Reducing Virus Infection Risk in Space Environments through Nutrient Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Differential Methylation Analysis
2.2. Differential Expression Analysis
2.3. Viral Infection-Related Nutrient Enrichment Analysis
2.4. Two Sample Mendelian Randomization Analysis
3. Results
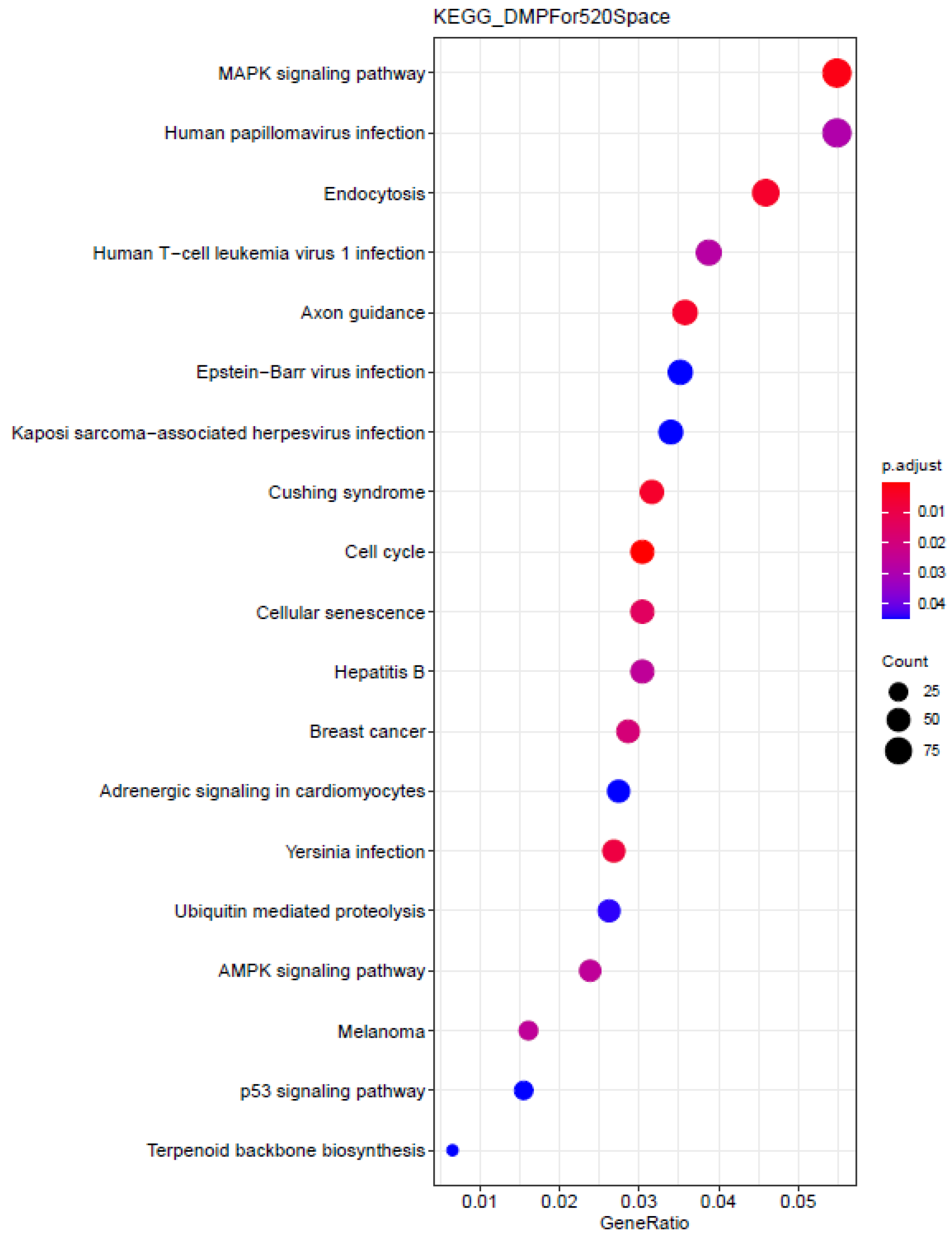
3.1. Differential Methylation Analysis of Mars 520 Samples
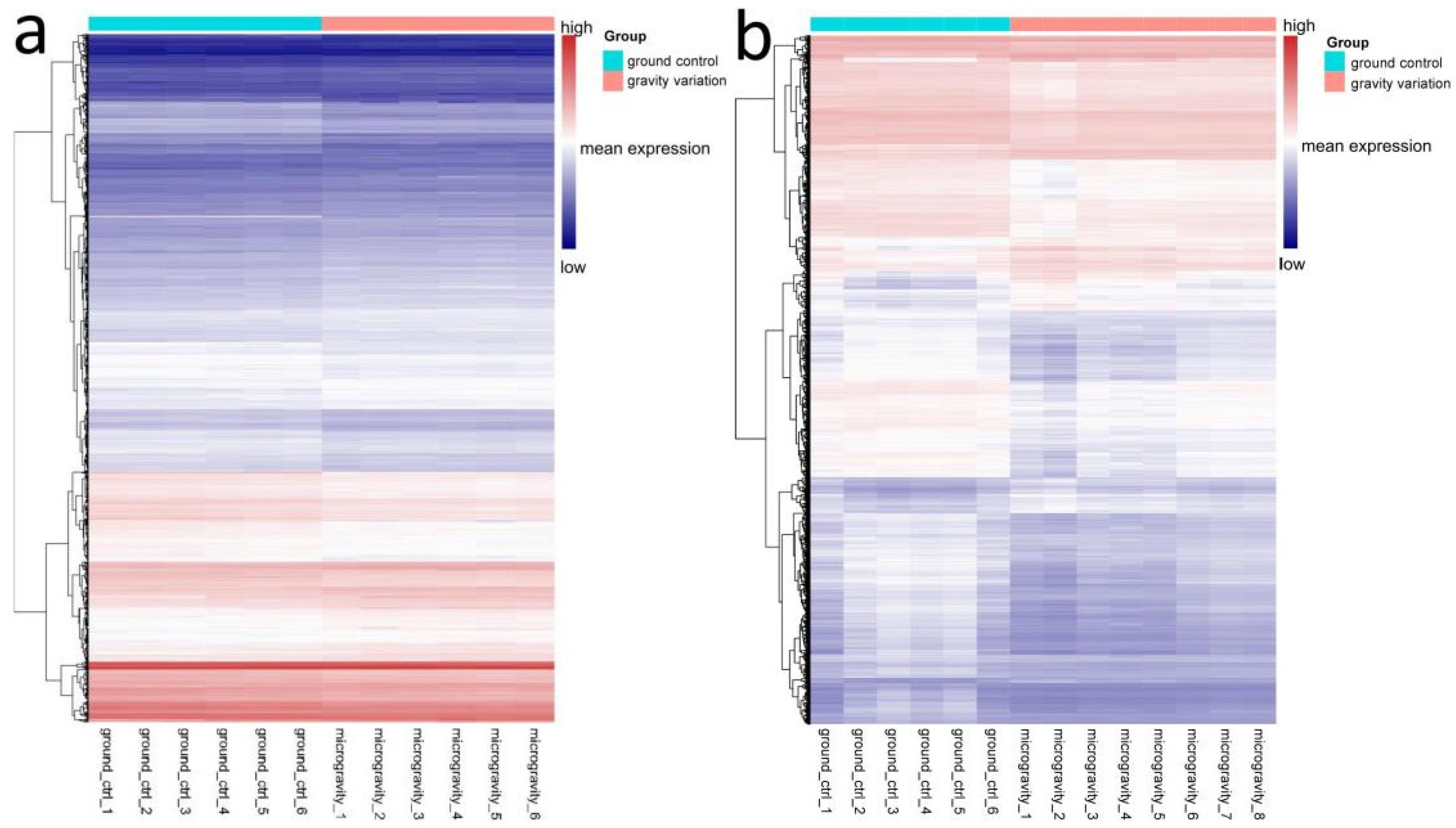
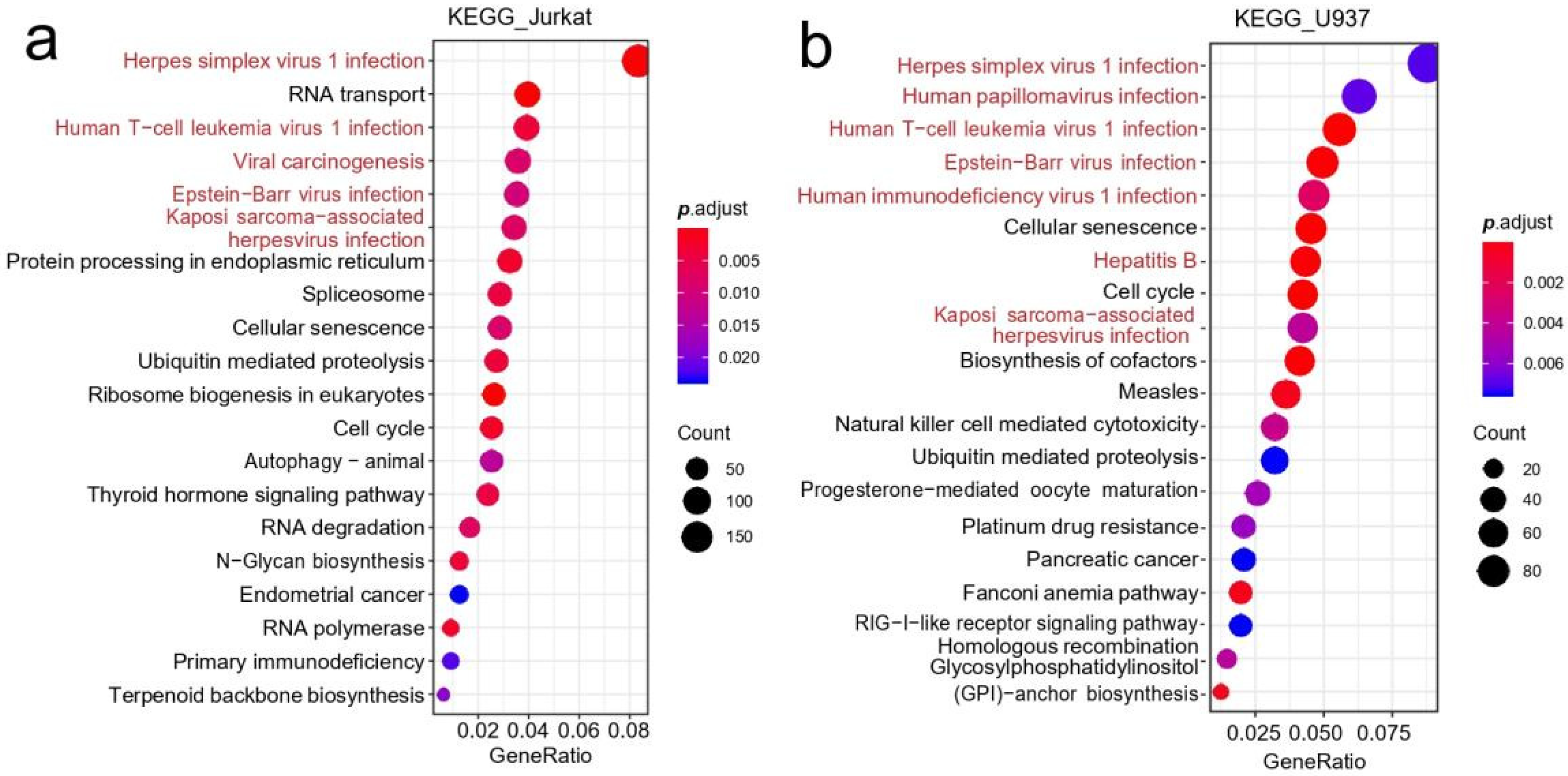
3.2. Differential Gene Expression Analysis of Immune Cells
3.3. Nutrient Enrichment Analysis
3.4. Mendelian Randomization Causal Association Analysis
4. Discussion
4.1. Higher Risk of Viral Infection in the Space Environments
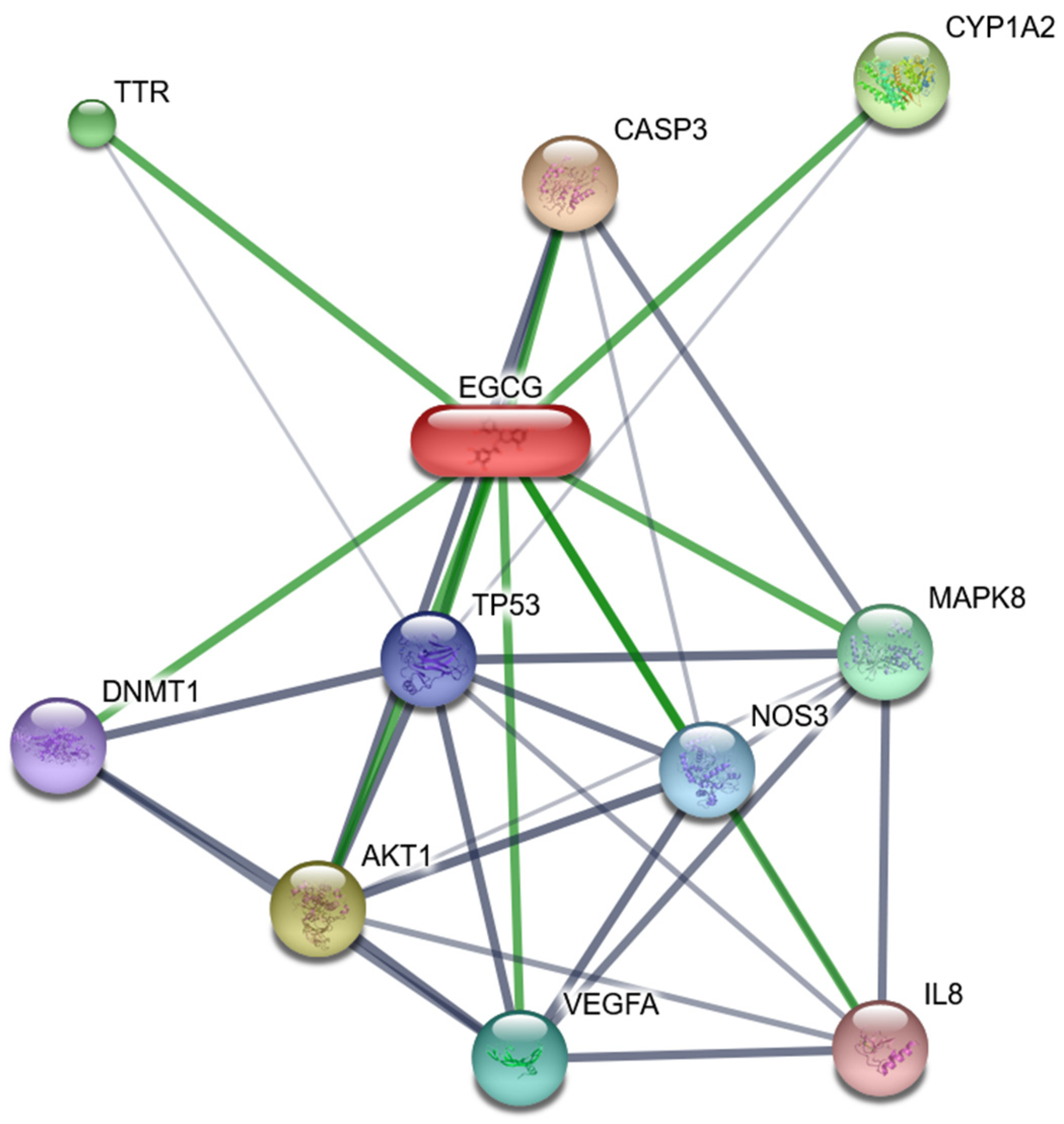
4.2. Positive Role of Vitamin D and EGCG in Reducing Viral Infection Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, F.; Lv, K.; Wang, Y.; Yuan, Y.; Lu, L.; Feng, Q.; Jing, X.; Wang, H.; Liu, C.; Rayner, S.; et al. Personalized Epigenome Remodeling Under Biochemical and Psychological Changes During Long-Term Isolation Environment. Front. Physiol. 2019, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a microgravity environment invited review: Microgravity and skeletal muscle. J. Appl. Physiol. 2000, 89, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D.; Evans, H.J.; Sung, H.G.; Spector, E.R.; Lang, T.F.; Oganov, V.S.; Bakulin, A.V.; Shackelford, L.C.; LeBlanc, A.D. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 2007, 41, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Adler, R.A.; Amin, S.; Binkley, N.; Lewiecki, E.M.; Petak, S.M.; Shapses, S.A.; Sinaki, M.; Watts, N.B.; Sibonga, J.D. Skeletal health in long-duration astronauts: Nature, assessment, and management recommendations from the NASA bone summit. J. Bone Miner. Res. 2013, 28, 1243–1255. [Google Scholar] [CrossRef]
- Parihar, V.K.; Maroso, M.; Syage, A.; Allen, B.D.; Angulo, M.C.; Soltesz, I.; Limoli, C.L. Persistent nature of alterations in cognition and neuronal circuit excitability after exposure to simulated cosmic radiation in mice. Exp. Neurol. 2018, 305, 44–55. [Google Scholar] [CrossRef]
- Luxton, J.J.; McKenna, M.J.; Taylor, L.E.; George, K.A.; Zwart, S.R.; Crucian, B.E.; Drel, V.R.; Garrett-Bakelman, F.E.; Mackay, M.J.; Butler, D.; et al. Temporal Telomere and DNA Damage Responses in the Space Radiation Environment. Cell Rep. 2020, 33, 108435. [Google Scholar] [CrossRef]
- Patel, S. The effects of microgravity and space radiation on cardiovascular health: From low-Earth orbit and beyond. IJC Heart Vasc. 2020, 30, 100595. [Google Scholar] [CrossRef]
- Illa-Bochaca, I.; Ouyang, H.; Tang, J.; Sebastiano, C.; Mao, J.-H.; Costes, S.V.; Demaria, S.; Barcellos-Hoff, M.H. Densely Ionizing Radiation Acts via the Microenvironment to Promote Aggressive Trp53-Null Mammary Carcinomas. Cancer Res. 2014, 74, 7137–7148. [Google Scholar] [CrossRef]
- Hughson, R.L.; Helm, A.; Durante, M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 2018, 15, 167–180. [Google Scholar] [CrossRef]
- Münzel, T.; Kröller-Schön, S.; Oelze, M.; Gori, T.; Schmidt, F.P.; Steven, S.; Hahad, O.; Röösli, M.; Wunderli, J.-M.; Daiber, A.; et al. Adverse Cardiovascular Effects of Traffic Noise with a Focus on Nighttime Noise and the New WHO Noise Guidelines. Annu. Rev. Public Health 2020, 41, 309–328. [Google Scholar] [CrossRef] [Green Version]
- Beheshti, A.; Cekanaviciute, E.; Smith, D.J.; Costes, S.V. Global transcriptomic analysis suggests carbon dioxide as an environmental stressor in spaceflight: A systems biology GeneLab case study. Sci. Rep. 2018, 8, 4191. [Google Scholar] [CrossRef] [PubMed]
- White, R.A.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef]
- Levine, D.S.; Greenleaf, J.E. Immunosuppression during spaceflight deconditioning. Aviat. Space Environ. Med. 1998, 69, 172–177. [Google Scholar]
- Mermel, L.A. Infection Prevention and Control During Prolonged Human Space Travel. Clin. Infect. Dis. 2012, 56, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Sams, C.F.; Pierson, D.L. Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav. Immun. 2014, 41, 210–217. [Google Scholar] [CrossRef]
- Stowe, R.P.; Kozlova, E.V.; Sams, C.F.; Pierson, D.L.; Walling, D.M. Latent and lytic Epstein-Barr virus gene expression in the peripheral blood of astronauts. J. Med. Virol. 2011, 83, 1071–1077. [Google Scholar] [CrossRef]
- Crucian, B.; Johnston, S.; Mehta, S.; Stowe, R.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Laudenslager, M.L.; Sams, C. A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International Space Station. J. Allergy Clin. Immunol. Pract. 2016, 4, 759–762.e8. [Google Scholar]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef]
- Green, M.J.; Aylott, J.W.; Williams, P.; Ghaemmaghami, A.M.; Williams, P.M. Immunity in Space: Prokaryote Adaptations and Immune Response in Microgravity. Life 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Pool, S.L.; Davis, J.R. Space medicine roots: A historical perspective for the current direction. Aviat. Space Environ. Med. 2007, 78 (Suppl. S4), A3–A4. [Google Scholar] [PubMed]
- Baisden, D.L.; Beven, G.E.; Campbell, M.R.; Charles, J.B.; Dervay, J.P.; Foster, E.; Gray, G.W.; Hamilton, D.R.; Holland, D.A.; Jennings, R.T.; et al. Human health and performance for long-duration spaceflight. Aviat. Space Environ. Med. 2008, 79, 629–635. [Google Scholar] [PubMed]
- Law, J.; Mathers, C.H.; Fondy, S.R.; Vanderploeg, J.M.; Kerstman, E.L. NASA’s Human System Risk Management Approach and Its Applicability to Commercial Spaceflight. Aviat. Space Environ. Med. 2013, 84, 68–73. [Google Scholar]
- Basner, M.; Dinges, D.F.; Mollicone, D.J.; Savelev, I.; Ecker, A.J.; Di Antonio, A.; Jones, C.W.; Hyder, E.C.; Kan, K.; Morukov, B.V.; et al. Psychological and Behavioral Changes during Confinement in a 520-Day Simulated Interplanetary Mission to Mars. PLoS ONE 2014, 9, e93298. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef]
- Yet, I.; Tsai, P.C.; Castillo-Fernandez, J.E.; Carnero-Montoro, E.; Bell, J.T. Genetic and environmental impacts on DNA methylation levels in twins. Epigenomics 2016, 8, 105–117. [Google Scholar] [CrossRef]
- Komaki, S.; Ohmomo, H.; Hachiya, T.; Sutoh, Y.; Ono, K.; Furukawa, R.; Umekage, S.; Otsuka-Yamasaki, Y.; Tanno, K.; Sasaki, M.; et al. Longitudinal DNA methylation dynamics as a practical indicator in clinical epigenetics. Clin. Epigenetics 2021, 13, 219. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F.; Mollicone, D.; Ecker, A.; Jones, C.W.; Hyder, E.C.; Di Antonio, A.; Savelev, I.; Kan, K.; Goel, N.; et al. Mars 520-d mission simulation reveals protracted crew hypokinesis and alterations of sleep duration and timing. Proc. Natl. Acad. Sci. USA 2013, 110, 2635–2640. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Ebner, B.F.; Chueng, T.; Martinez, C.A. Epigenetics, HIV, and Cardiovascular Disease Risk. Curr. Probl. Cardiol. 2020, 46, 100615. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Jaeger, P.A.; Kreisberg, J.F.; Licon, K.; Jepsen, K.L.; Khosroheidari, M.; Morsey, B.M.; Swindells, S.; Shen, H.; Ng, C.T.; et al. Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol. Cell 2016, 62, 157–168. [Google Scholar] [PubMed]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitó, M.; Konstantinidou, V. Nutritional Genomics and the Mediterranean Diet’s Effects on Human Cardiovascular Health. Nutrients 2016, 8, 218. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Reglero, G.; Ordovás, J.M.; Dávalos, A. NutriGenomeDB: A nutrigenomics exploratory and analytical platform. Database-Oxford 2019, 2019, baz097. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.-P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q. Identification of Core Genes and Screening of Potential Targets in Glioblastoma Multiforme by Integrated Bioinformatic Analysis. Front Oncol. 2021, 10, 615976. [Google Scholar] [CrossRef]
- Xiong, A.; Clarke-Katzenberg, R.H.; Valenzuela, G.; Izumi, K.M.; Millan, M.T. Epstein-Barr Virus Latent Membrane Protein 1 Activates Nuclear Factor-κB in Human Endothelial Cells and Inhibits Apoptosis. Transplantation 2004, 78, 41–49. [Google Scholar] [CrossRef]
- Limesand, K.H.; Schwertfeger, K.L.; Anderson, S.M. MDM2 Is Required for Suppression of Apoptosis by Activated Akt1 in Salivary Acinar Cells. Mol. Cell. Biol. 2006, 26, 8840–8856. [Google Scholar] [CrossRef]
- Rönnstrand, L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol. Life Sci. 2004, 61, 2535–2548. [Google Scholar] [CrossRef]
- Heron-Milhavet, L.; Khouya, N.; Fernandez, A.; Lamb, N.J. Akt1 and Akt2: Differentiating the aktion. Histol. Histopathol. 2011, 26, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ghias, K.; Ma, C.; Gandhi, V.; Platanias, L.C.; Krett, N.L.; Rosen, S.T. 8-Amino-adenosine induces loss of phosphorylation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, and Akt kinase: Role in induction of apoptosis in multiple myeloma. Mol. Cancer Ther. 2005, 4, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Morishita, R.; Narumiya, S.; Kato, K.; Asano, T. Gαq/11 signaling induces apoptosis through two pathways involving reduction of Akt phosphorylation and activation of RhoA in HeLa cells. Exp. Cell Res. 2004, 298, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Li, H.; Chou, H. FGFR3 promotes the growth and malignancy of melanoma by influencing EMT and the phosphorylation of ERK, AKT, and EGFR. BMC Cancer 2019, 19, 963. [Google Scholar] [CrossRef]
- Woo, M.G.; Xue, K.; Liu, J.; McBride, H.; Tsang, B.K. Calpain-mediated Processing of p53-associated Parkin-like Cytoplasmic Protein (PARC) Affects Chemosensitivity of Human Ovarian Cancer Cells by Promoting p53 Subcellular Trafficking. J. Biol. Chem. 2012, 287, 3963–3975. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and immune function: Understanding common pathways. Curr. Osteoporos. Rep. 2009, 7, 58–63. [Google Scholar]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for common colds: The pivotal role of Vitamin D, Vitamin C, Zinc, and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds-practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid.-Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2010, 55, 96–108. [Google Scholar]
- Herr, C.; Shaykhiev, R.; Bals, R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin. Biol. Ther. 2007, 7, 1449–1461. [Google Scholar]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Seidita, A.; Vitale, G.; Gangemi, S.; Iaria, C.; Cascio, A. Vitamin D Deficiency in HIV Infection: Not Only a Bone Disorder. BioMed Res. Int. 2015, 2015, 735615. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Murdaca, G.; Pioggia, G.; Negrini, S. Vitamin D and Covid-19: An update on evidence and potential therapeutic implications. Clin. Mol. Allergy 2020, 18, 23. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Mathias, A.; Du Pasquier, R.; Koneczny, I.; Disanto, G.; Kuhle, J.; Ramagopalan, S.; Damoiseaux, J.; Smolders, J.; et al. Exploring the effect of Vitamin D3 supplementation on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2018, 24, 1280–1287. [Google Scholar] [CrossRef]
- Hoan, N.X.; Khuyen, N.; Binh, M.T.; Giang, D.P.; Van Tong, H.; Hoan, P.Q.; Trung, N.T.; Anh, D.T.; Toan, N.L.; Meyer, C.G.; et al. Association of Vitamin D deficiency with hepatitis B virus—Related liver diseases. BMC Infect. Dis. 2016, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.S.; Hauschild, S.; Huge, A.; Tauber, S.; Lauber, B.A.; Polzer, J.; Paulsen, K.; Lier, H.; Engelmann, F.; Schmitz, B.; et al. Dynamic gene expression response to altered gravity in human T cells. Sci. Rep. 2017, 7, 5204. [Google Scholar] [CrossRef] [Green Version]
- Thiel, C.S.; Tauber, S.; Christoffel, S.; Huge, A.; Lauber, B.A.; Polzer, J.; Paulsen, K.; Lier, H.; Engelmann, F.; Schmitz, B.; et al. Rapid coupling between gravitational forces and the transcriptome in human myelomonocytic U937 cells. Sci. Rep. 2018, 8, 13267. [Google Scholar] [CrossRef]
- Crucian, B.; Sams, C. Immune system dysregulation during spaceflight: Clinical risk for exploration-class missions. J. Leukoc. Biol. 2009, 86, 1017–1018. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.; Mehta, S.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Sams, C. Immune System Dysregulation Occurs during Short Duration Spaceflight on Board the Space Shuttle. J. Clin. Immunol. 2012, 33, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Zwart, S.R.; Mehta, S.; Uchakin, P.; Quiriarte, H.D.; Pierson, D.; Sams, C.F.; Smith, S.M. Plasma Cytokine Concentrations Indicate That In Vivo Hormonal Regulation of Immunity Is Altered During Long-Duration Spaceflight. J. Interf. Cytokine Res. 2014, 34, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Takeda, T.; Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J. Med. 2005, 54, 55–59. [Google Scholar] [CrossRef]
- Pradhan, P.; Nguyen, M.L. Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate. Virus Res. 2018, 249, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Yu, Z.-Y.; Chen, Y.-C.; Hung, S.-L. Effects of epigallocatechin-3-gallate and acyclovir on herpes simplex virus type 1 infection in oral epithelial cells. J. Formos. Med. Assoc. 2020, 120, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Nance, C.L.; Siwak, E.B.; Shearer, W.T. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J. Allergy Clin. Immunol. 2009, 123, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Obregon, D.; Hou, H.; Zeng, J.; Sun, N.; Nikolic, V.; Ehrhart, J.; Shytle, D.; Fernandez, F.; Tan, J. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-γ: Role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006, 1123, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef]





| Virus | Epigallocatechin-3-Gallate | Vitamin D | Ethyl Alcohol | Lycopene |
|---|---|---|---|---|
| HSV Infection | 1.79 × 10−10 | 1.55 × 10−4 | 7.11 × 10−5 | |
| EBV Infection | 5.40 × 10−17 | 3.95 × 10−3 | 1.91 × 10−15 | |
| KSHV Infection | 2.94 × 10−17 | 1.22 × 10−4 | 9.49 × 10−9 | 1.52 × 10−3 |
| HPV Infection | 1.07 × 10−2 | 5.19 × 10−4 | ||
| HIV Infection | 1.74 × 10−14 | 1.05 × 10−7 | 1.25 × 10−5 | 1.43 × 10−2 |
| HBV Infection | 7.86 × 10−17 | 6.18 × 10−5 | 3.76 × 10−15 | 9.26 × 10−3 |
| Nutrients | Trait | HPV E7 Type 18 (p-Value) | EBNA IgG Levels (p-Value) | HBs IgG Levels (p-Value) |
|---|---|---|---|---|
| Vitamin D | Vitamin D levels | 0.3050 | 0.0283 | 0.0069 |
| Calcium | Calcium levels | 0.0039 | 0.3088 | 0.8635 |
| Selenium | Blood trace element (Se levels) | 0.4299 | 0.0715 | 0.2474 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xue, Y.-W.; Quan, Y.; Zhang, H.-Y. Reducing Virus Infection Risk in Space Environments through Nutrient Supplementation. Genes 2022, 13, 1536. https://doi.org/10.3390/genes13091536
Li H, Xue Y-W, Quan Y, Zhang H-Y. Reducing Virus Infection Risk in Space Environments through Nutrient Supplementation. Genes. 2022; 13(9):1536. https://doi.org/10.3390/genes13091536
Chicago/Turabian StyleLi, Hui, Ya-Wen Xue, Yuan Quan, and Hong-Yu Zhang. 2022. "Reducing Virus Infection Risk in Space Environments through Nutrient Supplementation" Genes 13, no. 9: 1536. https://doi.org/10.3390/genes13091536
APA StyleLi, H., Xue, Y.-W., Quan, Y., & Zhang, H.-Y. (2022). Reducing Virus Infection Risk in Space Environments through Nutrient Supplementation. Genes, 13(9), 1536. https://doi.org/10.3390/genes13091536







