Investigation of NLR Genes Reveals Divergent Evolution on NLRome in Diploid and Polyploid Species in Genus Trifolium
Abstract
1. Introduction
2. Materials and Methods
2.1. Mining of NLR Genes in Arachis Species
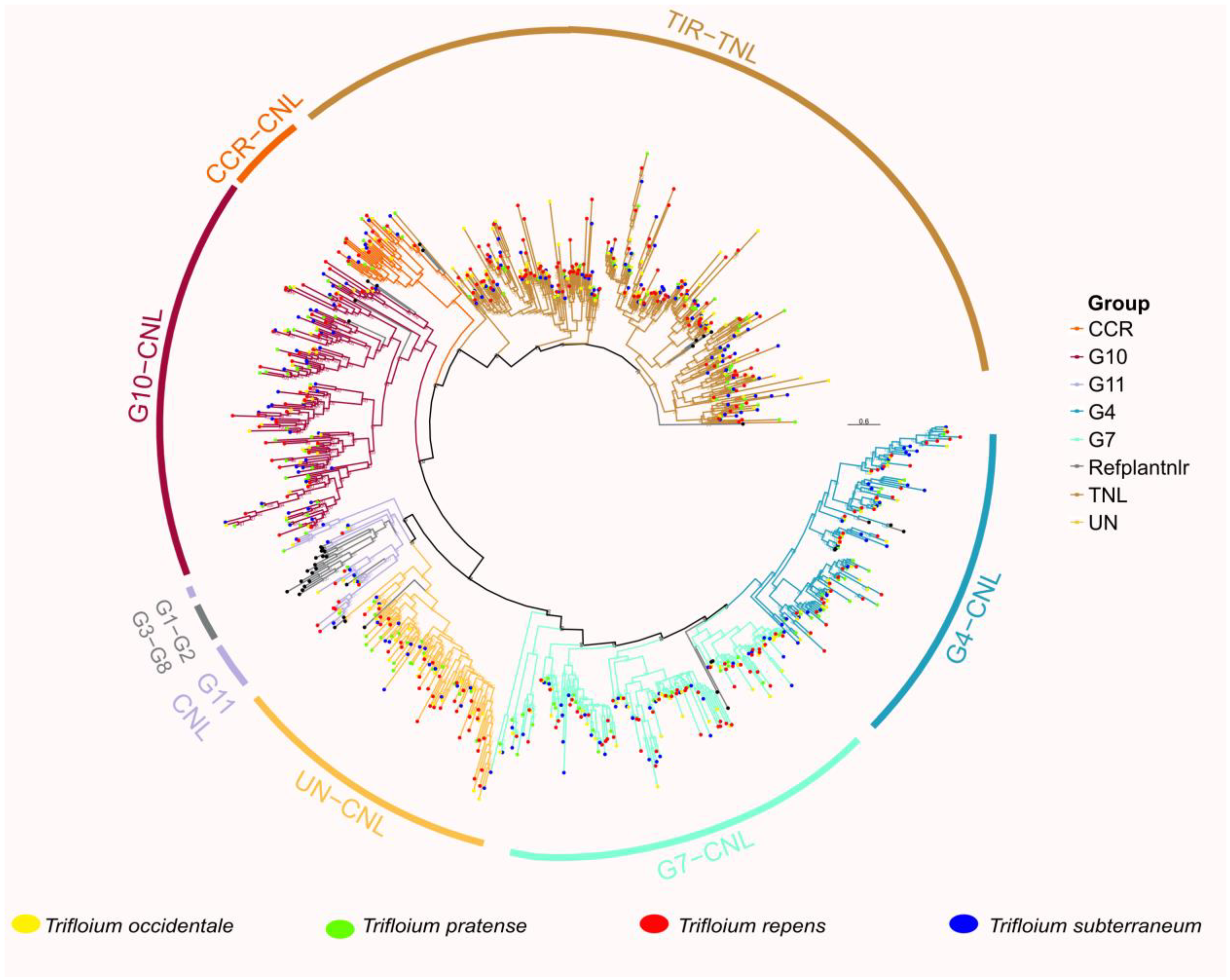
2.2. Classification and Phylogenetic Analysis
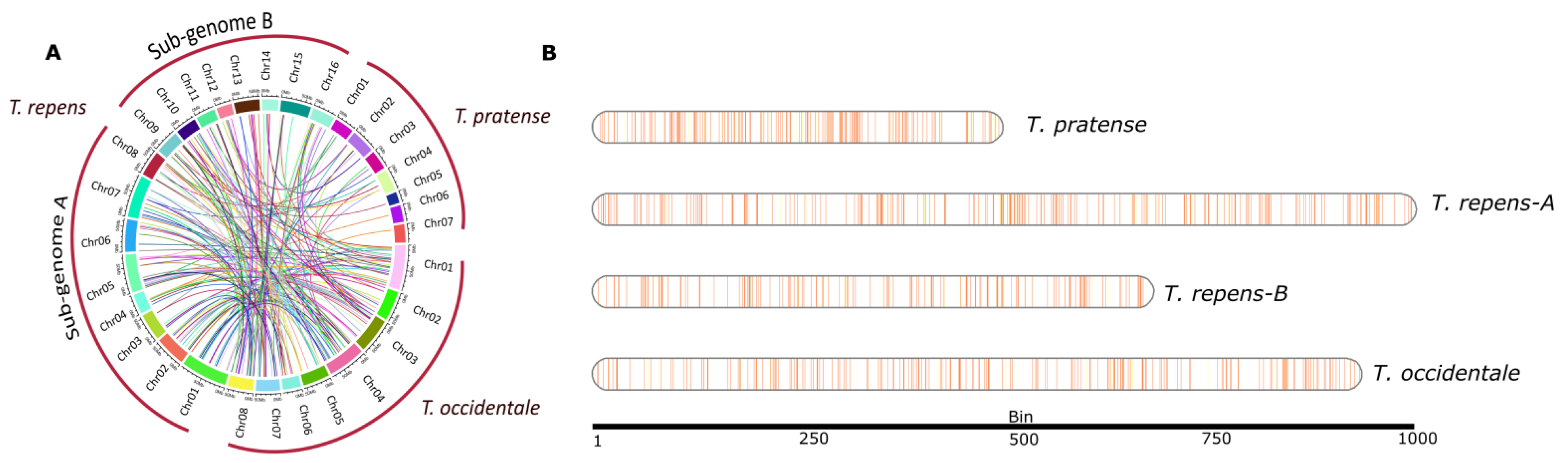
2.3. Loci Maps and Syntenic Maps of NLR-Genes
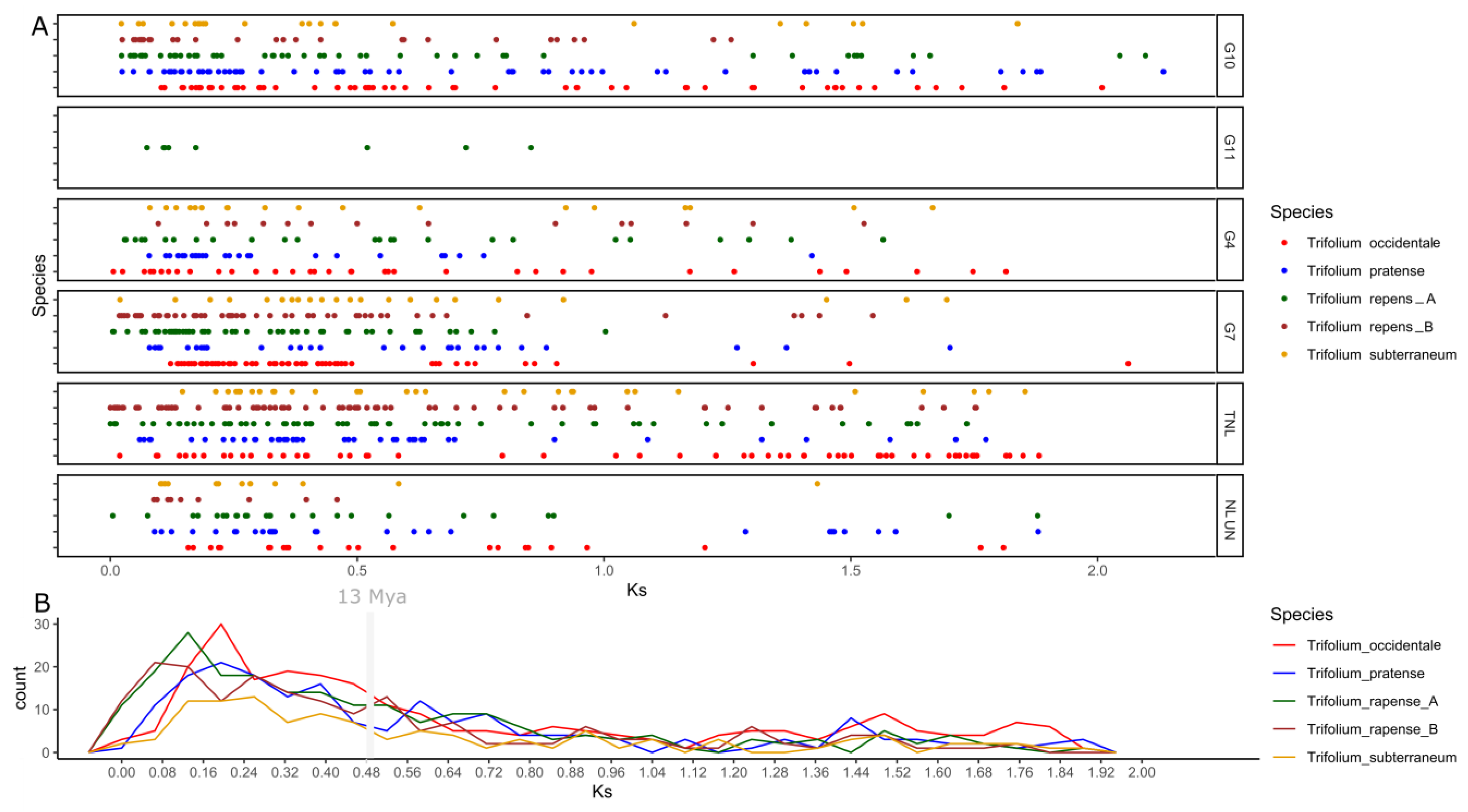
2.4. Evolutionary Analysis in Trifolium NLRs
2.5. RNA-Seq Based Expression Analysis
3. Results
3.1. Gene Mining of NLR Genes in Trifolium Species
3.2. Landscape of NLR Genes among Trifolium Species
3.3. Phylogenetic Analysis and Classification of NLR Genes
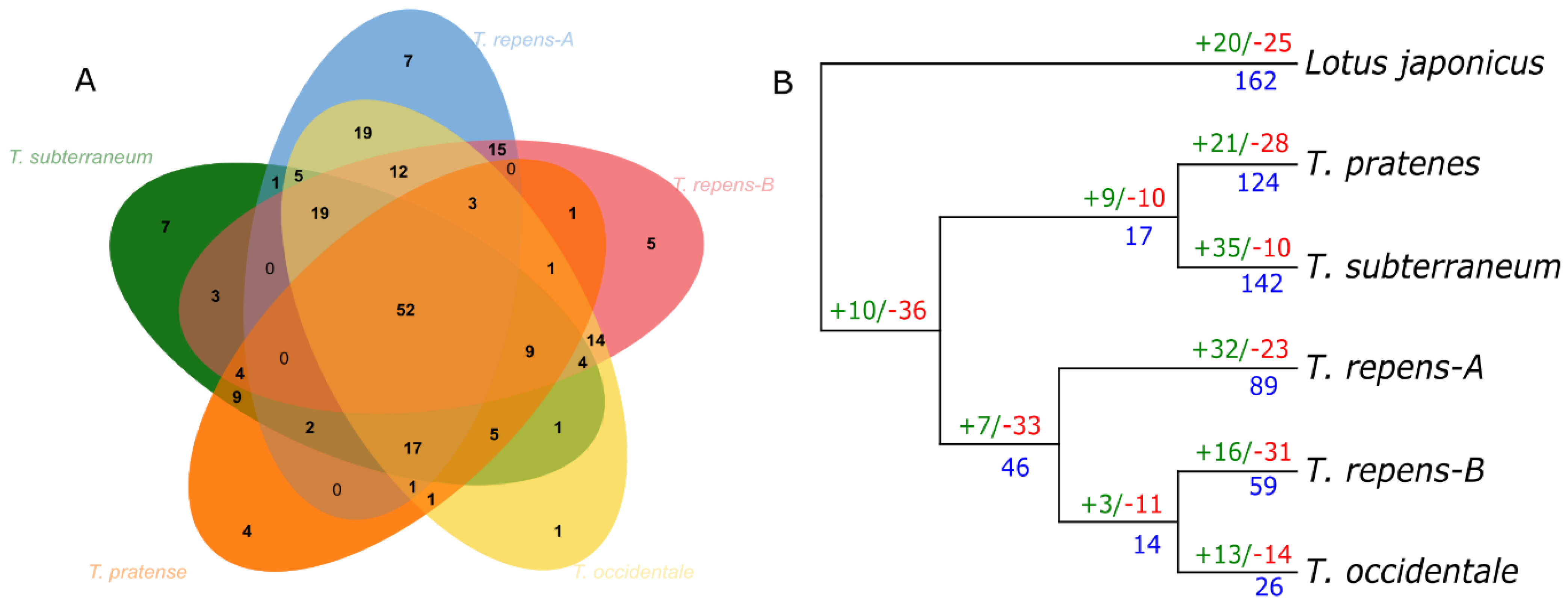
3.4. NLR Gene Birth and Death Ratio
3.5. Duplication Assay
3.6. Expression of Identified NLR Genes in Trifolium Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellison, N.W.; Liston, A.; Steiner, J.J.; Williams, W.M.; Taylor, N.L. Molecular Phylogenetics of the Clover Genus (Trifolium-Leguminosae). Mol. Phylogenet. Evol. 2006, 39, 688–705. [Google Scholar] [CrossRef]
- Tözsér, J. Comparative Studies on Retroviral Proteases: Substrate Specificity. Viruses 2010, 2, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Kaur, P.; Shirasawa, K.; Nichols, P.; Nagano, S.; Appels, R.; Erskine, W.; Isobe, S.N. Draft Genome Sequence of Subterranean Clover, a Reference for Genus Trifolium. Sci. Rep. 2016, 6, 30358. [Google Scholar] [CrossRef] [PubMed]
- Lukjanová, E.; Řepková, J. Chromosome and Genome Diversity in the Genus Trifolium (Fabaceae). Plants 2021, 10, 2518. [Google Scholar] [CrossRef] [PubMed]
- Sayed Ahmed, H.I.; Badr, A.; El-Shazly, H.H.; Watson, L.; Fuoad, A.S.; Ellmouni, F.Y. Molecular Phylogeny of Trifolium L. Section Trifolium with Reference to Chromosome Number and Subsections Delimitation. Plants 2021, 10, 1985. [Google Scholar] [CrossRef]
- Leitch, A.R.; Leitch, I.J. Genomic Plasticity and the Diversity of Polyploid Plants. Science 2008, 320, 481–483. [Google Scholar] [CrossRef]
- Williams, W.M.; Ellison, N.W.; Ansari, H.A.; Verry, I.M.; Hussain, S.W. Experimental Evidence for the Ancestry of Allotetraploid Trifolium Repens and Creation of Synthetic Forms with Value for Plant Breeding. BMC Plant Biol. 2012, 12, 55. [Google Scholar] [CrossRef]
- Griffiths, A.G.; Moraga, R.; Tausen, M.; Gupta, V.; Bilton, T.P.; Campbell, M.A.; Ashby, R.; Nagy, I.; Khan, A.; Larking, A.; et al. Breaking Free: The Genomics of Allopolyploidy-Facilitated Niche Expansion in White Clover. Plant Cell 2019, 31, 1466–1487. [Google Scholar] [CrossRef]
- Gross, B.L.; Kane, N.C.; Lexer, C.; Ludwig, F.; Rosenthal, D.M.; Donovan, L.A.; Rieseberg, L.H. Reconstructing the Origin of Helianthus deserticola: Survival and Selection on the Desert Floor. Am. Nat. 2004, 164, 145–156. [Google Scholar] [CrossRef]
- Fawcett, J.A.; Maere, S.; van de Peer, Y. Plants with Double Genomes Might Have Had a Better Chance to Survive the Cretaceous-Tertiary Extinction Event. Proc. Natl. Acad. Sci. USA 2009, 106, 5737–5742. [Google Scholar] [CrossRef]
- Vanneste, K.; Maere, S.; van de Peer, Y. Tangled up in Two: A Burst of Genome Duplications at the End of the Cretaceous and the Consequences for Plant Evolution. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130353. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.M.; Maruvka, Y.E.; Richmond, P.A.; Guillet, M.; Shoresh, N.; Sorenson, A.L.; De, S.; Kishony, R.; Michor, F.; Dowell, R.; et al. Polyploidy Can Drive Rapid Adaptation in Yeast. Nature 2015, 519, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.C.; Thomas, B.J. Incidence and Severity of Pest and Disease Damage to White Clover Foliage at 16 Sites in England and Wales. Ann. Appl. Biol. 1991, 118, 1–8. [Google Scholar] [CrossRef]
- Kahraman, A.; Pandey, A.; Khan, M.K.; Lindsay, D.; Moenga, S.; Vance, L.; Bergmann, E.; Carrasquilla-Garcia, N.; Shin, M.G.; Chang, P.L.; et al. Distinct Subgroups of Cicer Echinospermum Are Associated with Hybrid Sterility and Breakdown in Interspecific Crosses with Cultivated Chickpea. Crop Sci. 2017, 57, 3101–3111. [Google Scholar] [CrossRef]
- Calle García, J.; Guadagno, A.; Paytuvi-Gallart, A.; Saera-Vila, A.; Amoroso, C.G.; D’esposito, D.; Andolfo, G.; Aiese Cigliano, R.; Sanseverino, W.; Ercolano, M.R.; et al. PRGdb 4.0: An Updated Database Dedicated to Genes Involved in Plant Disease Resistance Process. Nucleic Acids Res. 2022, 50, D1483–D1490. [Google Scholar] [CrossRef]
- Kourelis, J.; Sakai, T.; Adachi, H.; Kamoun, S. RefPlantNLR Is a Comprehensive Collection of Experimentally Validated Plant Disease Resistance Proteins from the NLR Family. PLoS Biol. 2021, 19, e3001124. [Google Scholar] [CrossRef]
- Stanke, M.; Diekhans, M.; Baertsch, R.; Haussler, D. Using Native and Syntenically Mapped CDNA Alignments to Improve de Novo Gene Finding. Bioinformatics 2008, 24, 637–644. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Jupe, F.; Pritchard, L.; Etherington, G.J.; MacKenzie, K.; Cock, P.J.A.; Wright, F.; Sharma, S.K.; Bolser, D.; Bryan, G.J.; Jones, J.D.G.; et al. Identification and Localisation of the NB-LRR Gene Family within the Potato Genome. BMC Genom. 2012, 13, 75. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Rani, S.; Zahra, R.; Bakar, A.; Rizwan, M.; Sultan, A.-B.; Zain, M.; Mehmood, A.; Danial, M.; Shakoor, S.; Saleem, F.; et al. Dynamic Evolution of NLR Genes in Dalbergioids. Genes 2023, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kim, S.; Yeom, S.I.; Choi, D. Genome-Wide Comparative Analyses Reveal the Dynamic Evolution of Nucleotide-Binding Leucine-Rich Repeat Gene Family among Solanaceae Plants. Front. Plant Sci. 2016, 7, 1205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Lv, D.; Ge, Y.; Shi, J.; Weijers, D.; Yu, G.; Chen, J. RIdeogram: Drawing SVG Graphics to Visualize and Map Genome-Wide Data on the Idiograms. PeerJ Comput. Sci. 2020, 6, e251. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust Conversion of Protein Sequence Alignments into the Corresponding Codon Alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef]
- Zhang, Z. KaKs_calculator 3.0: Calculating Selective Pressure on Coding and Non-Coding Sequences. Genom. Proteom. Bioinform. 2022, 20, 536–540. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A Web Server for Whole-Genome Comparison and Annotation of Orthologous Clusters across Multiple Species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.K.; Vanderpool, D.; Fulton, B.; Hahn, M.W. CAFE 5 Models Variation in Evolutionary Rates among Gene Families. Bioinformatics 2020, 36, 5516–5518. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Ji, C.; Song, Q.; Zhang, W.; Zhang, X.; Zhao, K.; Chen, C.Y.; Wang, C.; He, G.; Liang, Z.; et al. Comparison of Arachis Monticola with Diploid and Cultivated Tetraploid Genomes Reveals Asymmetric Subgenome Evolution and Improvement of Peanut. Adv. Sci. 2020, 7, 1901672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wu, H.; Zhang, R.; Li, S.; He, W.; Wong, F.L.; Li, G.; Zhao, S.; Lam, H.M. Molecular Phylogeny and Dynamic Evolution of Disease Resistance Genes in the Legume Family. BMC Genom. 2016, 17, 402. [Google Scholar] [CrossRef] [PubMed]
- Ashfield, T.; Egan, A.N.; Pfeil, B.E.; Chen, N.W.G.; Podicheti, R.; Ratnaparkhe, M.B.; Ameline-Torregrosa, C.; Denny, R.; Cannon, S.; Doyle, J.J. Evolution of a Complex Disease Resistance Gene Cluster in Diploid Phaseolus and Tetraploid Glycine. Plant Physiol. 2012, 159, 336–354. [Google Scholar] [CrossRef]
- Yin, D.; Ji, C.; Ma, X.; Li, H.; Zhang, W.; Li, S.; Liu, F.; Zhao, K.; Li, F.; Li, K.; et al. Genome of an Allotetraploid Wild Peanut Arachis monticola: A de Novo Assembly. Gigascience 2018, 7, giy066. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areej, A.; Nawaz, H.; Aslam, I.; Danial, M.; Qayyum, Z.; Rasool, U.A.; Asif, J.; Khalid, A.; Serfraz, S.; Saleem, F.; et al. Investigation of NLR Genes Reveals Divergent Evolution on NLRome in Diploid and Polyploid Species in Genus Trifolium. Genes 2023, 14, 867. https://doi.org/10.3390/genes14040867
Areej A, Nawaz H, Aslam I, Danial M, Qayyum Z, Rasool UA, Asif J, Khalid A, Serfraz S, Saleem F, et al. Investigation of NLR Genes Reveals Divergent Evolution on NLRome in Diploid and Polyploid Species in Genus Trifolium. Genes. 2023; 14(4):867. https://doi.org/10.3390/genes14040867
Chicago/Turabian StyleAreej, Amna, Hummera Nawaz, Iqra Aslam, Muhammad Danial, Zohaib Qayyum, Usama Akhtar Rasool, Jehanzaib Asif, Afia Khalid, Saad Serfraz, Fozia Saleem, and et al. 2023. "Investigation of NLR Genes Reveals Divergent Evolution on NLRome in Diploid and Polyploid Species in Genus Trifolium" Genes 14, no. 4: 867. https://doi.org/10.3390/genes14040867
APA StyleAreej, A., Nawaz, H., Aslam, I., Danial, M., Qayyum, Z., Rasool, U. A., Asif, J., Khalid, A., Serfraz, S., Saleem, F., Mubin, M., Shoaib, M., Shahnawaz-ul-Rehman, M., Nahid, N., & Alkahtani, S. (2023). Investigation of NLR Genes Reveals Divergent Evolution on NLRome in Diploid and Polyploid Species in Genus Trifolium. Genes, 14(4), 867. https://doi.org/10.3390/genes14040867