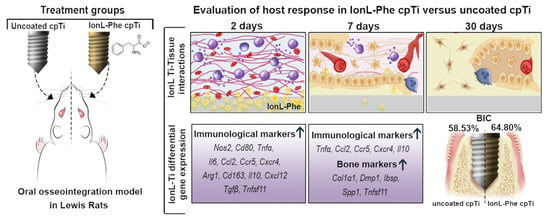
Effects of Dicationic Imidazolium-Based Ionic Liquid Coatings on Oral Osseointegration of Titanium Implants: A Biocompatibility Study in Multiple Rat Demographics
Abstract
:1. Introduction
2. Materials and Methods
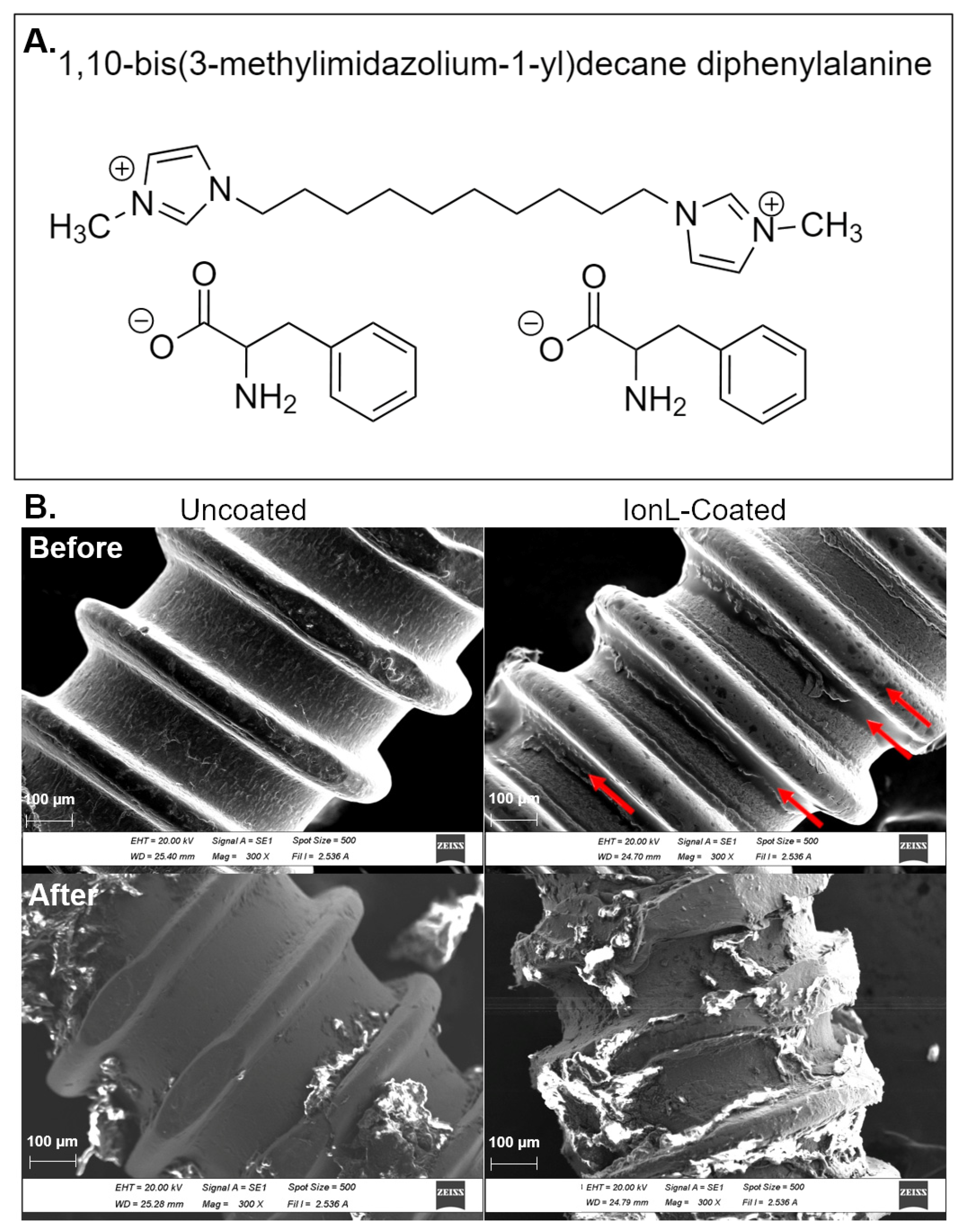
2.1. Selection of Ionic Liquid Formulation
2.2. Preparation of Implant and IonL-Phe Coating
2.3. Animals
2.4. Surgical Procedure
2.5. MicroCT Imaging and Analysis
2.6. Histological Processing
2.7. Histopathological and Histolomorphometric Analysis
- The entire length of the implant under the crest of the maxillary bone and above the crest of the maxillary sinus (Implant Length).
- The length of the implant in contact with bone under the crest of the maxillary bone and above the maxillary sinus (Bone Contact).
2.8. Immunohistochemistry
2.9. Inflammatory Scoring
2.10. Molecular Analysis
2.11. Statistical Analysis
3. Results
3.1. SEM and Clinical Analysis
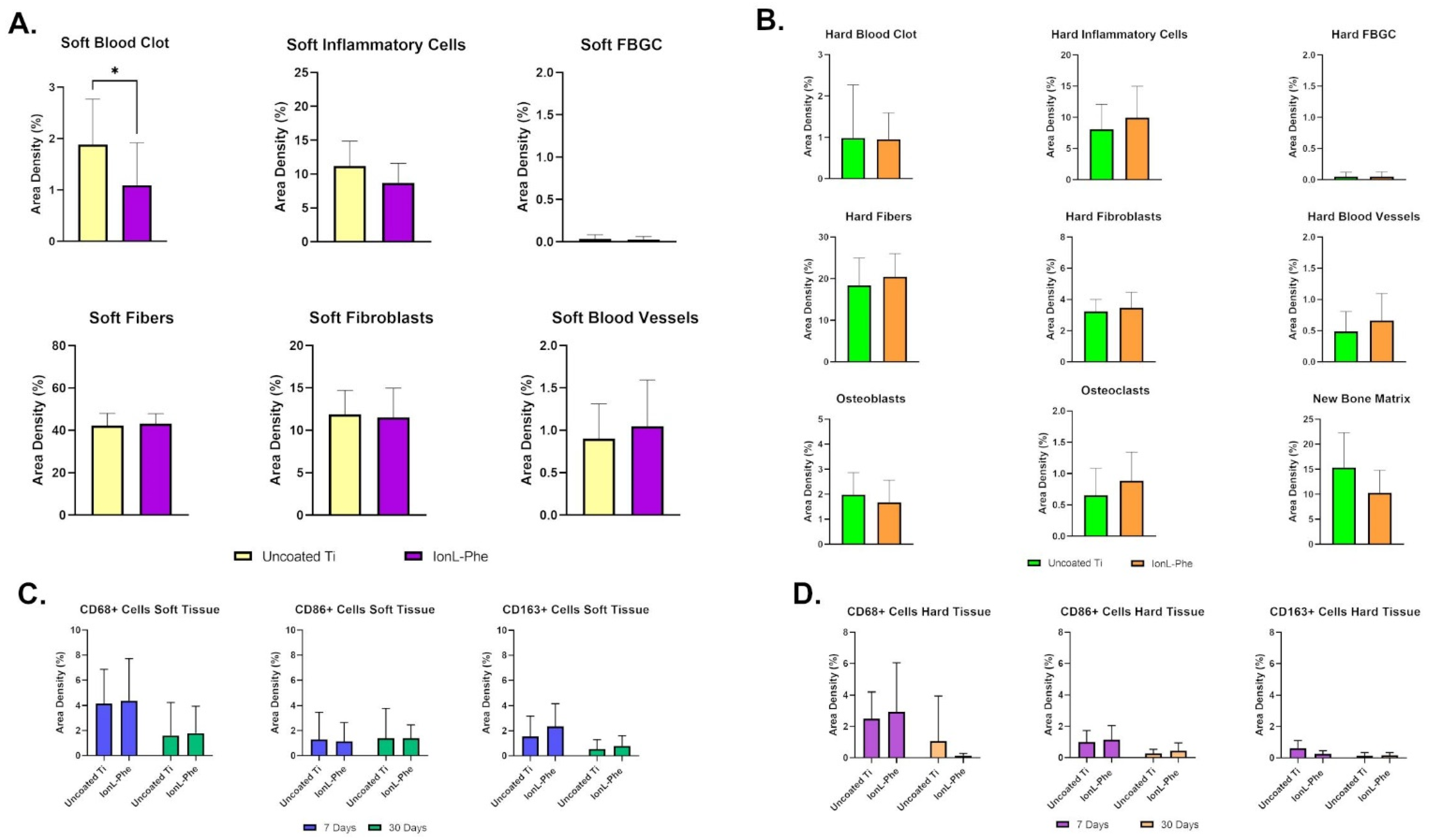
3.2. Histopathological Analysis and Inflammatory Scoring
3.3. BIC% and Success Rate
| Young Males (n = 9) | Young Females a (n = 7) | Old Males a (n = 10) | OVX Females (n = 10) | |||||
|---|---|---|---|---|---|---|---|---|
| Uncoated Ti | IonL-Phe | Uncoated Ti | IonL-Phe | Uncoated Ti | IonL-Phe | Uncoated Ti | IonL-Phe | |
| BIC (%) | 58.53 ± 20.94 | 64.80 ± 8.466 d | 17.85 ± 30.01 | 33.37 ± 36.86 | 21.88 ± 23.06 | 24.70 ± 12.72 | 40.89 ± 24.64 | 43.54 ± 26.12 |
| Success Rate (>60% BIC) | 66.67% | 77.78% | 28.57% | 28.57% | 10.00% | 0.00% | 20.00% | 50.00% * |
3.4. Histomorphometric Analysis in Young Males
3.5. Molecular Analysis in Young Males
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Elani, H.W.; Starr, J.R.; da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.-I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-Osseous Anchorage of Dental Prostheses: I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.O.; Jemt, T.; Stenport, V. Early Implant Failures in Edentulous Patients: A Multivariable Regression Analysis of 4615 Consecutively Treated Jaws. A Retrospective Study. J. Prosthodont. 2018, 27, 803–812. [Google Scholar] [CrossRef]
- Howe, M.-S.; Keys, W.; Richards, D. Long-Term (10-Year) Dental Implant Survival: A Systematic Review and Sensitivity Meta-Analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Noguerol, B.; Muñoz, R.; Mesa, F.; Luna, J.D.D.; O’Valle, F. Early Implant Failure. Prognostic Capacity of Periotest®: Retrospective Study of a Large Sample. Clin. Oral Implants Res. 2006, 17, 459–464. [Google Scholar] [CrossRef]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors Affecting the Survival Rate of Dental Implants: A Retrospective Study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef]
- Olmedo-Gaya, M.V.; Manzano-Moreno, F.J.; Cañaveral-Cavero, E.; Luna-Del Castillo, J.D.D.; Vallecillo-Capilla, M. Risk Factors Associated with Early Implant Failure: A 5-Year Retrospective Clinical Study. J. Prosthet. Dent. 2016, 115, 150–155. [Google Scholar] [CrossRef]
- Grisar, K.; Sinha, D.; Schoenaers, J.; Dormaar, T.; Politis, C. Retrospective Analysis of Dental Implants Placed Between 2012 and 2014: Indications, Risk Factors, and Early Survival. Int. J. Oral Maxillofac. Implants 2017, 32, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors Influencing Early Dental Implant Failures. J. Dent. Res. 2016, 95, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for Failures of Oral Implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Thomsen, P.; Ericson, L.E.; Lekholm, U. Histopathologic Observations on Early Oral Implant Failures. Int. J. Oral Maxillofac. Implants 2000, 14, 798–810. [Google Scholar]
- Esposito, M.; Thomsen, P.; Mölne, J.; Gretzer, C.; Ericson, L.E.; Lekholm, U. Immunohistochemistry of Soft Tissues Surrounding Late Failures of Brånemark Implants. Clin. Oral Implants Res. 1997, 8, 352–366. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.-M.; Lekholm, U.; Thomsen, P. Biological Factors Contributing to Failures of Osseointegrated Oral Implants, (I). Success Criteria and Epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Wennerberg, A. Current Concepts for the Biological Basis of Dental Implants. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 175–183. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Expanded Applications, Shifting Paradigms and an Improved Understanding of Host–Biomaterial Interactions. Acta Biomater. 2013, 9, 4948–4955. [Google Scholar] [CrossRef]
- Charles, J.F.; Nakamura, M.C. Bone and the Innate Immune System. Curr. Osteoporos. Rep. 2014, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Biguetti, C.C.; Cavalla, F.; Silveira, E.V.; Tabanez, A.P.; Francisconi, C.F.; Taga, R.; Campanelli, A.P.; Trombone, A.P.F.; Rodrigues, D.C.; Garlet, G.P. HGMB1 and RAGE as Essential Components of Ti Osseointegration Process in Mice. Front. Immunol. 2019, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.A.; Liu, S.; Agrawal, V.; Brown, B.N.; Johnson, S.A.; Medberry, C.J.; Badylak, S.F. Damage Associated Molecular Patterns within Xenogeneic Biologic Scaffolds and Their Effects on Host Remodeling. Biomaterials 2012, 33, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key Players around Bone Biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Feng, Y.; Cheng, H.; Li, D. The Role of Macrophages in Osseointegration of Dental Implants: An Experimental Study in Vivo. J. Biomed. Mater. Res. Part A 2020, 108, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Raggatt, L.-J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal Tissue Macrophages Are Intercalated throughout Human and Mouse Bone Lining Tissues and Regulate Osteoblast Function In Vitro and In Vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biguetti, C.C.; Cavalla, F.; Silveira, E.M.; Fonseca, A.C.; Vieira, A.E.; Tabanez, A.P.; Rodrigues, D.C.; Trombone, A.P.F.; Garlet, G.P. Oral Implant Osseointegration Model in C57Bl/6 Mice: Microtomographic, Histological, Histomorphometric and Molecular Characterization. J. Appl. Oral Sci. 2018, 26, e20170601. [Google Scholar] [CrossRef] [PubMed]
- Biguetti, C.C.; Cavalla, F.; Fonseca, A.C.; Tabanez, A.P.; Siddiqui, D.A.; Wheelis, S.E.; Taga, R.; Fakhouri, W.D.; Silva, R.M.; Rodrigues, D.C.; et al. Effects of Titanium Corrosion Products on In Vivo Biological Response: A Basis for the Understanding of Osseointegration Failures Mechanisms. Front. Mater. Sci. 2021, 8, 651970. [Google Scholar] [CrossRef]
- Yeo, I.-S.; Kim, H.-Y.; Lim, K.S.; Han, J.-S. Implant Surface Factors and Bacterial Adhesion: A Review of the Literature. Int. J. Artif. Organs 2012, 35, 762–772. [Google Scholar] [CrossRef]
- Zhao, B.; van der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft Tissue Integration versus Early Biofilm Formation on Different Dental Implant Materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef]
- Malm, M.O.; Jemt, T.; Stenport, V.F. Patient Factors Related to Early Implant Failures in the Edentulous Jaw: A Large Retrospective Case Control Study. Clin. Implant Dent. Relat. Res. 2021, 23, 466–476. [Google Scholar] [CrossRef]
- Chrcanovic, B.; Albrektsson, T.; Wennerberg, A. Bone Quality and Quantity and Dental Implant Failure: A Systematic Review and Meta-Analysis. Int. J. Prosthodont. 2017, 30, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, N.; Xu, X.; Qu, X.; Lu, E. Smoking, Radiotherapy, Diabetes and Osteoporosis as Risk Factors for Dental Implant Failure: A Meta-Analysis. PLoS ONE 2013, 8, e71955. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.; Bedi, G.; Garg, A. Implant Surface Modifications: A Review. J. Clin. Diagn. Res. 2012, 6, 319–324. [Google Scholar]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A Critical Review of Multifunctional Titanium Surfaces: New Frontiers for Improving Osseointegration and Host Response, Avoiding Bacteria Contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional Coatings to Simultaneously Promote Osseointegration and Prevent Infection of Orthopaedic Implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface Engineering of Biomaterials in Orthopedic and Dental Implants: Strategies to Improve Osteointegration, Bacteriostatic and Bactericidal Activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Bhise, N.S.; Evangelista, M.B.; Rouwkema, J.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Vrana, N.E.; Khademhosseini, A. Engineering Immunomodulatory Biomaterials to Tune the Inflammatory Response. Trends Biotechnol. 2016, 34, 470–482. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium Surface Characteristics, Including Topography and Wettability, Alter Macrophage Activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, X.; Ma, X.; Ma, Z.; Zhang, Y.; Lu, Y.; Li, X.; Lei, W.; Feng, Y. Promotion of Osteointegration under Diabetic Conditions by Tantalum Coating-Based Surface Modification on 3-Dimensional Printed Porous Titanium Implants. Colloids Surf. B 2016, 148, 440–452. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, G.; Zheng, H.; Lu, Z.; Zhong, X.; Cheng, X.; Zreiqat, H. Delicate Refinement of Surface Nanotopography by Adjusting TiO2 Coating Chemical Composition for Enhanced Interfacial Biocompatibility. ACS Appl. Mater. Interfaces 2013, 5, 8203–8209. [Google Scholar] [CrossRef] [PubMed]
- Yuran, S.; Dolid, A.; Reches, M. Resisting Bacteria and Attracting Cells: Spontaneous Formation of a Bifunctional Peptide-Based Coating by On-Surface Assembly Approach. ACS Biomater. Sci. Eng. 2018, 4, 4051–4061. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human Innate Immunosenescence: Causes and Consequences for Immunity in Old Age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Haschak, M.J.; Lopresti, S.T.; Stahl, E.C. Effects of Age-Related Shifts in Cellular Function and Local Microenvironment upon the Innate Immune Response to Implants. Semin. Immunol. 2017, 29, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Wendler, S.; Schlundt, C.; Bucher, C.H.; Birkigt, J.; Schipp, C.J.; Volk, H.-D.; Duda, G.N.; Schmidt-Bleek, K. Immune Modulation to Enhance Bone Healing—A New Concept to Induce Bone Using Prostacyclin to Locally Modulate Immunity. Front. Immunol. 2019, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Bosco, R.; Iafisco, M.; Tampieri, A.; Jansen, J.A.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P. Hydroxyapatite Nanocrystals Functionalized with Alendronate as Bioactive Components for Bone Implant Coatings to Decrease Osteoclastic Activity. Appl. Surf. Sci. 2015, 328, 516–524. [Google Scholar] [CrossRef]
- Korn, P.; Kramer, I.; Schlottig, F.; Tödtmann, N.; Eckelt, U.; Bürki, A.; Ferguson, S.; Kautz, A.; Schnabelrauch, M.; Range, U.; et al. Systemic Sclerostin Antibody Treatment Increases Osseointegration and Biomechanical Competence of Zoledronic-Acid-Coated Dental Implants in a Rat Osteoporosis Model. Eur. Cells Mater. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Xing, H.; Wang, X.; Xiao, S.; Zhang, G.; Li, M.; Wang, P.; Shi, Q.; Qiao, P. Osseointegration of Layer-by-Layer Polyelectrolyte Multilayers Loaded with IGF1 and Coated on Titanium Implant under Osteoporotic Condition. Int. J. Nanomed. 2017, 12, 7709–7720. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xi, Y.; Bai, J.; Jiang, Z.; Wang, S.; Zhang, H.; Dai, W.; Chen, C.; Gou, Z.; Yang, G.; et al. Covalent Grafting of Hyperbranched Poly-L-Lysine on Ti-Based Implants Achieves Dual Functions of Antibacteria and Promoted Osteointegration in Vivo. Biomaterials 2021, 269, 120534. [Google Scholar] [CrossRef]
- Ma, T.; Ge, X.-Y.; Hao, K.-Y.; Jiang, X.; Zheng, Y.; Lin, Y.; Zhang, Y. Titanium Discs Coated with 3,4-Dihydroxy-l-Phenylalanine Promote Osteogenic Differentiation of Human Bone Mesenchymal Stem Cells: In Vitro. RSC Adv. 2019, 9, 9117–9125. [Google Scholar] [CrossRef] [Green Version]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Dicationic Imidazolium-Based Ionic Liquids: A New Strategy for Non-Toxic and Antimicrobial Materials. RSC Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic Liquid Lubricants: Designed Chemistry for Engineering Applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.-D.; Jiménez, A.-E.; Sanes, J.; Carrión, F.-J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J. Properties of Ionic Liquid Solvents for Catalysis. J. Mol. Catal. A Chem. 2004, 214, 11–17. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The Third Evolution of Ionic Liquids: Active Pharmaceutical Ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Ionic Liquid Coatings for Titanium Surfaces: Effect of IL Structure on Coating Profile. ACS Appl. Mater. Interfaces 2015, 7, 27421–27431. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C.; Davis, C.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Improvement of Tribological and Anti-Corrosive Performance of Titanium Surfaces Coated with Dicationic Imidazolium-Based Ionic Liquids. RSC Adv. 2016, 6, 78795–78802. [Google Scholar] [CrossRef]
- Gindri, I.M.; Palmer, K.L.; Siddiqui, D.A.; Aghyarian, S.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Evaluation of Mammalian and Bacterial Cell Activity on Titanium Surface Coated with Dicationic Imidazolium-Based Ionic Liquids. RSC Adv. 2016, 6, 36475–36483. [Google Scholar] [CrossRef]
- Wheelis, S.E.; Biguetti, C.C.; Natarajan, S.; Guida, L.; Hedden, B.; Garlet, G.P.; Rodrigues, D.C. Investigation of the Early Healing Response to Dicationic Imidazolium-Based Ionic Liquids: A Biocompatible Coating for Titanium Implants. ACS Biomater. Sci. Eng. 2020, 6, 984–994. [Google Scholar] [CrossRef]
- Du, Z.; Lee, R.S.B.; Hamlet, S.; Doan, N.; Ivanovski, S.; Xiao, Y. Evaluation of the First Maxillary Molar Post-Extraction Socket as a Model for Dental Implant Osseointegration Research. Clin. Oral Implants Res. 2016, 27, 1469–1478. [Google Scholar] [CrossRef]
- Wheelis, S.E.; Biguetti, C.C.; Natarajan, S.; Arteaga, A.; el Allami, J.; Chandrashekar, B.L.; Garlet, G.P.; Rodrigues, D.C. Cellular and Molecular Dynamics during Early Oral Osseointegration: A Comprehensive Characterization in the Lewis Rat. ACS Biomater. Sci. Eng. 2021, 7, 2392–2407. [Google Scholar] [CrossRef]
- Mouraret, S.; Hunter, D.J.; Bardet, C.; Brunski, J.B.; Bouchard, P.; Helms, J.A. A Pre-Clinical Murine Model of Oral Implant Osseointegration. Bone 2014, 58, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Du, Z.; Steck, R.; Doan, N.; Woodruff, M.A.; Ivanovski, S.; Xiao, Y. Estrogen Deficiency-Associated Bone Loss in the Maxilla: A Methodology to Quantify the Changes in the Maxillary Intra-Radicular Alveolar Bone in an Ovariectomized Rat Osteoporosis Model. Tissue Eng. Part C 2015, 21, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Só, B.B.; Silveira, F.M.; Llantada, G.S.; Jardim, L.C.; Calcagnotto, T.; Martins, M.A.T.; Martins, M.D. Effects of Osteoporosis on Alveolar Bone Repair after Tooth Extraction: A Systematic Review of Preclinical Studies. Arch. Oral Biol. 2021, 125, 105054. [Google Scholar] [CrossRef]
- Nyman, J.S. Age-Related Changes to Bone Structure and Quality in Rodent Models. In Conn’s Handbook of Models for Human Aging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 919–936. [Google Scholar]
- Albrektsson, T.; Eriksson, A.R.; Friberg, B.; Lekholm, U.; Lindahl, L.; Nevins, M.; Oikarinen, V.; Roos, J.; Sennerby, L.; Astrand, P. Histologic Investigations on 33 Retrieved Nobelpharma Implants. Clin. Mater. 1993, 12, 1–9. [Google Scholar] [CrossRef]
- ISO/TC194 ISO 10993-6:2016; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. ISO: Geneva, Switzerland, 2016.
- Carter, L.E.; Kilroy, G.; Gimble, J.M.; Floyd, Z.E. An Improved Method for Isolation of RNA from Bone. BMC Biotechnol. 2012, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, D.A.; Gindri, I.M.; Rodrigues, D.C. Corrosion and Wear Performance of Titanium and Cobalt Chromium Molybdenum Alloys Coated with Dicationic Imidazolium-Based Ionic Liquids. J. Bio- Tribo-Corros. 2016, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Kyriakides, T.R.; Foster, M.J.; Keeney, G.E.; Tsai, A.; Giachelli, C.M.; Clark-Lewis, I.; Rollins, B.J.; Bornstein, P. The CC Chemokine Ligand, CCL2/MCP1, Participates in Macrophage Fusion and Foreign Body Giant Cell Formation. Am. J. Pathol. 2004, 165, 2157–2166. [Google Scholar] [CrossRef] [Green Version]
- Alfarsi, M.A.; Hamlet, S.M.; Ivanovski, S. Titanium Surface Hydrophilicity Modulates the Human Macrophage Inflammatory Cytokine Response. J. Biomed. Mater. Res. Part A 2014, 102, 60–67. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.D.J.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L.P. Systematic Validation of Specific Phenotypic Markers for in Vitro Polarized Human Macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of INOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Wang, W.; Nassiri, S.; Kwan, T.; Dang, C.; Liu, W.; Spiller, K.L. Temporal and Spatial Distribution of Macrophage Phenotype Markers in the Foreign Body Response to Glutaraldehyde-Crosslinked Gelatin Hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 721–742. [Google Scholar] [CrossRef]
- Etzerodt, A.; Moestrup, S.K. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid. Redox Signal. 2013, 18, 2352–2363. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.-I.; Kim, M.R.; Jeong, H.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal Stem Cells Reciprocally Regulate the M1/M2 Balance in Mouse Bone Marrow-Derived Macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in Vitro Comparative Model of Osteogenic and Inflammatory Cell Response to Dental Implants. Dent. Mater. 2019, 35, 176–184. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.-Y.; Salih, E.; Xu, L.; Wunderlich, L.; Gu, X.; Hofstaetter, J.G.; Torres, M.; Glimcher, M.J. Site-Specific In Vivo Calcification and Osteogenesis Stimulated by Bone Sialoprotein. Calcif. Tissue Int. 2006, 79, 179–189. [Google Scholar] [CrossRef]
- He, G.; George, A. Dentin Matrix Protein 1 Immobilized on Type I Collagen Fibrils Facilitates Apatite Deposition in Vitro. J. Biol. Chem. 2004, 279, 11649–11656. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Regulation of Bone Development and Extracellular Matrix Protein Genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Cao, Y.; Zhan, D.; Wang, D.; Wang, B.; Liu, Y.; Li, G.; He, W.; Wang, H.; Xu, L. Influence of DNA Methylation on the Expression of OPG/RANKL in Primary Osteoporosis. Int. J. Med. Sci. 2018, 15, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Fiorellini, J.; Glindmann, S.; Salcedo, J.; Weber, H.-P.; Park, C.-J.; Sarmiento, H. The Effect of Osteopontin and an Osteopontin-Derived Synthetic Peptide Coating on Osseointegration of Implants in a Canine Model. Int. J. Periodontics Restor. Dent. 2016, 36, e88–e94. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Moreno, P.; Hernandez-Cortes, P.; Padial-Molina, M.; Vizoso, M.L.; Crespo-Lora, V.; O’Valle, F. Immunohistochemical Osteopontin Expression in Bone Xenograft in Clinical Series of Maxillary Sinus Lift. J. Oral Rehabil. 2015, 1, 42–50. [Google Scholar]
- Bakshi, K.; Mitra, S.; Sharma, V.K.; Jayadev, M.S.K.; Sakai, V.G.; Mukhopadhyay, R.; Gupta, A.; Ghosh, S.K. Imidazolium-Based Ionic Liquids Cause Mammalian Cell Death Due to Modulated Structures and Dynamics of Cellular Membrane. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183103. [Google Scholar] [CrossRef]
- Shahnazari, M.; Chu, V.; Wronski, T.J.; Nissenson, R.A.; Halloran, B.P. CXCL12/CXCR4 Signaling in the Osteoblast Regulates the Mesenchymal Stem Cell and Osteoclast Lineage Populations. FASEB J. 2013, 27, 3505–3513. [Google Scholar] [CrossRef]
- Yellowley, C. CXCL12/CXCR4 Signaling and Other Recruitment and Homing Pathways in Fracture Repair. BoneKEy Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.L.; Razali, M.; Ngeow, W.C. Dimension and Structures of Biological Seal of Peri-Implant Tissues. In Dental Implantology and Biomaterial; IntechOpen: London, UK, 2016. [Google Scholar]
- Sun, J.; Eberhard, J.; Glage, S.; Held, N.; Voigt, H.; Schwabe, K.; Winkel, A.; Stiesch, M. Development of a Peri-implantitis Model in the Rat. Clin. Oral Implants Res. 2020, 31, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.C.; Sridhar, S.; Gindri, I.M.; Siddiqui, D.; Valderrama, P.; Wilson, T.G., Jr.; Wadhwani, C.; Chung, A. Spectroscopic and Microscopic Investigation of the Effects of Bacteria on Implant Surfaces. RSC Adv. 2016, 6, 48283–48293. [Google Scholar] [CrossRef]
- Dereka, X.; Calciolari, E.; Donos, N.; Mardas, N. Osseointegration in Osteoporotic-like Condition: A Systematic Review of Preclinical Studies. J. Periodontal Res. 2018, 53, 933–940. [Google Scholar] [CrossRef]
- Knowles, H.J.; Athanasou, N.A. Canonical and Non-Canonical Pathways of Osteoclast Formation. Histol. Histopathol. 2009, 24, 337–346. [Google Scholar] [CrossRef]
- Du, Z.; Xiao, Y.; Hashimi, S.; Hamlet, S.M.; Ivanovski, S. The Effects of Implant Topography on Osseointegration under Estrogen Deficiency Induced Osteoporotic Conditions: Histomorphometric, Transcriptional and Ultrastructural Analysis. Acta Biomater. 2016, 42, 351–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G. Too Old to Fight? Aging and Its Toll on Innate Immunity. Mol. Oral Microbiol. 2010, 25, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Löffler, J.; Sass, F.A.; Filter, S.; Rose, A.; Ellinghaus, A.; Duda, G.N.; Dienelt, A. Compromised Bone Healing in Aged Rats Is Associated with Impaired M2 Macrophage Function. Front. Immunol. 2019, 10, 2443. [Google Scholar] [CrossRef] [PubMed]




| Demographic Group | Age Range (Weeks) | Average Weight at Surgery (g) | Average Weight at Sacrifice (g) | Average Weight Change |
|---|---|---|---|---|
| Young Male (YM) | 10–12 | 295.82 ± 39.80 | 354.64 ± 53.48 | 19.88% |
| Young Female (OF) | 10–12 | 180.33 ± 10.60 | 206.71 ± 16.01 | 9.180% |
| Old Males (OM) | 52–78 | 529.87 ± 45.03 | 524.95 ± 45.57 | −0.9257% |
| Ovariectomized Females (OVXF) | 26–28 | 290.55 ± 11.42 | 305.2 ± 16.85 | 4.378% |
| Type of Analysis | Outcome | Groups | Healing Period | Result | Statistical Test |
|---|---|---|---|---|---|
| Clinical Evaluation | Overall health evaluation and resolution of inflammation at implant site | Young Males, Young Females, OVX Females, and Old Males | 7 and 30 days | Figure 2, Table 1. | N/A |
| X-ray Microtomography | Supplemental evaluation of bone quality in femur. | Young Males, Young Females, OVX Females, and Old Males | N/A | Figure S2. | One-Way ANOVA with Tukey’s multiple comparisons test (YM vs. YF, YF vs OVXF, and YM vs. OM only) |
| Histology (H&E) | Qualitative progression of inflammation, wound healing, and osseointegration | Young Males, Young Females, OVX Females, and Old Males | 7 and 30 days | Figure 3 and Figure 4. | N/A |
| Histomorphometry (H&E-BIC%) | Implant success rate | Young Males, Young Females, OVX Females, and Old Males | 30 days | Table 4. | Yate’s Chi-Square–Success Rate (YM vs YF, YF vs OVXF, and YM vs. OM only) |
| Two-Way ANOVA with Tukey’s multiple comparisons test (Demographic and Coating as factors, YM vs. YF, YF vs. OVXF, and YM vs. OM only) | |||||
| Paired t-test or Wilcoxon signed-rank test (Two-tailed, YM only) -BIC% | |||||
| Equivalence (YM only) -BIC% | |||||
| Cell Histomorphometry (H&E) | Quantitative progression of inflammation, wound healing, and osseointegration | Young Males | 7 days | Figure 5. | Paired t-test or Wilcoxon signed-rank test (Two-tailed) |
| Inflammatory Scoring (H&E) | Semiquantitative measure of degree of inflammation | Young Males, Young Females, OVX Females, and Old Males | 7 and 30 days | Table 3, Figure 4. | Two-Way ANOVA with Tukey’s multiple comparisons test (Demographic and Coating as factors) |
| IHC | CD68 (Pan-Macrophage) | Young Males | 7 and 30 days | Figure 5. | Paired t-test or Wilcoxon signed-rank test (Two-tailed) |
| CD86 (M1 Macrophages) | |||||
| CD163 (M2 Macrophage) | |||||
| RT-qPCR | Fold change in genes associated with inflammation, wound healing, and osseointegration | Young Males | 2 and 7 Days | Figure 6. | Unpaired t-test or Mann–Whitney U test (Two-tailed) |
| Score | |||||
|---|---|---|---|---|---|
| Cell Type | 0 | 1 | 2 | 3 | 4 |
| Polymorphonuclear Cells (PMNs) | 0 | 1–5 cells/per field (pf) 1 | 5–10 cells/pf | Heavy Infiltrate (>10 cells/pf) | Densely Packed |
| Macrophages | 0 | 1–5 cells/pf | 5–10 cells/pf | Heavy Infiltrate (>10 cells/pf) | Densely Packed |
| Lymphocytes | 0 | 1–5 cells/pf | 5–10 cells/pf | Heavy Infiltrate (>10 cells/pf) | Densely Packed |
| Plasma Cells | 0 | 1–5 cells/pf | 5–10 cells/pf | Heavy Infiltrate (>10 cells/pf) | Densely Packed |
| Foreign Body Giant Cells | 0 | 1–2 cells/pf | 3–5 cells/pf | Heavy Infiltrate (>5 cells/pf) | Densely Packed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wheelis, S.E.; Biguetti, C.C.; Natarajan, S.; Chandrashekar, B.L.; Arteaga, A.; Allami, J.E.; Garlet, G.P.; Rodrigues, D.C. Effects of Dicationic Imidazolium-Based Ionic Liquid Coatings on Oral Osseointegration of Titanium Implants: A Biocompatibility Study in Multiple Rat Demographics. Genes 2022, 13, 642. https://doi.org/10.3390/genes13040642
Wheelis SE, Biguetti CC, Natarajan S, Chandrashekar BL, Arteaga A, Allami JE, Garlet GP, Rodrigues DC. Effects of Dicationic Imidazolium-Based Ionic Liquid Coatings on Oral Osseointegration of Titanium Implants: A Biocompatibility Study in Multiple Rat Demographics. Genes. 2022; 13(4):642. https://doi.org/10.3390/genes13040642
Chicago/Turabian StyleWheelis, Sutton E., Claudia C. Biguetti, Shruti Natarajan, Bhuvana Lakkasetter Chandrashekar, Alexandra Arteaga, Jihad El Allami, Gustavo P. Garlet, and Danieli C. Rodrigues. 2022. "Effects of Dicationic Imidazolium-Based Ionic Liquid Coatings on Oral Osseointegration of Titanium Implants: A Biocompatibility Study in Multiple Rat Demographics" Genes 13, no. 4: 642. https://doi.org/10.3390/genes13040642
APA StyleWheelis, S. E., Biguetti, C. C., Natarajan, S., Chandrashekar, B. L., Arteaga, A., Allami, J. E., Garlet, G. P., & Rodrigues, D. C. (2022). Effects of Dicationic Imidazolium-Based Ionic Liquid Coatings on Oral Osseointegration of Titanium Implants: A Biocompatibility Study in Multiple Rat Demographics. Genes, 13(4), 642. https://doi.org/10.3390/genes13040642