Evaluation of Antioxidant Defence Systems and Inflammatory Status in Basketball Elite Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Experimental Approach
2.3. Collection Data
2.4. Dosage of CK, LDH, AST, ALT and GGT
2.5. Urine Evaluation
2.6. GSH and GSSG Assay
2.7. RNA Extraction and cDNA Synthesis
2.8. Gene Expression using Real-Time PCR
2.9. Evaluation of Vitamin A and E
2.10. Statistical Analysis
3. Results
3.1. Dosage of CK, LDH, AST, ALT and GGT
3.2. Urine Analysis
3.3. Evaluation of GSH and GSSG Levels
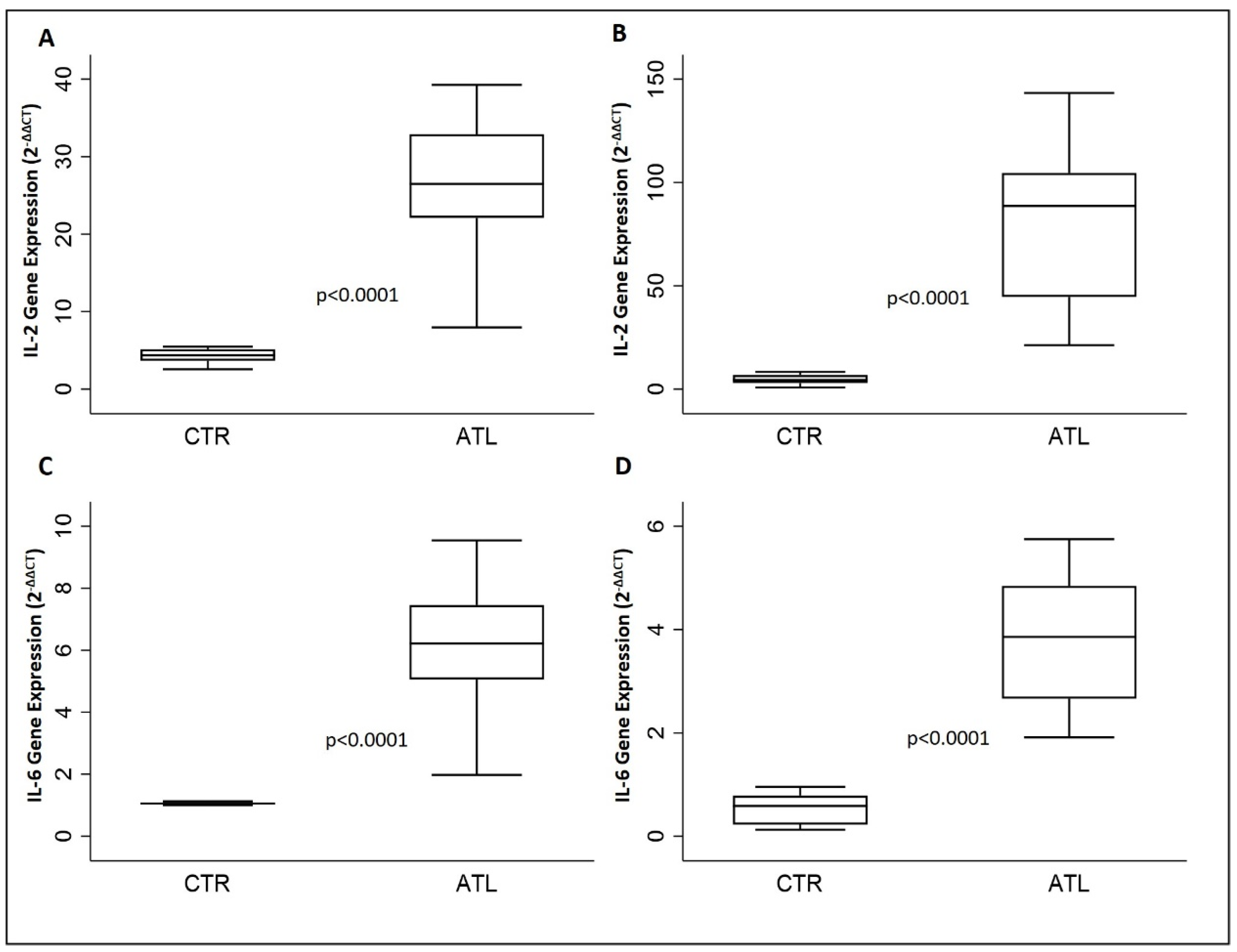
3.4. The Influence of Physical Activity on Gene Expression of IL-2, IL-6, IL-8 and IL-10
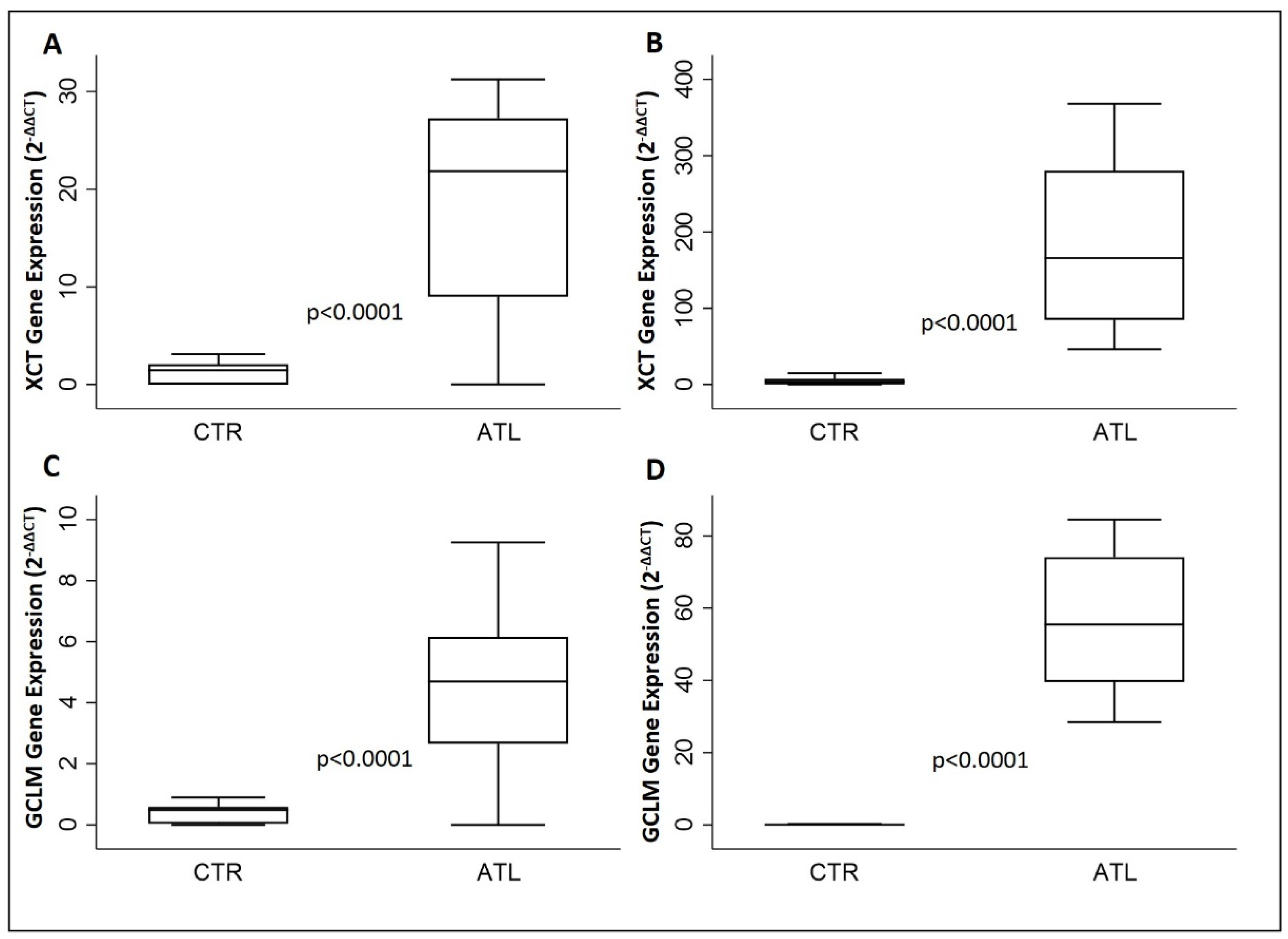
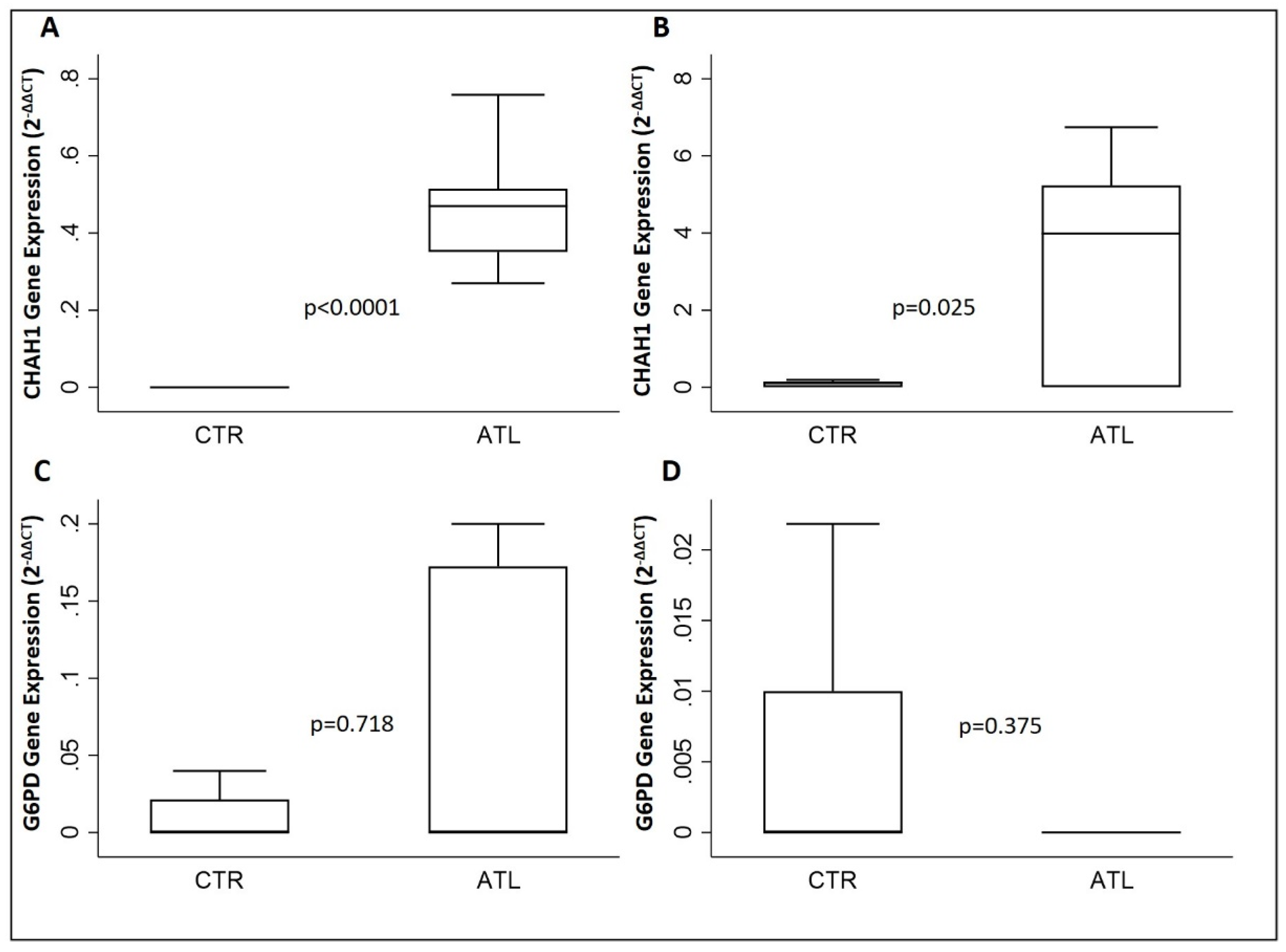
3.5. Effects of Physical Activity on xCT, GCLM, CHAC1 and G6PD
3.6. The Impact of Physical Exercise on Vitamins A and E
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’Alicandro, G.; et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Caiazza, M.; Gragnano, F.; Ranieri, A.; D’Alicandro, G.; Tinto, N.; et al. Dietary Thiols: A Potential Supporting Strategy against Oxidative Stress in Heart Failure and Muscular Damage during Sports Activity. Int. J. Environ. Res. Public Health 2020, 17, 9424. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Mennitti, C.; Gentile, A.; Correale, L.; Buzzachera, C.F.; Ferraris, C.; Montomoli, C.; Frisso, G.; Borrelli, P.; Scudiero, O. Effects of the COVID-19 Pandemic on Job Activity, Dietary Behaviours and Physical Activity Habits of University Population of Naples, Federico II-Italy. Int. J. Environ. Res. Public Health 2021, 18, 1502. [Google Scholar] [CrossRef]
- Scudiero, O.; Lombardo, B.; Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Gentile, L.; Moscarella, E.; Amodio, F.; Ranieri, A.; et al. Exercise, Immune System, Nutrition, Respiratory and Cardiovascular Diseases during COVID-19: A Complex Combination. Int. J. Environ. Res. Public Health 2021, 18, 904. [Google Scholar] [CrossRef]
- Wyszyńska, J.; Ring-Dimitriou, S.; Thivel, D.; Weghuber, D.; Hadjipanayis, A.; Grossman, Z.; Ross-Russell, R.; Dereń, K.; Mazur, A. Physical Activity in the Prevention of Childhood Obesity: The Position of the European Childhood Obesity Group and the European Academy of Pediatrics. Front. Pediatr. 2020, 8, 535705. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Laneri, S.; Brancaccio, M.; Mennitti, C.; De Biasi, M.G.; Pero, M.E.; Pisanelli, G.; Scudiero, O.; Pero, R. Antimicrobial Peptides and Physical Activity: A Great Hope against COVID-19. Microorganisms 2021, 9, 1415. [Google Scholar] [CrossRef]
- Malm, C.; Jakobsson, J.; Isaksson, A. Physical Activity and Sports-Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports 2019, 7, 127. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Monda, E.; D’Argenio, V.; Casaburi, G.; Mazzaccara, C.; Ranieri, A.; Fimiani, F.; Barretta, F.; et al. Multidisciplinary In-Depth Investigation in a Young Athlete Suffering from Syncope Caused by Myocardial Bridge. Diagnostics 2021, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Mennitti, C.; Laneri, S.; Franco, A.; De Biasi, M.G.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Gragnano, F.; Mazzaccara, C.; et al. Methicillin-Resistant Staphylococcus aureus: Risk for General Infection and Endocarditis among Athletes. Antibiotics 2020, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Brancaccio, M.; Mennitti, C.; Gentile, L.; Arpino, S.; De Falco, R.; Leggiero, E.; Ranieri, A.; Pagliuca, C.; Colicchio, R.; et al. Urinary Biomarkers: Diagnostic Tools for Monitoring Athletes’ Health Status. Int. J. Environ. Res. Public Health 2020, 17, 6065. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, C.; Brancaccio, M.; Gentile, L.; Ranieri, A.; Terracciano, D.; Cennamo, M.; La Civita, E.; Liotti, A.; D’Alicandro, G.; Mazzaccara, C.; et al. Athlete’s Passport: Prevention of Infections, Inflammations, Injuries and Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2540. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Brancaccio, M.; Mennitti, C.; Gentile, L.; Franco, A.; Laneri, S.; De Biasi, M.G.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. HNP-1 and HBD-1 as Biomarkers for the Immune Systems of Elite Basketball Athletes. Antibiotics 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar]
- Shin, K.A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.J. Comparison of Changes in Biochemical Markers for Skeletal Muscles, Hepatic Metabolism, and Renal Function after Three Types of Long-distance Running: Observational Study. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Noakes, T.D. Effect of Exercise on Serum Enzyme Activities in Humans. Sports Med. 1987, 4, 245–267. [Google Scholar] [CrossRef]
- Nowakowska, A.; Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Blood Biomarkers of Recovery Efficiency in Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 3279. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81, 209–230. [Google Scholar] [CrossRef]
- Brancaccio, P.; Limongelli, F.M.; Maffulli, N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 2006, 40, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses after Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet 2017, 58, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 2008, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Pavletic, A.J.; Pao, M. Exercise-induced elevation of liver enzymes in a healthy female research volunteer. Psychosomatics 2015, 56, 604–606. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Montagnana, M.; Salvagno, G.L.; Banfi, G.; Guidi, G.C. Significant variation of traditional markers of liver injury after a half-marathon run. Eur. J. Intern. Med. 2011, 22, e36–e38. [Google Scholar] [CrossRef]
- Lombardo, B.; Izzo, V.; Terracciano, D.; Ranieri, A.; Mazzaccara, C.; Fimiani, F.; Cesaro, A.; Gentile, L.; Leggiero, E.; Pero, R.; et al. Laboratory medicine: Health evaluation in elite athletes. Clin. Chem. Lab. Med. 2019, 57, 1450–1473. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, H.B.; Li, P.S.; Yuan, W.-X.; Liu, B.; Liu, S.-T.; Qin, K.-R. ROS and NO Dynamics in Endothelial Cells Exposed to Exercise-Induced Wall Shear Stress. Cell Mol. Bioeng. 2018, 12, 107–120. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Spe-cies: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Hadžović-Džuvo, A.; Valjevac, A.; Lepara, O.; Pjanić, S.; Hadžimuratović, A.; Mekić, A. Oxidative stress status in elite athletes engaged in different sport disciplines. Bosn. J. Basic Med. Sci. 2014, 14, 56–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox. Biol. 2020, 32, 101500. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Sen, C.K. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol. Cell Biochem. 1999, 196, 31–42. [Google Scholar] [CrossRef]
- Sastre, J.; Asensi, M.; Gascó, E.; Pallardó, F.V.; Ferrero, J.A.; Furukawa, T.; Viña, J. Exhaustive physical exercise causes oxidation of glutathione status in blood: Prevention by antioxidant administration. Am. J. Physiol. 1992, 263 Pt 2, R992–R995. [Google Scholar] [CrossRef]
- Karolkiewicz, J.; Szczêsniak, L.; Deskur-Smielecka, E.; Nowak, A.; Stemplewski, R.; Szeklicki, R. Oxidative stress and antioxidant defense system in healthy, elderly men: Relationship to physical activity. Aging Male 2003, 6, 100–105. [Google Scholar] [CrossRef]
- Qi, J.H.; Dong, F.X. The relevant targets of anti-oxidative stress: A review. J. Drug Target 2021, 29, 677–686. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Lim, J.; Nakamura, B.N.; Mohar, I.; Kavanagh, T.J.; Luderer, U. Glutamate Cysteine Ligase Modifier Subunit (Gclm) Null Mice Have Increased Ovarian Oxidative Stress and Accelerated Age-Related Ovarian Failure. Endocrinology 2015, 156, 3329–3343. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Sakellariou, G.K.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. Comparison between glucose-6-phosphate dehydrogenase-deficient and normal individuals after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschalis, V.; Kostaropoulos, I.A.; Kladi-Skandali, A.; Balamitsi, V.; Koutedakis, Y.; Kouretas, D. Exercise-induced oxidative stress in G6PD-deficient individuals. Med. Sci. Sports Exerc. 2006, 38, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.R.; Prescott, E.T.; Sylvester, C.F.; Higdon, A.N.; Shan, J.; Kilberg, M.S.; Mungrue, I.N. Human CHAC1 Protein Degrades Glutathione, and mRNA Induction Is Regulated by the Transcription Factors ATF4 and ATF3 and a Bipartite ATF/CRE Regulatory Element. J. Biol. Chem. 2015, 290, 15878–15891. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Verma, H.K.; Pande, B.; Costanzo, V.; Ye, W.; Cai, Y.; Bhaskar, L.V.K.S. Physical Activity and Nutritional Influence on Immune Function: An Important Strategy to Improve Immunity and Health Status. Front. Physiol. 2021, 12, 751374. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Pedersen, B.K. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Exercise and interleukin-6. Curr. Opin. Hematol. 2001, 8, 137–141. [Google Scholar] [CrossRef]
- Bendickova, K.; Fric, J. Roles of IL-2 in bridging adaptive and innate immunity, and as a tool for cellular immunotherapy. J. Leukoc. Biol. 2020, 108, 427–437. [Google Scholar] [CrossRef]
- Kaya, O. Effect of a four-week exercise program on the secretion of IFN-γ, TNF-α, IL-2 and IL-6 cytokines in elite Taekwondo athletes. Biomed. Rep. 2016, 5, 367–370. [Google Scholar] [CrossRef][Green Version]
- Cabral-Santos, C.; de Lima Junior, E.A.; Fernandes, I.M.C.; Pinto, R.Z.; Rosa-Neto, J.C.; Bishop, N.C.; Lira, F.S. Interleukin-10 responses from acuteexercise in healthy subjects: A systematic review. J. Cell Physiol. 2019, 234, 9956–9965. [Google Scholar] [CrossRef]
- Russeau, A.-S.; Hininger, I.; Palazzetti, S.; Faure, H.; Roussel, A.-M.; Margaritis, I. Antioxidant vitamin status in high exposure to oxidative stress in competitive athletes. Br. J. Nutr. 2004, 92, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, N.; Mohamad Shalan, N.A.A. The potential effects of vitamin E in sport performance. Int. J. Curr. Res. Biosci. Plantbiol. 2018, 5, 17–27. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.M.; Dantas, E.H.M.; De Salles, B.F.; Miranda, H.L.; Koch, A.J.; Willardson, J.M.; Simão, R. Creatine kinase and lactate dehydrogenase responses after upper-body resistance exercise with different rest intervals. J. Strength Cond. Res. 2010, 24, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Giakas, G.; Baltzopoulos, V.; Jamurtas, A.Z.; Theoharis, V.; Kotzamanidis, C.; Koutedakis, Y. The effects of muscle damage following eccentric exercise on gait biomechanics. Gait. Posture 2007, 25, 236242. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Tricoli, V. Mechanisms involved in delayed onset muscle soreness etiology. Rev. Bras. Ciên. Mov. 2001, 9, 39–44. [Google Scholar]
- Lieber, R.L.; Shah, S.; Fridén, J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin. Orthop. Relat. Res. 2002, 403, S90–S99. [Google Scholar] [CrossRef]
- Mougios, V. Reference intervals for serum creatine kinase in athletes. Br. J. Sports Med. 2007, 41, 674–678. [Google Scholar] [CrossRef]
- Rosales, X.Q.; Chu, M.L.; Shilling, C.; Wall, C.; Pastores, G.M.; Mendell, J.R. Fidelity of γ-glutamyl transferase (GGT) in differentiating skeletal muscle from liver damage. J. Child Neurol. 2008, 23, 748–751. [Google Scholar] [CrossRef]
- Lim, A.K. Abnormal liver function tests associated with severe rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Dearlove, D.J.; Harrison, O.K.; Hodson, L.; Jefferson, A.; Clarke, K.; Cox, P.J. The Effect of Blood Ketone Concentration and Exercise Intensity on Exogenous Ketone Oxidation Rates in Athletes. Med. Sci. Sports Exerc. 2021, 53, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.Z.; Cubrilo, D.G.; Barudzic, N.S.; Vuletic, M.S.; Zivkovic, V.I.; Nesic, M.; Radovanovic, D.; Djuric, D.M.; Jakovljevic, V.L.j. Comparison of blood pro/antioxidant levels before and after acute exercise in athletes and non-athletes. Gen. Physiol. Biophys. 2012, 31, 211–219. [Google Scholar] [CrossRef]
- Stark, A.A.; Porat, N.; Volohonsky, G.; Komlosh, A.; Bluvshtein, E.; Tubi, C.; Steinberg, P. The role of γ-glutamyl transpeptidase in the biosynthesis of glutathione. Biofactors 2003, 17, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, N.; Samiei, A.; Afshar, R.; Saremi, A.; Sheikhhoseini, R. The effect of exercise on urinary γ-glutamyltransferase and protein levels in elite female karate athletes. Asian J. Sports Med. 2012, 3, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E. Free Radic Biol. Med. 2021, 176, 1–15. [Google Scholar] [CrossRef]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X.; McKenna, M.J. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Phisiol. 2004, 97, 1477–1485. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, S.N.; Monga, Y.P.; Ray, U.S. Improvement of Glutathione and Total Antioxidant Status with Yoga. J. Altern. Complement. Med. 2007, 13, 1085–1090. [Google Scholar] [CrossRef]


| Gene | Accession Numbers | Primer Forward | Primer Reverse |
|---|---|---|---|
| RLP0 | NM_053275.4 | 5′-TGGCAGCATCTACAACCCTG-3′ | 5′-GACAAGGCCAGGACTCGTTT-3′ |
| IL-2 | NM_000586.4 | 5′-AACCTCAACTCCTGCCACAA-3′ | 5′-GCATCCTGGTGAGTTTGGGA-3′ |
| IL-6 | NM_001318095.2 | 5′-CATCCTCGACGG-CATCTCAG-3′ | 5′-TCACCAGGCAAGTCTCCTCA-3 |
| IL-8 | NM_001354840.3 | 5′-AAACCCAGGTGAGAGCTG-3′ | 5′-TCTGAGATCCCGTCAGAGC-3′ |
| IL-10 | NM_001382624.1 | 5′-TCCATTCCAAGCCTGACCAC-3′ | 5′-AATCCCTCCGAGACACTGGA-3′ |
| xCT | NM_014331.4 | 5′-TGAAATCCCTGAACTTGCGAT-3′ | 5′-TCTGGATCCGGGCGCT-3′ |
| GCLM | NM_002061.4 | 5′-GACAAAACACAGTTGGAACAGC-3′ | 5′-CAGTCAAATCTGGTGGCATC-3′ |
| CHAC1 | NM_024111.6 | 5′-TTCTGGCAGGGAGACACCTT-3′ | 5′-GCCTCTCGCACATTCAGGTA–3′ |
| G6PD | NM_001360016.2 | 5′-ACATGAATGCCCTCCACCTG-3′ | 5′-GGTAGTGGTCGATGCGGTAG-3′ |
| Parameters | Ctr (0 Months) | Athletes (0 Months) | p-Value (Ctr vs. 0) | Ctr (2 Months) | Athletes (2 Months) | p-Value § (Ctr vs. 2) | p-Value * (0 vs. 2 Months for Athletes) |
|---|---|---|---|---|---|---|---|
| CK (30–200 U/L) * | 138.0 (112.0–158.0) | 254.0 (141.0–375.0) | 0.003 | 129.0 (112.0–148.0) | 869.0 (551.0–1045.0) | <0.001 | <0.001 |
| LDH (125–243 U/L) * | 159.0 (148.0–187.0) | 208.0 (186.0–257.0) | <0.001 | 175.0 (148.0–210.0) | 267.0 (226.0–302.0) | <0.001 | 0.053 |
| AST (0–34 U/L) * | 21.0 (17.0–28.0) | 28.0 (21.0–33.0) | 0.048 | 23.0 (19.0–26.0) | 39.0 (34.0–45.0) | <0.001 | <0.001 |
| ALT (0–55 U/L) * | 34.0 (17.0–42.0) | 22.0 (12.0–31.0) | 0.094 | 32.0 (20.0–43.0) | 27.0 (21.0–34.0) | 0.090 | 0.037 |
| GGT (12–64 U/L) * | 28.0 (19.0–36.0) | 15.0 (13.0–19.0) | 0.001 | 36.0 (26.0–41.0) | 17.0 (15.0–19.0) | <0.001 | 0.568 |
| Parameters | Value | Ctr (0 Months) | Athletes (0 Months) | p-Value (Ctr vs. 0) | Ctr (2 Months) | Athletes (2 Months) | p-Value (Ctr vs. 2) |
|---|---|---|---|---|---|---|---|
| Glucose | Absent | Absent | Absent | Absent | Absent | ||
| Ketones | Negative | Negative | Negative | Negative | Negative | ||
| Bilirubin | Absent | Absent | Absent | Absent | Absent | ||
| Hemoglobin | Absent | Absent | Absent | Absent | Absent | ||
| Nitrite | Absent | Absent | Absent | Absent | Absent | ||
| Leukocyte esterase | Absent | Absent | Absent | Absent | Absent | ||
| Urobilinogen | ≤1.0 mg/dL | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 1.000 | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 1.000 |
| Proteins | Absent | Absent | Absent | Absent | Absent | ||
| pH | 5.5–7.0 mg/dL | 5.5 (5.5–6.0) | 6.00 (5.5–6.5) | 0.514 | 5.5 (5.5–6.0) | 6.00 (5.5–6.5) | 0.015 |
| Bacteria | 0–1000 n/µL | 1.0 (1.0–3.0) | 12.0 (6.0–19.0) | <0.001 | 7.0 (2.0–9.0) | 13.0 (4.0–29.0) | 0.006 |
| Leucocytes | 0–18 n/µL | 4.0 (3.0–8.0) | 5.0 (4.0–9.0) | 0.438 | 6.0 (4.0–9.0) | 7.0 (3.0–9.0) | 0.910 |
| Erythrocytes | 0–14 n/µL | 5.0 (3.0–9.0) | 3.0 (1.0–7.0) | 0.052 | 6.0 (4.0–8.0) | 2.0 (0.0–3.0) | <0.001 |
| Parameters | Ctr (0 Months) | Athletes (0 Months) | p-Value (Ctr vs. 0) | Ctr (2 Months) | Athletes (2 Months) | p-Value § (Ctr vs. 2) | p-Value * (0 vs. 2 Months for Athletes) |
|---|---|---|---|---|---|---|---|
| GSH (µM/µL) | 0.3 (0.3–0.4) | 0.8 (0.5–1.6) | <0.001 | 0.3 (0.3–0.4) | 0.8 (0.5–0.9) | <0.001 | 0.330 |
| GSSG (µM/µL) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 0.774 | 0.2 (0.2–0.2) | 0.1 (0.1–0.2) | 0.002 | 0.359 |
| GSH/GSSG (µM/µL) | 2.6 (2.2–2.9) | 6.8 (3.1–13.5) | 0.001 | 1.9 (1.6–2.2) | 6.2 (4.4–10.2) | <0.001 | 0.890 |
| Parameters | Ctr (0 Months) | Athletes (0 Months) | p-Value (Ctr vs. 0) | Ctr (2 Months) | Athletes (2 Months) | p-Value § (Ctr vs. 2) | p-Value * (0 vs. 2 Months for Athletes) |
|---|---|---|---|---|---|---|---|
| GSH (µM/µL) | 5.2 (4.2–6.6) | 3.7 (3.2–4.2) | 0.002 | 5.7 (4.2–9.1) | 3.9 (3.5–4.7) | 0.003 | 0.761 |
| GSSG (µM/µL) | 0.9 (0.8–1.0) | 0.7 (0.5–0.9) | 0.026 | 0.6 (0.5–0.6) | 0.6 (0.5–0.7) | 0.566 | 0.083 |
| GSH/GSSG (µM/µL) | 5.8 (5.3–7.7) | 5.1 (4.0–7.5) | 0.232 | 11.1 (8.2–16.0) | 6.5 (5.9–7.9) | <0.001 | 0.168 |
| Gene | 0 Months | 2 Months | p-Value |
|---|---|---|---|
| IL-2 | 26.5 (22.1–32.9) | 88.6 (44.6–104.6) | <0.0001 |
| IL-6 | 6.2 (5.1–7.5) | 3.9 (2.7–4.8) | <0.0001 |
| IL-8 | 150.3 (136.0–174.9) | 23.6 (21.2–27.9) | <0.0001 |
| IL-10 | 378.2 (329.3–536.5) | 20.1 (19.3–21.9 | <0.0001 |
| Gene | 0 Months | 2 Months | p-Value |
|---|---|---|---|
| XCT | 21.9 (9.0–27.3) | 165.6 (84.5–280.3) | <0.0001 |
| GCLM | 4.7 (2.7–6.2) | 55.5 (39.6–74.2) | <0.0001 |
| CHAC1 | 0.5 (0.4–0.5) | 4.0 (0.0–5.2) | 0.007 |
| G6PD | 0.0 (0.0–0.2) | 0.0 (0.0–0.0) | 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, A.; Punziano, C.; Calvanese, M.; De Falco, R.; Gentile, L.; D’Alicandro, G.; Miele, C.; Capasso, F.; Pero, R.; Mazzaccara, C.; et al. Evaluation of Antioxidant Defence Systems and Inflammatory Status in Basketball Elite Athletes. Genes 2023, 14, 1891. https://doi.org/10.3390/genes14101891
Gentile A, Punziano C, Calvanese M, De Falco R, Gentile L, D’Alicandro G, Miele C, Capasso F, Pero R, Mazzaccara C, et al. Evaluation of Antioxidant Defence Systems and Inflammatory Status in Basketball Elite Athletes. Genes. 2023; 14(10):1891. https://doi.org/10.3390/genes14101891
Chicago/Turabian StyleGentile, Alessandro, Carolina Punziano, Mariella Calvanese, Renato De Falco, Luca Gentile, Giovanni D’Alicandro, Ciro Miele, Filomena Capasso, Raffaela Pero, Cristina Mazzaccara, and et al. 2023. "Evaluation of Antioxidant Defence Systems and Inflammatory Status in Basketball Elite Athletes" Genes 14, no. 10: 1891. https://doi.org/10.3390/genes14101891
APA StyleGentile, A., Punziano, C., Calvanese, M., De Falco, R., Gentile, L., D’Alicandro, G., Miele, C., Capasso, F., Pero, R., Mazzaccara, C., Lombardo, B., Frisso, G., Borrelli, P., Mennitti, C., Scudiero, O., & Faraonio, R. (2023). Evaluation of Antioxidant Defence Systems and Inflammatory Status in Basketball Elite Athletes. Genes, 14(10), 1891. https://doi.org/10.3390/genes14101891












