Abstract
Indoor residual spray (IRS), mainly employing pyrethroid insecticides, is the most common intervention for preventing malaria transmission in many regions of Latin America; the use of long-lasting insecticidal nets (LLINs) has been more limited. Knockdown resistance (kdr) is a well-characterized target-site resistance mechanism associated with pyrethroid and DDT resistance. Most mutations detected in acetylcholinesterase-1 (Ace-1) and voltage-gated sodium channel (VGSC) genes are non-synonymous, resulting in a change in amino acid, leading to the non-binding of the insecticide. In the present study, we analyzed target-site resistance in Nyssorhynchus darlingi, the primary malaria vector in the Amazon, in multiple malaria endemic localities. We screened 988 wild-caught specimens of Ny. darlingi from three localities in Amazonian Peru and four in Amazonian Brazil. Collections were conducted between 2014 and 2021. The criteria were Amazonian localities with a recent history as malaria hotspots, primary transmission by Ny. darlingi, and the use of both IRS and LLINs as interventions. Fragments of Ace-1 (456 bp) and VGSC (228 bp) were amplified, sequenced, and aligned with Ny. darlingi sequences available in GenBank. We detected only synonymous mutations in the frequently reported Ace-1 codon 280 known to confer resistance to organophosphates and carbamates, but detected three non-synonymous mutations in other regions of the gene. Similarly, no mutations linked to insecticide resistance were detected in the frequently reported codon (995) at the S6 segment of domain II of VGSC. The lack of genotypic detection of insecticide resistance mutations by sequencing the Ace-1 and VGSC genes from multiple Ny. darlingi populations in Brazil and Peru could be associated with low-intensity resistance, or possibly the main resistance mechanism is metabolic.
1. Introduction
Indoor residual spray (IRS) and, more recently, long-lasting insecticidal nets (LLINs), have been used as the primary malaria vector control interventions due to their cost-effectiveness and protection against vectors that feed and rest mainly indoors [1,2]. In contrast, in Latin America, many vectors feed and rest primarily outdoors (exophagy and exophily, respectively). This behavior, together with the widespread existence of houses with incomplete walls, communities where unprotected travel-related occupations are common [3,4,5], and the temporally irregular application of IRS in many communities diminishes the effectiveness of insecticide-based interventions throughout Latin America in relation to some other endemic malaria regions [6,7].
In 2007, the World Health Organization’s (WHO) recommendations for LLIN distribution were broadened to include all individuals in endemic malaria areas, rather than solely pregnant women and children under the age of 5 [8]. Simultaneously, the WHO guidelines called for endemic malaria nations to adopt triennial LLIN mass distribution campaigns, which would allocate one LLIN for every two people per household in a given region, aspiring to universal coverage [9]. Following these updates, mass distribution campaigns became more prevalent in Latin America, particularly in the Amazon region. In Peru, the Project for Malaria Control in Andean Border Areas (PAMAFRO) was mainly responsible for a 70% decrease in cases from 2005 to 2011 [9]. Since the program was discontinued, malaria cases have increased fairly steadily due to a decline in international and domestic funding [10]. The sharpest increase in cases throughout the Latin American region occurred between 2014 and 2017, when case incidence nearly doubled [11]; ironically, incidence has apparently decreased with the recent COVID-19 pandemic [12]. An encouraging sign is that the Ministry Health, Peru, adopted a new plan as of January 2022 to eliminate malaria by 2030 [13].
Between 2010 and 2019, an estimated 28 endemic malaria countries (of 82) detected insecticide resistance to all four of the most commonly used insecticides (pyrethroids, organochlorines, carbamates, and organophosphates), and nearly all (73/82) have reported resistance to at least one class [14,15]. Worldwide, the exposure of mosquitoes to any of these classes of insecticides [16], whether for public health or agricultural use, has the potential to be a strong selective force that favors the survival of resistant populations [17]. In Latin America, there is relatively little entomological surveillance and few published studies on insecticide resistance (IR), except for Nyssorhynchus albimanus [18]. The primary malaria vector in the Amazon, Ny. darlingi, is generally susceptible to insecticides [17,19] and exhibits exo- and endophagic behavior, depending on its local circumstances [20]. Nevertheless, resistance based on bioassays using WHO paper bioassays [21] or CDC bottle bioassays [22,23] has been reported for Ny. darlingi to pyrethroids and carbamates (Bolivia), pyrethroids (Brazil), pyrethroids and organochlorines (Colombia), and pyrethroids, carbamates, and organophosphates (Peru) [24].
IR in mosquitoes can result from several mechanisms. One of the best-characterized is knockdown resistance (kdr) or target-site point mutations, commonly associated with pyrethroid and DDT resistance [25,26]. The site of most kdr mutations is either the voltage-gated channel (VGSC) corresponding to the VGSC gene, located in the transmembrane segment IIS6 or the linker regions that connect domains III and IV, or the acetylcholinesterase-1 (Ace-1) gene in anophelinae and other mosquito species. The Asian malaria vector Anopheles culicifacies was one of the earliest anophelines to be found to be resistant to DDT in Gujerat State, India, using DDT-impregnated paper [27]. On the other hand, the first instance of DDT resistance in Ny. darlingi was not detected until 1990, using WHO techniques for susceptibility [28] in the Choco region of northwestern Colombia [29]. The second mechanism, behavioral modification, is a change in behavior, such as the avoidance of or repellence in response to insecticide-impregnated surfaces, or a modification in location (outdoors vs. indoors; dispersion to untreated houses), or time of day of blood seeking. This has been called qualitative behavioral resistance recently [30]. The first systematic study of Ny. darlingi behavioral modification was a demonstration of repellency to DDT-impregnated house surfaces in Amazonian Brazil [31]. A second example is the detection of fewer Ny. darlingi biting outdoors and more indoors between the distributions of LLINs (as the nets aged and became non-repellent) in Amazonian Peru [32]. Another mechanism is increased metabolism (detoxification/sequestration) through mixed-function oxidases (MFO) and non-specific esterases (NSE) that have developed during insect evolutionary history as protection against a range of plant toxins [33,34].
Of these mechanisms, only target-site—considered to be the most accurate indicator of resistance—can be identified using molecular assays [35]. These assays detect amino acid substitutions that give rise to non-synonymous amino acid changes in insecticide targets, ultimately preventing the insecticide from binding, leading to resistance [36]. Among the genes of interest are voltage-gated sodium channel (VGSC) and acetylcholinesterase-1 (Ace-1), which encode for the target binding of pyrethroids/organochlorines and carbamates/organophosphates, respectively [26,36]. Mutations linked most frequently with IR are L995F and L995S in VGSC [37] and G280S in Ace-1 [38]. Other mutations include L995C, L995W, V991L, and V994S in VGSC [38], and, in Ace-1, A221T and S216T [18]. Codon 995 in VGSC and codon 280 in Ace-1 were referred to formerly as 1014 and 119, respectively [18]. Despite the accuracy of molecular assays, susceptibility (or phenotypic) bioassays are advantageous because hundreds of mosquitoes can be tested simultaneously with relatively simple equipment [24,35]. Having data from both molecular assays and phenotypic bioassays is ideal for assessing resistance frequency [36].
The combination of resurgent cases of malaria since 2014 and the widespread increase in the use of insecticides, yet the scarce reporting of resistant vectors, suggest a potential knowledge gap in IR throughout the Latin American region. IR detection requires the strategic selection of localities in which mosquito samples are collected and analyzed. Commonly used selection criteria from previous reports include accessibility by land or water, a history of insecticide use, a high malaria prevalence, and a sample size sufficient even for a population with a low frequency of resistant vectors [39,40]. This study aims to help close the knowledge gap in Ny. darlingi by using molecular assays for a relatively large sample size covering multiple localities, uncovering novel codon mutations, and providing insight that may assist in improving malaria vector control interventions.
2. Materials and Methods
2.1. Sample Collection
Samples of Ny. darlingi were collected in several rural and riverine localities throughout Amazonian Brazil and Peru in 2014–2021 (Table 1; Figure 1). Sample locations were based on information from local health officials about exposure to IRS or LLINs. In Cruzeiro do Sul, Acre, Brazil, local public health personnel (Brazil Ministry of Health and Instituto Evandro Chagas) demonstrated phenotypic resistance via discriminating concentration bioassays to deltamethrin and cypermethrin from 2012 to 2014 [41]. In the villages of Gamitanacocha and Zungarococha, Peru, local health authorities (Laboratorio Referencial de Salud Pública Loreto) demonstrated phenotypic resistance and possible resistance, respectively, to pyrethroids in 2018 [41]. Furthermore, Zungarococha and Cahuide are both along a highway where extensive IRS was used during a major malaria outbreak in 2012 [10].

Table 1.
Summary of Ny. darlingi samples sequenced for the Ace-1 and VGSC genes.
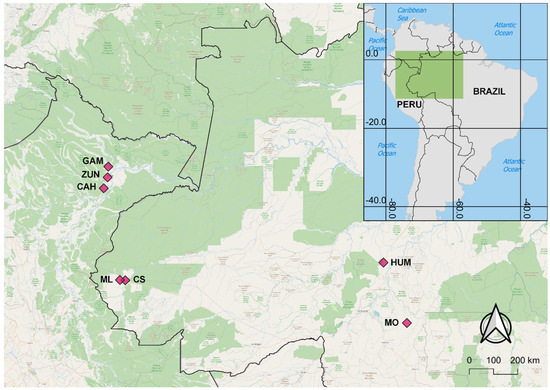
Figure 1.
Map of Ny. darlingi collection sites in Amazonian Peru (GAM: Gamitanacocha; ZUN: Zungarococha; and CAH: Cahuide) and Brazil (ML: Mâncio Lima; CS: Cruzeiro do Sul; HUM: Humaitá; and MO: Machadinho d’Oeste).
Mosquito collections were performed indoors, peridomestically, or in forest edge settings during various time periods throughout the evening using human landing catch, barrier screen sampling, or Shannon traps [32,42,43]. Details on the forest cover level [42], field collection protocols [32,43], and Plasmodium vivax malaria incidence [43] have been reported previously. Collected mosquitoes were identified to species morphologically by trained personnel using regional taxonomic keys [44,45,46] and stored on silica gel until a genetic analysis was conducted. Genomic DNA was extracted from whole mosquitoes using the Qiagen DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD, USA).
2.2. Molecular Analysis
Samples used for the genetic analysis were selected randomly from the available specimens in storage using a random number generator [47]. As year-round samples from Cahuide were available, we selected specimens from both the rainy and dry seasons [32]. The amplification of a 228 bp fragment of the kdr region of the VGSC gene, between exons 20 and 21, was performed in a 20 μL PCR mixture containing a 1.0–15 ng/μL DNA template, 1× AllTaq Master Mix (Qiagen), and 0.5 μM of each primer: AAKDRF2 and AAKDRR2, with the same cycling conditions as in [16]. Amplification of a 456 bp fragment of Exon 2 in the Ace-1 gene was performed in a 25 μL PCR mixture containing a 1.0–15 ng/μL DNA template [43], 2 U of Taq DNA Polymerase (Qiagen), 10× PCR Buffer (Qiagen) containing 1.5 μM of MgCl2, a supplemental 0.5 μM of MgCl2 totaling 2.0 μM of MgCl2, 0.2 μM of dNTPs, and 0.4 μM of each primer ACE1DAF and ACE1DAR, with the same cycling conditions as in [18].
SSamples were Sanger sequenced in forward and reverse directions at the Wadsworth Center Advanced Genomic Technologies Core (New York State Department of Health). Chromatograms of each sample were cleaned, converted into consensus sequences, translated, and exported to FASTA files using Geneious Prime Version 2020.2 [48]. Consensus sequences were aligned using ClustalW in MegaX Version 10.1.7, then analyzed in comparison to existing VGSC and Ace-1 sequences of Ny. darlingi from GenBank [16]. Unique sequences for VGSC were deposited in GenBank under the accession numbers: OR260704–OR260712 and Ace-1 under the accession numbers: OR260713–OR260857. The sSequences for both genes were examined for non-synonymous (amino acid change) and synonymous (no amino acid change) point mutations, with special focus on the documented codons known to convey insecticide resistance within these gene regions.
3. Results
3.1. Detection of Voltage-Gated Sodium Channel (VGSC) Mutations
A total of 495 wild-caught Ny. darlingi, 296 from Peru and 199 from Brazil, were successfully sequenced for the kdr target-site resistance region of the VGSC gene (Table 1). SSequences were aligned with the aid of the Ny. darlingi VGSC sequences available in Genbank [16]. We detected nine unique genotypes (denoted as V1–V9, corresponding to Genbank IDs OR260704–OR260712), with only the susceptible genotype TTA (leucine) observed at codon position 995 (Figure 2). The only point mutations detected were in the intron and primer regions.
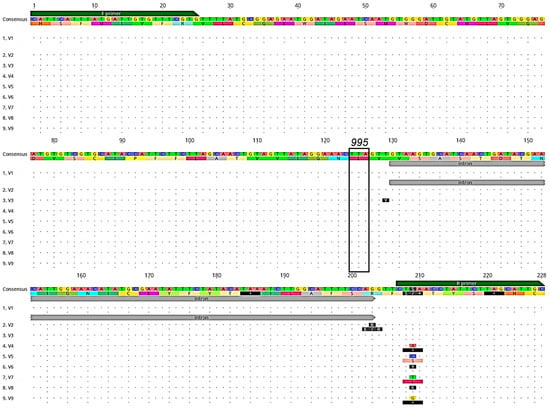
Figure 2.
Alignment of DNA genotypes (V1-V9) and amino acid translations (below each DNA sequence) of the VGSC gene kdr region sequenced from Ny. darlingi collected in Peru and Brazil. Dots indicate no base or amino acid change from consensus sequence. Forward (F primer) and reverse primer (R primer) regions (green boxes), intron position (gray), and codon 995 (black box), associated with pyrethroid and DDT resistance are denoted (image obtained from Geneious version 2020.2 created by Biomatters).
3.2. Detection of Acetylcholinesterase-1 (Ace-1) Mutations
We successfully sequenced 493 Ny. darlingi, 295 from Peru and 198 from Brazil, for the Ace-1 gene (Table 1), and detected 145 unique genotypes (named AC1–AC145, corresponding to Genbank IDs OR260713–OR260857). The susceptible genotype GGG/GGG or GGG/GGC (glycine) was observed at codon 280 across all samples. Three non-synonymous mutations were detected in samples from three populations (Humaitá and Mâncio Lima, Brazil; and Cahuide, Peru) within other regions of the Ace-1 gene not known to convey insecticide resistance (Figure 3).
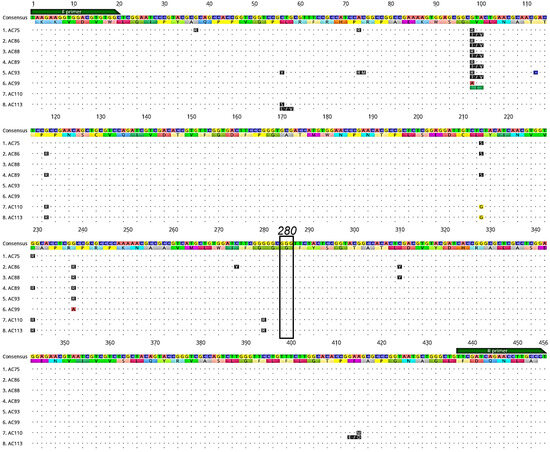
Figure 3.
Alignment of a subset of Ace-1 DNA genotypes and amino acid translations (below each DNA sequence) displaying non-synonymous mutations (blue lines) detected in Ny. darlingi samples sequenced from Peru and Brazil. Dots indicate no base or amino acid change from consensus sequence. Forward (F primer) and reverse primer (R primer) regions (green boxes) and codon 280 (black box), which is associated with organophosphate and carbamate resistance, are denoted (image obtained from Geneious version 2020.2 created by Biomatters).
3.3. Data Management
Unique sequences of Ny. darlingi for VGSC were deposited in GenBank under accession numbers: OR260704–OR260712, and for Ace-1, under accession numbers: OR260713–OR260857. All sample results will be deposited in VectorBase pending publication.
4. Discussion
A molecular analysis of a ~228 bp fragment that encodes for the kdr target-site resistance region of the VGSC gene and a ~456 bp fragment of the Ace-1 gene did not detect any non-synonymous mutations in the specimens of Ny. darlingi from endemic malaria areas of Brazil and Peru in the current study, similar to results of a recent analysis of Ny. darlingi from locations in Brazil (Manaus, Unini River, Jau River in Amazonas State, and Porto Velho, Rondônia state) and Colombia (Tagachi and Chocó Department) [49]. An investigation of specimens of Ny. darlingi from Chocó Department, Colombia, that had been demonstrated to be phenotypically susceptible and resistant, sequenced for the same region of the VGSC gene, also did not reveal any kdr mutations [16]. However, the classic L1014F kdr mutation has been detected in other important anopheline malaria vectors, i.e., Nyssorhynchus albimanus [16,50,51] and Nyssorhynchus albitarsis s.s. [52]. Other species of Latin American malaria vectors evaluated with these molecular assays include Anopheles vestitipennis and Anopheles pseudopunctipennis, both of which exhibited genotypic susceptibility [16]. The lack of genotypic evidence of IR in Ny. darlingi could be a reflection of limited regional data, rather than the absence of resistance [53]. On the other hand, Floch [54] suggested that frequent reintroduction of wild susceptible populations of Ny. darlingi from forest into village populations could reduce selection for insecticide resistance. This hypothesis received some support from a study in the Porto Velho area in Rondônia state, Brazil, that detected seasonal gene flow between forested and urban populations of Ny. darlingi [55].
Several previous studies of Anopheles malaria vectors have attributed a proportion of recent malaria case resurgence to increased outdoor biting and insecticide resistance (IR) following mass distribution campaigns [56,57,58,59], although there is no evidence in support of this latter trend for Ny. darlingi across Latin America (the scale-up of LLIN distribution has been limited compared with Africa) or after the completion of the intensive PAMAFRO project in Peru. Even though daytime biting behavior in members of the An. gambiae complex has been hypothesized to increase transmission [60], in Latin America, there has been scant investigation into this phenomenon, except for observations of Ny. darlingi biting during the day in forested French Guiana malaria hotspots [61].
The hot, rainy climate of the Amazon basin is optimal for mosquito habitats [62]; however, anthropogenic landscape changes—namely, forest fragmentation and an increased ecotone density—suggest that vector behavior (mainly exophagy and exophily in Ny. darlingi) and distribution (i.e., along ecotones for Ny. darlingi) in one location may not be generalizable to an entire region [63,64]. For example, during a recent malaria outbreak in French Guiana, Ny. darlingi was the only anopheline collected both outdoors and indoors, and its abundance was exceptionally high, possibly attributed to regional deforestation, and/or the higher than average rainy and dry seasons in 2017 [65]. Ny. darlingi has also been collected biting during the day in French Guiana forests [66,67] and along the Maroni River, a former malaria hotspot, in Suriname [68]. This appears to be a focal phenomenon in Ny. darlingi, perhaps a behavioral avoidance response to IRS or LLINs.
The majority of IR reports in Latin America are based on bioassay data from the Amazon Basin or Central America [24,36]. However, the recent genotypic reporting of several Brazilian samples of Ny. albitarsis s.s. showed heterozygous L995F mutations in VGSC [52], and a sample of Guatemalan Ny. albimanus had a heterozygous G280S mutation in Ace-1 [69]. Both of these reports inferred that agricultural insecticide use was the driver of IR, and a recent review of the contribution of agricultural insecticides and increasing insecticide resistance in malaria vectors found a strong association across Africa that could be affected by crop type (especially cotton and vegetables), urban development, and the strategies undertaken for farm pest management [70]. Questionnaires and insecticide susceptibility bioassays utilized in a field study in two South Côte d’Ivoire communities determined that local mosquito vectors were resistant to three of four insecticides tested, and the authors highlighted the need for collaboration between the public health and agricultural sectors to develop interventions that would benefit both [71]. Resistance in the important regional malaria vector, Ny. albimanus, has been detected in Central America, Panama, and northwestern coastal Peru, linked mainly to agriculture in general and rice cultivation in coastal Peru in particular [17].
Public health insecticide use can exert comparable selective pressure on malaria vectors [72], including, for example, the organophosphate malathion used in Brazilian public health for the arboviral vectors Aedes aegypti and Aedes albopictus to reduce the transmission of viruses such as dengue, chikungunya, and Zika [73]. Resistance in the vector Culex quinquefasciatus in Brazil has been detected for organophosphates, carbamate, DDT, pyrethroids, and biolarvicides; the concern for such resistance to arise in malaria vectors in Brazil, where they co-occur with Cx. quinquefasciatus, is limited to Fortaleza, Ceará state, and parts of Mato Grosso state [74].
For control of adult mosquitoes, IRS on interior house walls will kill resting mosquitoes; some also repel mosquitoes such that they modify their behavior and rest outdoors [17]. Based on an evaluation of the residual effects of four insecticides (deltamethrin, pyrethroids, lambda-cyhalothrin, and etofenprox) used on a range of wall materials in Amazonian Brazil [75], the Brazilian National Malaria Control Plan has been consistently using etofenprox PM 20% for residual spray in houses since 2013 [19], although a similar study of six insecticides in Amapá, Brazil, by Correa et al. [76] found that deltamethrin WG at 0.025 gm/m2 had the highest residual effects. In Peru, pyrethroid deltamethrin 5% is the most commonly applied insecticide for IRS [32].
Other approaches to tackling insecticide resistance consider biological control in general [77] or the replacement of synthetic compounds with plant-based compounds formulated as bioinsecticides, reviewed in Demirak and Canpolat [15]. The classes of compounds described and discussed were phytochemicals, pheromones, microbial pesticides, and plant-incorporated protectants; selected candidate compounds demonstrate larvicidal, adulticidal, and repellent properties. In general, their advantages are, compared to synthetic compounds, a lower toxicity, target specificity, being highly effectivity in small quantities, and they are biodegradable. Despite considerable promise, these products remain in various stages of development, and have not yet been field-tested for use against malaria vectors. As many target insects have evolved successful resistance mechanisms to most classes of insecticides, the evolution of different modes of action against plant-based insecticides could temper the early enthusiasm for such novel products [33].
Author Contributions
J.E.C., M.A.M.S., D.G., S.A.B. and J.M.V. conceived and designed the study. F.A., L.S.M.C., M.A.M.S. and D.G. provided guidance on field site selection. Field site collections conducted by E.S.B., M.A.M.S., L.S.M.C., G.A.D.R., C.A.M., C.T.R., M.P.S., J.D.J. and S.A.B. conducted the study and analyzed the data. J.E.C., S.A.B. and J.D.J. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
NIH-NIAID grants U19AI089681 to J.M.V. and R01AI110112 to J.E.C.
Institutional Review Board Statement
Human Landing Catch technique is considered to be a risk management issue by the New York State Institutional Review Board, and appropriate safeguards were put in place prior to and during all mosquito collections.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences were deposited into GenBank (detailed above) and relevant mosquito data will be added to VectorBase post-publication.
Acknowledgments
We thank all the residents and the local authorities of Cruzeiro do Sul, Humaitá, Machadinho d’Oeste, Mâncio Lima, in Brazil, and Gamitanacocha, Zungaracocha and Cahuide in Peru for their support. This publication has been possible thanks to the authorization and permits (no. 0424-2012-AG-DGFFS-DGEFFS) from Dirección de Gestion Forestal y de Fauna Silvestre y la Dirección General Forestal y de Fauna Silvestre del Ministerio de Agricultura de la Republica del Peru and from Brazil: Ministério do Meio Ambiente Conselho de Gestão do Patrimônio Genético, Cadastro No. R280EA1; and Comissão Nacional de Ética em Pesquisa, CAAE: 33671220.9.1001.5421. Sample DNA was sequenced at the Advanced Genomic Technologies Core, Wadsworth Center, Albany, NY.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexander, N.; DE LA Hoz, F.; Cruz, J.; Hall, A.J.; Prieto, G.; Arroyo, J.A.; Rodríguez, M.; Suárez, M.; Pérez, L.; Cotacio, M.C.; et al. Case-control study of mosquito nets against malaria in the Amazon region of Colombia. Am. J. Trop. Med. Hyg. 2005, 73, 140–148. [Google Scholar] [CrossRef]
- Sherrard-Smith, E.; Skarp, J.E.; Beale, A.D.; Fornadel, C.; Norris, L.C.; Moore, S.J.; Mihreteab, S.; Charlwood, J.D.; Bhatt, S.; Winskill, P.; et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. USA 2019, 116, 15086–15095. [Google Scholar] [CrossRef]
- Parker, B.S.; Paredes Olortegui, M.; Penataro Yori, P.; Escobedo, K.; Florin, D.; Rengifo Pinedo, S.; Cardenas Greffa, R.; Capcha Vega, L.; Rodriguez Ferrucci, H.; Pan, W.K.; et al. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar. J. 2013, 12, 178. [Google Scholar] [CrossRef]
- Carrasco-Escobar, G.; Gamboa, D.; Castro, M.C.; Bangdiwala, S.I.; Rodriguez, H.; Contreras-Mancilla, J.; Alava, F.; Speybroeck, N.; Lescano, A.G.; Vinetz, J.M.; et al. Micro-epidemiology and spatial heterogeneity of P. vivax parasitaemia in riverine communities of the Peruvian Amazon: A multilevel analysis. Sci. Rep. 2017, 7, 8082. [Google Scholar] [CrossRef]
- Moreno, J.E.; Rubio-Palis, Y.; Paez, E.; Perez, E.; Sanchez, V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med. Vet. Entomol. 2007, 21, 339–349. [Google Scholar] [CrossRef]
- Moreno, M.; Saavedra, M.P.; Bickersmith, S.A.; Lainhart, W.; Tong, C.; Alava, F.; Vinetz, J.M.; Conn, J.E. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar. J. 2015, 14, 290. [Google Scholar] [CrossRef]
- Durnez, L.; Coosemans, M. Residual transmission of malaria: An old issue for new approaches. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; Intech Open: Rijeka, Croatia, 2013; pp. 671–704. [Google Scholar]
- Sousa, J.O.; de Albuquerque, B.C.; Coura, J.R.; Suárez-Mutis, M.C. Use and retention of long-lasting insecticidal nets (LLINs) in a malaria risk area in the Brazilian Amazon: A 5-year follow-up intervention. Malar. J. 2019, 18, 100. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Soto-Calle, V.; Rosas-Aguirre, A.; Llanos-Cuentas, A.; Abatih, E.; DeDeken, R.; Rodriguez, H.; Rosanas-Urgell, A.; Gamboa, D.; Alessandro, U.D.; Erhart, A.; et al. Spatio-temporal analysis of malaria incidence in the Peruvian Amazon Region between 2002 and 2013. Sci. Rep. 2017, 7, 40350. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; p. 322. [Google Scholar]
- Gabaldón-Figueira, J.C.; Villegas, L.; Grillet, M.E.; Lezaun, J.; Pocaterra, L.; Bevilacqua, M.; Paniz-Mondolfi, A.; González, O.N.; Chaccour, C. Malaria in Venezuela: Gabaldón’s legacy scattered to the winds. Lancet Glob. Health 2021, 9, e584–e585. [Google Scholar] [CrossRef]
- Ministerio de Salud. Documento Technico: Plan Hacia la Malaria en el Peru 2022–2030; Ministerio de Salud: Lima, Pero, 2022; p. 60.
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020; p. 247. [Google Scholar]
- Şengül Demirak, M.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef]
- Orjuela, L.I.; Álvarez-Diaz, D.A.; Morales, J.A.; Grisales, N.; Ahumada, M.L.; Venegas, H.J.; Quiñones, M.L.; Yasnot, M.F. Absence of knockdown mutations in pyrethroid and DDT resistant populations of the main malaria vectors in Colombia. Malar. J. 2019, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.L.; Norris, D.E.; Conn, J.E.; Moreno, M.; Burkot, T.R.; Bugoro, H.; Keven, J.B.; Cooper, R.; Yan, G.; Rosas, A.; et al. Insecticide resistance in areas under investigation by the International Centers of Excellence for Malaria Research: A challenge for malaria control and elimination. Am. J. Trop. Med. Hyg. 2015, 93, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lol, J.C.; Castañeda, D.; Mackenzie-Impoinvil, L.; Romero, C.G.; Lenhart, A.; Padilla, N.R. Development of molecular assays to detect target-site mechanisms associated with insecticide resistance in malaria vectors from Latin America. Malar. J. 2019, 18, 202. [Google Scholar] [CrossRef]
- Baia-da-Silva, D.C.; Brito-Sousa, J.D.; Rodovalho, S.R.; Peterka, C.; Moresco, G.; Lapouble, O.M.M.; Melo, G.C.; Sampaio, V.S.; Alecrim, M.; Pimenta, P.; et al. Current vector control challenges in the fight against malaria in Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180542. [Google Scholar] [CrossRef]
- Hiwat, H.; Bretas, G. Ecology of Anopheles darlingi Root with respect to vector importance: A review. Parasit. Vectors 2011, 4, 177. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces: Report of the WHO Informal Consultation; World Health Organization: Geneva, Switzerland, 1998; p. 43. [Google Scholar]
- Lenhart, A.; Chan, A.; Vizcaino, L.; Brogdon, W. Manual for Evaluating Insecticide Resistance Using the CDC Bottle Bioassay; CDC: Atlanta, GA, USA, 2023; p. 31. [Google Scholar]
- Brogdon, W.; Chan, A. Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassa; CDC: Atlanta, GA, USA, 2011; p. 28. [Google Scholar]
- World Health Organization. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Weill, M.; Lutfalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Insecticide resistance in mosquito vectors. Nature 2003, 423, 136–137. [Google Scholar] [CrossRef]
- Davies, T.G.; Field, L.M.; Usherwood, P.N.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.C.; Shalaby, A.M. Preliminary note on the development of DDT-resistance in Anopheles culicifacies Giles in Panchmahals District, Gujerat State, India. Bull. World Health Organ. 1962, 26, 128–134. [Google Scholar]
- World Health Organization. Division of Vector, B.; Control. Instructions for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticides; World Health Organization: Geneva, Switzerland, 1981. [Google Scholar]
- Suarez, M.F.; Quiñones, M.L.; Palacios, J.D.; Carrillo, A. First record of DDT resistance in Anopheles darlingi. J. Am. Mosq. Control Assoc. 1990, 6, 72–74. [Google Scholar]
- Carrasco, D.; Lefèvre, T.; Moiroux, N.; Pennetier, C.; Chandre, F.; Cohuet, A. Behavioural adaptations of mosquito vectors to insecticide control. Curr. Opin. Insect Sci. 2019, 34, 48–54. [Google Scholar] [CrossRef]
- Roberts, D.R.; Alecrim, W.D. Behavioral response of Anopheles darlingi to DDT-sprayed house walls in Amazonia. Bull. Pan Am. Health Organ. 1991, 25, 210–217. [Google Scholar] [PubMed]
- Panini, M.; Manicardi, G.C.; Moores, G.D.; Mazzoni, E. An overview of the main pathways of metabolic resistance in insects. Invertebrate Surviv. J. 2016, 13, 326–335. [Google Scholar]
- Vontas, J.; Katsavou, E.; Mavridis, K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: Muddying the waters. Pestic. Biochem. Physiol. 2020, 170, 104666. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.R.; Rockett, K.A.; Lynd, A.; Essandoh, J.; Grisales, N.; Kemei, B.; Njoroge, H.; Hubbart, C.; Rippon, E.J.; Morgan, J.; et al. A high throughput multi-locus insecticide resistance marker panel for tracking resistance emergence and spread in Anopheles gambiae. Sci. Rep. 2019, 9, 13335. [Google Scholar] [CrossRef]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide resistance in malaria vectors: An update at a global scale. In Towards Malaria Elimination; Sylvie, M., Vas, D., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Silva, A.P.; Santos, J.M.; Martins, A.J. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids—A review. Parasit. Vectors 2014, 7, 450. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, K.; Wipf, N.; Müller, P.; Traoré, M.M.; Muller, G.; Vontas, J. Detection and monitoring of insecticide resistance mutations in Anopheles gambiae: Individual vs. pooled specimens. Genes 2018, 9, 479. [Google Scholar] [CrossRef]
- Kisinza, W.; Kabula, B.; Tungu, P.; Sindato, C.; Mweya, C.; Massue, D.; Emidi, B.; Kitau, J.; Chacha, M.; Batengana, B.; et al. Detection and Monitoring of Insecticide Resistance in Malaria Vectors in Tanzania Mainland; Technical Report of the National Institute for Medical Research, Tanzania: Muheza, Tanzania, 2011. [Google Scholar]
- World Health Organization. Malaria Threats Map. Available online: http://www.who.int/teams/global-malaria-programme/surveillance/malaria-threats-map (accessed on 13 June 2023).
- Laporta, G.Z.; Ilacqua, R.C.; Bergo, E.S.; Chaves, L.S.M.; Rodovalho, S.R.; Moresco, G.G.; Figueira, E.A.G.; Massad, E.; de Oliveira, T.M.P.; Bickersmith, S.A.; et al. Malaria transmission in landscapes with varying deforestation levels and timelines in the Amazon: A longitudinal spatiotemporal study. Sci. Rep. 2021, 11, 6477. [Google Scholar] [CrossRef]
- Sallum, M.A.M.; Conn, J.E.; Bergo, E.S.; Laporta, G.Z.; Chaves, L.S.M.; Bickersmith, S.A.; de Oliveira, T.M.P.; Figueira, E.A.G.; Moresco, G.; Olívêr, L.; et al. Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian Region of Brazil. Malar. J. 2019, 18, 117. [Google Scholar] [CrossRef]
- Prussing, C.; Moreno, M.; Saavedra, M.P.; Bickersmith, S.A.; Gamboa, D.; Alava, F.; Schlichting, C.D.; Emerson, K.J.; Vinetz, J.M.; Conn, J.E. Decreasing proportion of Anopheles darlingi biting outdoors between long-lasting insecticidal net distributions in peri-Iquitos, Amazonian Peru. Malar. J. 2018, 17, 86. [Google Scholar] [CrossRef]
- Consoli, R.A.; Lourenco-de-Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil; Editora Fiocruz: Fundação Oswaldo Cruz, Brazil, 1994; p. 228. [Google Scholar]
- Faran, M.E.; Linthicum, K.J. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq. Syst. 1981, 13, 1–81. [Google Scholar]
- Forattini, O.P. Entomologia Medica; Faculdade de Higiene e Sáude Publica: São Paulo, Brazil, 1962; Volume 1, p. 622. [Google Scholar]
- Berman, H.B. Stat Trek: Random Number Generator. Available online: http://stattrek.com/statistics/random-number-generator.aspx (accessed on 21 January 2021).
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Loureiro, A.C.; Araki, A.S.; Bruno, R.V.; Lima, J.B.P.; Ladeia-Andrade, S.; Santacoloma, L.; Martins, A.J. Molecular diversity of genes related to biological rhythms (period and timeless) and insecticide resistance (Na V and ace-1) in Anopheles darlingi. Mem. Inst. Oswaldo Cruz 2023, 118, e220159. [Google Scholar] [CrossRef]
- Lol, J.C.; Castellanos, M.E.; Liebman, K.A.; Lenhart, A.; Pennington, P.M.; Padilla, N.R. Molecular evidence for historical presence of knock-down resistance in Anopheles albimanus, a key malaria vector in Latin America. Parasit. Vectors 2013, 6, 268. [Google Scholar] [CrossRef]
- Mackenzie-Impoinvil, L.; Weedall, G.D.; Lol, J.C.; Pinto, J.; Vizcaino, L.; Dzuris, N.; Riveron, J.; Padilla, N.; Wondji, C.; Lenhart, A. Contrasting patterns of gene expression indicate differing pyrethroid resistance mechanisms across the range of the New World malaria vector Anopheles albimanus. PLoS ONE 2019, 14, e0210586. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.A.; Loureiro, A.C.; Lima, J.B.P.; Martins, A.J. Insecticide resistance in Anopheles albitarsis s.s. from a rice production field, with the first record of Kdr mutation. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Alimi, T.O.; Fuller, D.O.; Quinones, M.L.; Xue, R.D.; Herrera, S.V.; Arevalo-Herrera, M.; Ulrich, J.N.; Qualls, W.A.; Beier, J.C. Prospects and recommendations for risk mapping to improve strategies for effective malaria vector control interventions in Latin America. Malar. J. 2015, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Floch, H. [Antimalarial campaign in French Guiana. III. DDT campaigns and their results]. Riv. Malariol. 1955, 34, 77–92. [Google Scholar] [PubMed]
- Angella, A.F.; Gil, L.H.; Silva, L.H.; Ribolla, P.E. Population structure of the malaria vector Anopheles darlingi in Rondonia, Brazilian Amazon, based on mitochondrial DNA. Mem. Inst. Oswaldo Cruz 2007, 102, 953–958. [Google Scholar] [CrossRef]
- Ranson, H. Current and future prospects for preventing malaria transmission via the use of insecticides. Cold Spring Harb. Perspect. Med. 2017, 7, a026823. [Google Scholar] [CrossRef]
- Souris, M.; Marcombe, S.; Laforet, J.; Brey, P.T.; Corbel, V.; Overgaard, H.J. Modeling spatial variation in risk of presence and insecticide resistance for malaria vectors in Laos. PLoS ONE 2017, 12, e0177274. [Google Scholar] [CrossRef]
- Metelo-Matubi, E.; Zanga, J.; Binene, G.; Mvuama, N.; Ngamukie, S.; Nkey, J.; Schopp, P.; Bamba, M.; Irish, S.; Nguya-Kalemba-Maniania, J.; et al. The effect of a mass distribution of insecticide-treated nets on insecticide resistance and entomological inoculation rates of Anopheles gambiae s.l. in Bandundu City, Democratic Republic of Congo. Pan Afr. Med. J. 2021, 40, 118. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.; Nelli, L.; Guelbéogo, W.M.; Cissé, F.; Tapsoba, M.; Ouédraogo, P.; Sagnon, N.; Ranson, H.; Matthiopoulos, J.; Ferguson, H.M. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci. Rep. 2021, 11, 17569. [Google Scholar] [CrossRef]
- Sangbakembi-Ngounou, C.; Costantini, C.; Longo-Pendy, N.M.; Ngoagouni, C.; Akone-Ella, O.; Rahola, N.; Cornelie, S.; Kengne, P.; Nakouné, E.R.; Komas, N.P.; et al. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be “out of control”. Proc. Natl. Acad. Sci. USA 2022, 119, e2104282119. [Google Scholar] [CrossRef]
- Vezenegho, S.B.; Adde, A.; Pommier de Santi, V.; Issaly, J.; Carinci, R.; Gaborit, P.; Dusfour, I.; Girod, R.; Briolant, S. High malaria transmission in a forested malaria focus in French Guiana: How can exophagic Anopheles darlingi thwart vector control and prevention measures? Mem. Inst. Oswaldo Cruz 2016, 111, 561–569. [Google Scholar] [CrossRef]
- Iyer, M.; Skelton, J.; de Wildt, G.; Meza, G. A qualitative study on the use of long-lasting insecticidal nets (LLINs) for the prevention of malaria in the Peruvian Amazon. Malar. J. 2019, 18, 301. [Google Scholar] [CrossRef]
- Chaves, L.S.M.; Bergo, E.S.; Conn, J.E.; Laporta, G.Z.; Prist, P.R.; Sallum, M.A.M. Anthropogenic landscape decreases mosquito biodiversity and drives malaria vector proliferation in the Amazon rainforest. PLoS ONE 2021, 16, e0245087. [Google Scholar] [CrossRef]
- Escobar, D.; Ascencio, K.; Ortiz, A.; Palma, A.; Fontecha, G. Distribution and phylogenetic diversity of Anopheles species in malaria endemic areas of Honduras in an elimination setting. Parasit. Vectors 2020, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, E.; Dusfour, I.; Lacour, G.; Saldanha, R.; Guidez, A.; Gomes, M.S.; Sanna, A.; Epelboin, Y.; Restrepo, J.; Davy, D.; et al. Resurgence risk for malaria, and the characterization of a recent outbreak in an Amazonian border area between French Guiana and Brazil. BMC Infect. Dis. 2020, 20, 373. [Google Scholar] [CrossRef]
- Dusfour, I.; Carinci, R.; Issaly, J.; Gaborit, P.; Girod, R. A survey of adult anophelines in French Guiana: Enhanced descriptions of species distribution and biting responses. J. Vector Ecol. 2013, 38, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pommier de Santi, V.; Dusfour, I.; de Parseval, E.; Lespinet, B.; Nguyen, C.; Gaborit, P.; Carinci, R.; Hyvert, G.; Girod, R.; Briolant, S. Risk of daytime transmission of malaria in the French Guiana rain forest. Med. Sante Trop. 2017, 27, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Hiwat, H.; Issaly, J.; Gaborit, P.; Somai, A.; Samjhawan, A.; Sardjoe, P.; Soekhoe, T.; Girod, R. Behavioral heterogeneity of Anopheles darlingi (Diptera: Culicidae) and malaria transmission dynamics along the Maroni River, Suriname, French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Liebman, K.A.; Pinto, J.; Valle, J.; Palomino, M.; Vizcaino, L.; Brogdon, W.; Lenhart, A. Novel mutations on the ace-1 gene of the malaria vector Anopheles albimanus provide evidence for balancing selection in an area of high insecticide resistance in Peru. Malar. J. 2015, 14, 74. [Google Scholar] [CrossRef]
- Reid, M.C.; McKenzie, F.E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar. J. 2016, 15, 107. [Google Scholar] [CrossRef]
- Chouaïbou, M.S.; Fodjo, B.K.; Fokou, G.; Allassane, O.F.; Koudou, B.G.; David, J.P.; Antonio-Nkondjio, C.; Ranson, H.; Bonfoh, B. Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Côte d’Ivoire. Malar. J. 2016, 15, 426. [Google Scholar] [CrossRef]
- Miller, M.W.; Tren, R. Implications of public-health insecticide resistance and replacement costs for malaria control: Challenges and policy options for endemic countries and donors. Res. Rep. Trop. Med. 2012, 3, 1–19. [Google Scholar] [CrossRef]
- Campos, K.B.; Martins, A.J.; Rodovalho, C.M.; Bellinato, D.F.; Dias, L.D.S.; Macoris, M.; Andrighetti, M.T.M.; Lima, J.B.P.; Obara, M.T. Assessment of the susceptibility status of Aedes aegypti (Diptera: Culicidae) populations to pyriproxyfen and malathion in a nation-wide monitoring of insecticide resistance performed in Brazil from 2017 to 2018. Parasit. Vectors 2020, 13, 531. [Google Scholar] [CrossRef]
- Lopes, R.P.; Lima, J.B.P.; Martins, A.J. Insecticide resistance in Culex quinquefasciatus Say, 1823 in Brazil: A review. Parasit. Vectors 2019, 12, 591. [Google Scholar] [CrossRef]
- Santos, R.L.; Fayal Ada, S.; Aguiar, A.E.; Vieira, D.B.; Póvoa, M.M. [Evaluation of the residual effect of pyrethroids on Anopheles in the Brazilian Amazon]. Rev. Saude Publica 2007, 41, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.; Galardo, A.K.R.; Lima, L.A.; Câmara, D.C.P.; Müller, J.N.; Barroso, J.F.S.; Lapouble, O.M.M.; Rodovalho, C.M.; Ribeiro, K.A.N.; Lima, J.B.P. Efficacy of insecticides used in indoor residual spraying for malaria control: An experimental trial on various surfaces in a “test house”. Malar. J. 2019, 18, 345. [Google Scholar] [CrossRef]
- Kamareddine, L. The biological control of the malaria vector. Toxins 2012, 4, 748–767. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).