (rs3093301) Polymorphism and High Levels of IL-21 Are Associated with Rheumatoid Arthritis in Mexican Patients. Genes 2023, 14, 878. https://doi.org/10.3390/genes14040878
Carreño-Saavedra NM, Reyes-Pérez IV, Machado-Sulbaran AC, Martínez-Bonilla GE, Ramírez-Dueñas MG, Muñoz-Valle JF, Olaya-Valdiviezo V, García-Iglesias T, Martínez-García EA, Sánchez-Hernández PE. IL-21 (rs2055979 and rs2221903)/IL-21R (rs3093301) Polymorphism and High Levels of IL-21 Are Associated with Rheumatoid Arthritis in Mexican Patients. Genes. 2023; 14(4):878. https://doi.org/10.3390/genes14040878
Chicago/Turabian StyleCarreño-Saavedra, Noemi Magdalena, Itzel Viridiana Reyes-Pérez, Andrea Carolina Machado-Sulbaran, Gloria Esther Martínez-Bonilla, María Guadalupe Ramírez-Dueñas, José Francisco Muñoz-Valle, Valeria Olaya-Valdiviezo, Trinidad García-Iglesias, Erika Aurora Martínez-García, and Pedro Ernesto Sánchez-Hernández. 2023. "IL-21 (rs2055979 and rs2221903)/IL-21R (rs3093301) Polymorphism and High Levels of IL-21 Are Associated with Rheumatoid Arthritis in Mexican Patients" Genes 14, no. 4: 878. https://doi.org/10.3390/genes14040878
APA StyleCarreño-Saavedra, N. M., Reyes-Pérez, I. V., Machado-Sulbaran, A. C., Martínez-Bonilla, G. E., Ramírez-Dueñas, M. G., Muñoz-Valle, J. F., Olaya-Valdiviezo, V., García-Iglesias, T., Martínez-García, E. A., & Sánchez-Hernández, P. E. (2023). IL-21 (rs2055979 and rs2221903)/IL-21R (rs3093301) Polymorphism and High Levels of IL-21 Are Associated with Rheumatoid Arthritis in Mexican Patients. Genes, 14(4), 878. https://doi.org/10.3390/genes14040878








