Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Reagents
2.2. Generation of Tobacco Plants Expressing HsMT1L
2.3. Growth Conditions and Treatments
2.4. Determination of Zinc and Cadmium in Plants
2.5. Measurement of Antioxidant Enzyme Activity
2.6. Determination of Chlorophyll Content in Plants
2.7. Determination of Malondialdehyde Content in Plants
2.8. DAB Staining
2.9. Subcellular Localization of HsMT1L
2.10. Statistical Analysis
3. Results
3.1. Expression of HsMT1L in Tobacco
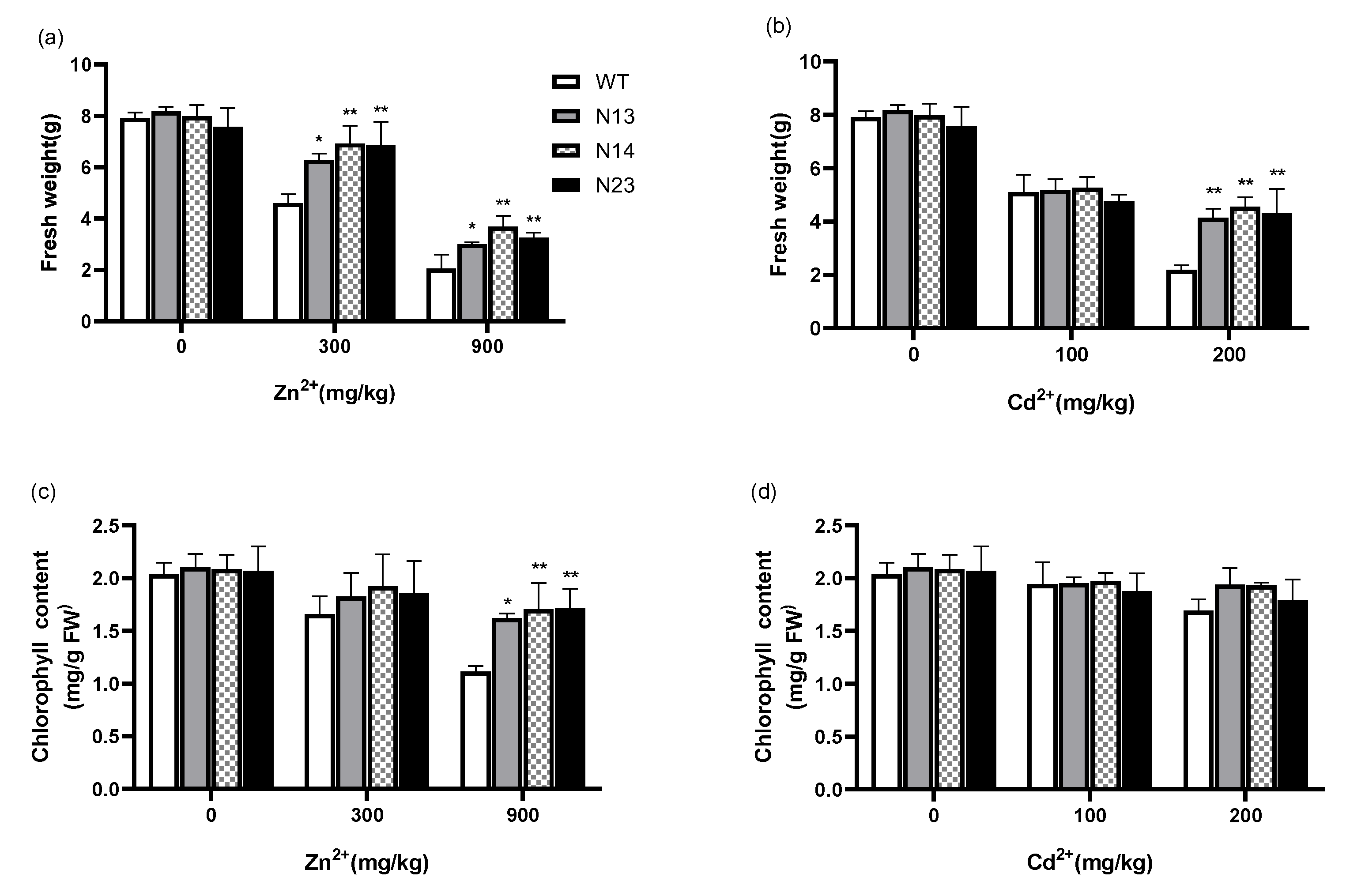
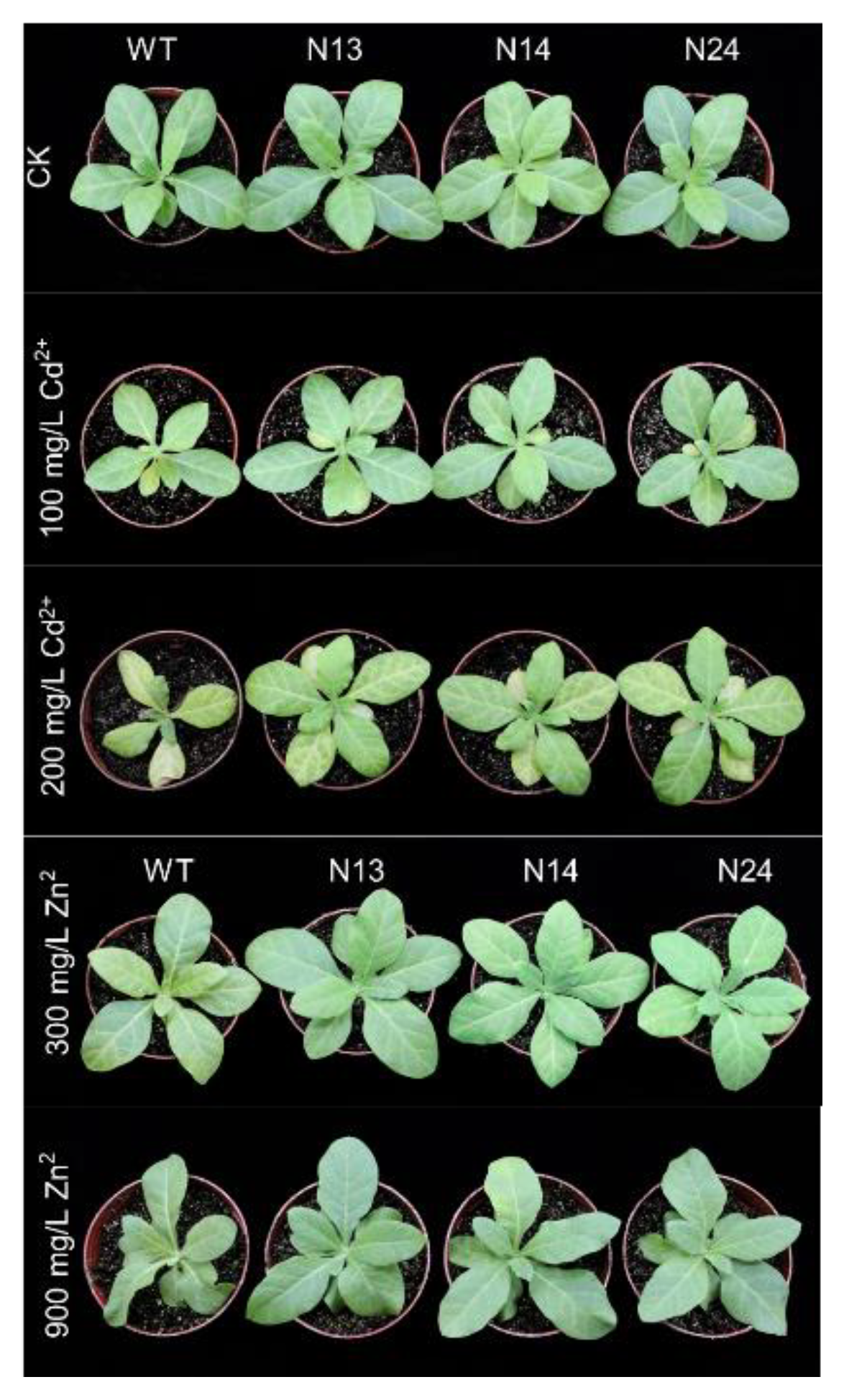
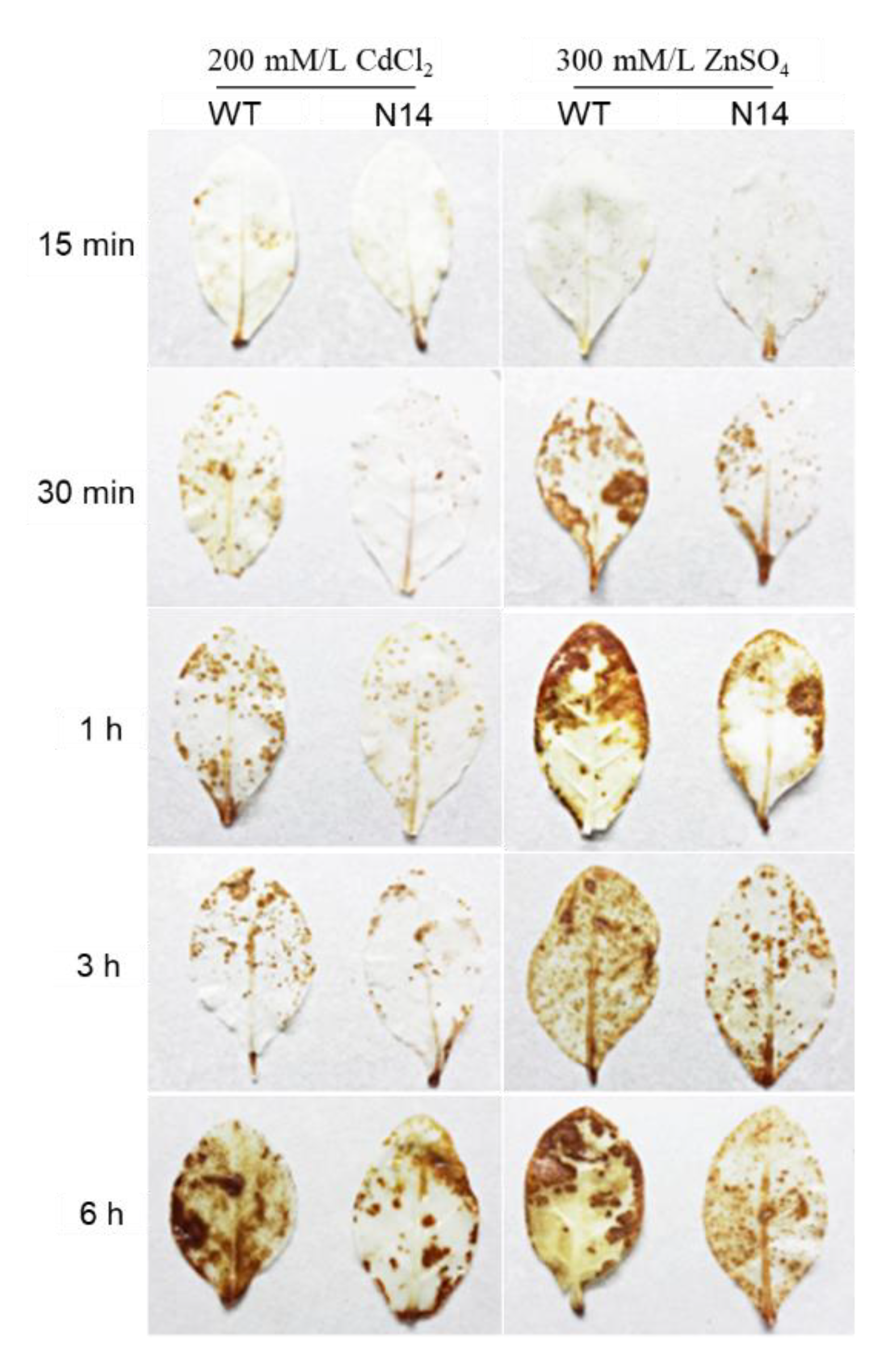
3.2. HsMT1L Gene Improved the Tolerance of Tobacco to Zn2+ and Cd2+
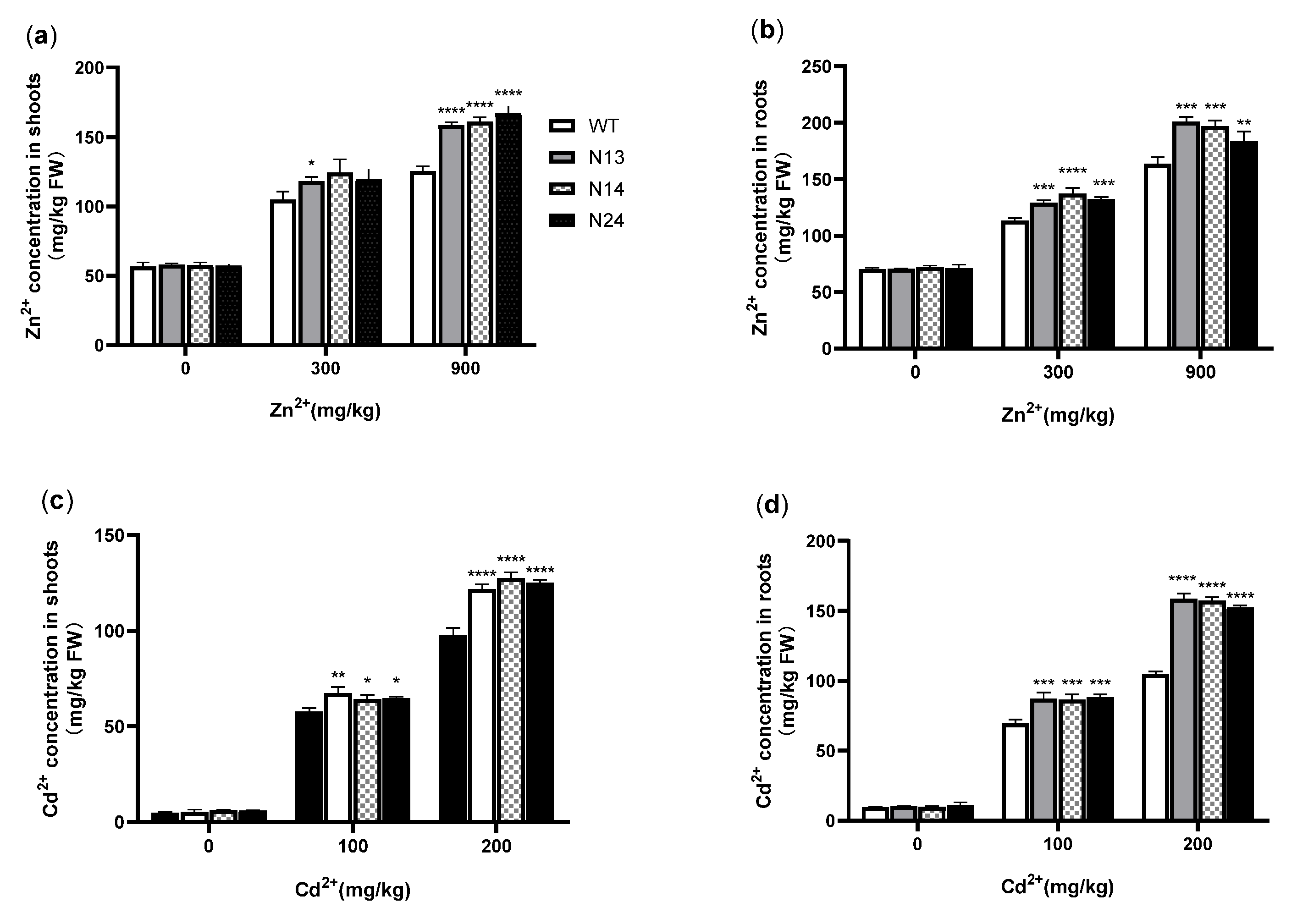
3.3. Heterologous Expression of HsMT1L Increased the Accumulation of Zn2+ and Cd2+
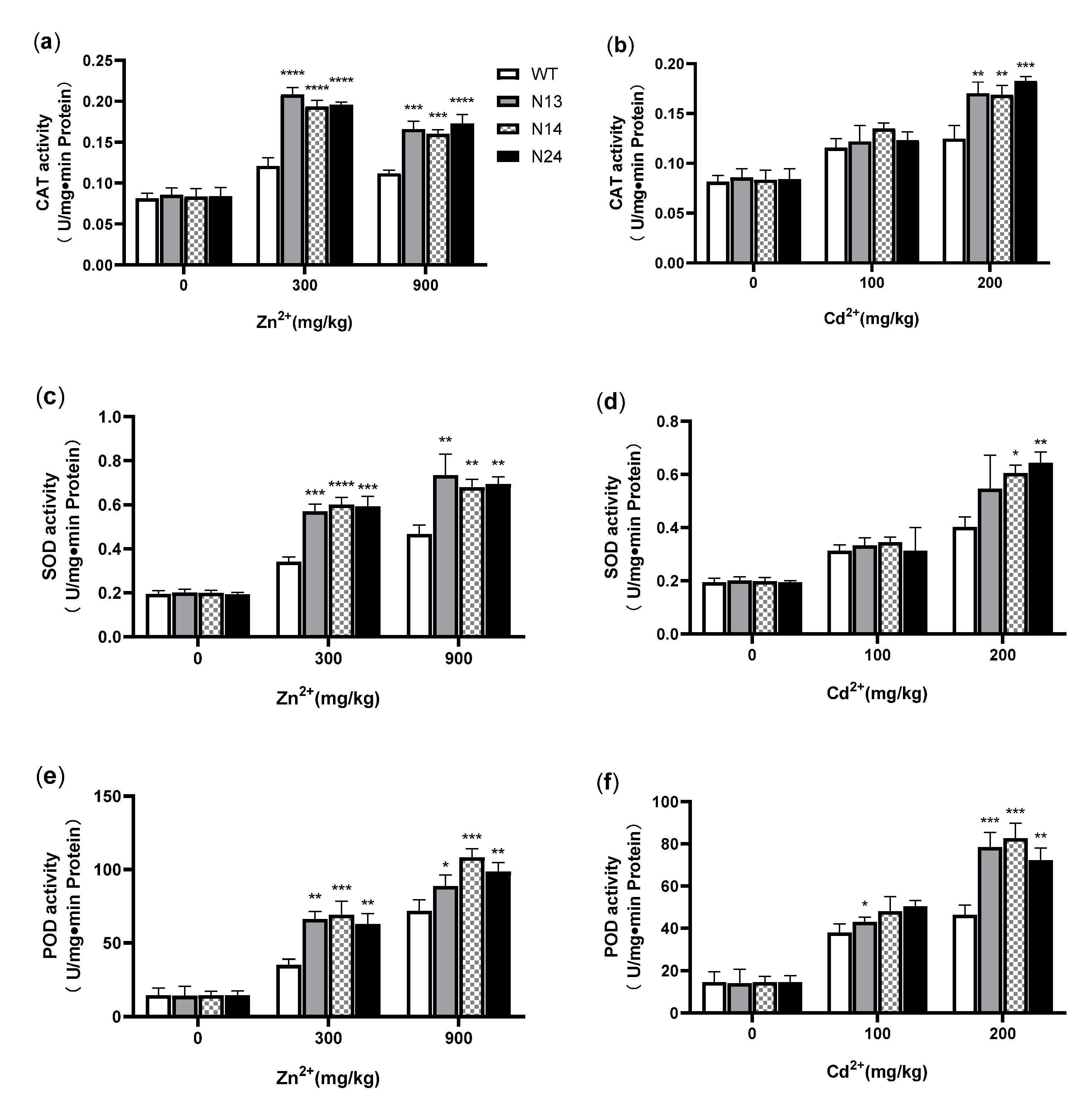
3.4. HsMT1L Enhances the Antioxidant Capacity of Transgenic Tobacco under Zn2+ or Cd2+ Stress
3.5. Subcellular Localization of HsMT1L in Transgenic Tobacco
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holtra, A.; Zamorska-Wojdyla, D. The pollution indices of trace elements in soils and plants close to the copper and zinc smelting works in Poland’s Lower Silesia. Environ. Sci. Pollut. Res. Int. 2020, 27, 16086–16099. [Google Scholar] [CrossRef] [PubMed]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated sites in Europe: Review of the current situation based on data collected through a European network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Zarei, A.; Esmaeilzadeh, M.; Taghavi, M.; Yousefi, M.; Yousefi, Z.; Sedighi, F.; Javan, S. Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur, Iran. Biol. Trace Elem. Res. 2020, 195, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; Djordjevic, D.; Polic, P. Trace and major element pollution originating from coal ash suspension and transport processes. Environ. Int. 2001, 26, 251–255. [Google Scholar] [CrossRef]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658–5668. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E. Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef]
- Solenkova, N.V.; Newman, J.D.; Berger, J.S.; Thurston, G.; Hochman, J.S.; Lamas, G.A. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. Am. Heart J. 2014, 168, 812–822. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. Int. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Macci, C.; Peruzzi, E.; Doni, S.; Poggio, G.; Masciandaro, G. The phytoremediation of an organic and inorganic polluted soil: A real scale experience. Int. J. Phytorem. 2016, 18, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Drouet, T.; Sterckeman, T.; Noret, N. Phytoremediation of urban soils contaminated with trace metals using Noccaea caerulescens: Comparing non-metallicolous populations to the metallicolous ‘Ganges’ in field trials. Environ. Sci. Pollut. Res. Int. 2017, 24, 8176–8188. [Google Scholar] [CrossRef]
- Pautot, V.; Brzezinski, R.; Tepfer, M. Expression of a mouse metallothionein gene in transgenic plant tissues. Gene 1989, 77, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Mahoney, M.; Koropatnick, J. Signaling events for metallothionein induction. Mutat. Res. 2003, 533, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Asselman, J.; Glaholt, S.P.; Smith, Z.; Smagghe, G.; Janssen, C.R.; Colbourne, J.K.; Shaw, J.R.; De Schamphelaere, K.A. Functional characterization of four metallothionein genes in Daphnia pulex exposed to environmental stressors. Aquat. Toxicol. 2012, 110–111, 54–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figueira, E.; Branco, D.; Antunes, S.C.; Goncalves, F.; Freitas, R. Are metallothioneins equally good biomarkers of metal and oxidative stress? Ecotoxicol. Environ. Saf. 2012, 84, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Metallothionein and cadmium toxicology—Historical review and commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Bremner, I. Oxygen free radicals and metallothionein. Free Radic. Biol. Med. 1993, 14, 325–337. [Google Scholar] [CrossRef]
- Romero-Isart, N.; Vasak, M. Advances in the structure and chemistry of metallothioneins. J. Inorg. Biochem. 2002, 88, 388–396. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Du, Z.Y.; Mjos, S.A.; Grung, B.; Midtbo, L.K. Methylmercury increases and eicosapentaenoic acid decreases the relative amounts of arachidonic acid-containing phospholipids in mouse brain. Lipids 2016, 51, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Laukens, D.; Waeytens, A.; De Bleser, P.; Cuvelier, C.; De Vos, M. Human metallothionein expression under normal and pathological conditions: Mechanisms of gene regulation based on in silico promoter analysis. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Rai, J.P. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Vasak, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Atrian-Blasco, E.; Santoro, A.; Pountney, D.L.; Meloni, G.; Hureau, C.; Faller, P. Chemistry of mammalian metallothioneins and their interaction with amyloidogenic peptides and proteins. Chem. Soc. Rev. 2017, 46, 7683–7693. [Google Scholar] [CrossRef]
- Scheller, J.S.; Irvine, G.W.; Stillman, M.J. Unravelling the mechanistic details of metal binding to mammalian metallothioneins from stoichiometric, kinetic, and binding affinity data. Dalton. Trans. 2018, 47, 3613–3637. [Google Scholar] [CrossRef]
- Conrad, C.C.; Walter, C.A.; Richardson, A.; Hanes, M.A.; Grabowski, D.T. Cadmium toxicity and distribution in metallothionein-I and -II deficient transgenic mice. J. Toxicol. Environ. Health 1997, 52, 527–543. [Google Scholar]
- Liu, J.; Liu, Y.; Habeebu, S.M.; Waalkes, M.P.; Klaassen, C.D. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology 2000, 147, 157–166. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Gao, Z.; Wen, Y.; Wang, W.; Liu, W.; Wang, X.; Zhu, F. Identification of three metallothioneins in the black soldier fly and their functions in Cd accumulation and detoxification. Environ. Pollut. 2021, 286, 117146. [Google Scholar] [CrossRef]
- Liu, J.; Klaassen, C.D. Absorption and distribution of cadmium in metallothionein-I transgenic mice. Fundam. Appl. Toxicol. 1996, 29, 294–300. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J. Metallothionein transgenic and knock-out mouse models in the study of cadmium toxicity. J. Toxicol. Sci. 1998, 23 (Suppl. 2), 97–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, S.; Wang, Y.; Luo, J.; Long, Y.; Mei, X.; Tang, Y. Role of metallothionein-1 and metallothionein-2 in the neuroprotective mechanism of sevoflurane preconditioning in mice. J. Mol. Neurosci. 2020, 70, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Moleirinho, A.; Carneiro, J.; Matthiesen, R.; Silva, R.M.; Amorim, A.; Azevedo, L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS ONE 2011, 6, e18487. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Forma, E.; Chwatko, G.; Jozwiak, P.; Szymczyk, A.; Wilkosz, J.; Rozanski, W.; Brys, M. Effect of metallothionein 2A gene polymorphism on allele-specific gene expression and metal content in prostate cancer. Toxicol. Appl. Pharmacol. 2013, 268, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mehus, A.A.; Muhonen, W.W.; Garrett, S.H.; Somji, S.; Sens, D.A.; Shabb, J.B. Quantitation of human metallothionein isoforms: A family of small, highly conserved, cysteine-rich proteins. Mol. Cell. Proteom. 2014, 13, 1020–1033. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Holloway, A.; Stennard, F.; West, A. Human metallothionein gene MT1L mRNA is present in several human tissues but is unlikely to produce a metallothionein protein. FEBS Lett. 1997, 404, 41–44. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Kelley, A.M.; Anderson, B.J.; Armstrong, N.J.; McClung, H.L.; Berryman, C.E.; Karl, J.P.; McClung, J.P. Sensitivity and reliability of zinc transporter and metallothionein gene expression in peripheral blood mononuclear cells as indicators of zinc status: Responses to ex vivo zinc exposure and habitual zinc intake in humans. Br. J. Nutr. 2021, 125, 361–368. [Google Scholar] [CrossRef]
- Lazo, G.R.; Stein, P.A.; Ludwig, R.A. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology 1991, 9, 963–967. [Google Scholar] [CrossRef]
- Svab, Z.; Maliga, P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc. Natl. Acad. Sci. USA 1993, 90, 913–917. [Google Scholar] [CrossRef]
- Shi, J.; Fu, X.Z.; Peng, T.; Huang, X.S.; Fan, Q.J.; Liu, J.H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Sprague, S.A.; Park, J.; Oh, M.; Rajashekar, C.B.; Koiwa, H.; Nakata, P.A.; Cheng, N.; Hirschi, K.D.; et al. Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. Hortic. Res. 2015, 2, 15051–15061. [Google Scholar] [CrossRef]
- Vasak, M.; Hasler, D.W. Metallothioneins: New functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Kaur, S.; Bhardwaj, R. Physiological and biochemical changes in Brassica juncea plants under Cd-induced stress. Biomed. Res. Int. 2014, 2014, 726070. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta 2016, 1864, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Sekhar, K.; Priyanka, B.; Reddy, V.D.; Rao, K.V. Metallothionein 1 (CcMT1) of pigeonpea (Cajanus cajan L.) confers enhanced tolerance to copper and cadmium in Escherichia coli and Arabidopsis thaliana. Environ. Exp. Bot. 2011, 72, 131–139. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, T.; Cheng, J.-s.; Yi, Y.-j.; Han, J.-j.; Cheng, H.-l.; Li, Q.; Tang, N.; Liang, M.-X. Heterologous expression of the metallothionein PpMT2 gene from Physcomitrella patens confers enhanced tolerance to heavy metal stress on transgenic Arabidopsis plants. Plant Growth Regul. 2020, 90, 63–72. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Tian, S.; Lu, L.; Shohag, M.; Yang, X. Metallothionein 2 (SaMT2) from Sedum alfredii Hance confers increased Cd tolerance and accumulation in yeast and tobacco. PLoS ONE 2014, 9, e102750. [Google Scholar] [CrossRef]
- Xue, T.; Li, X.; Zhu, W.; Wu, C.; Yang, G.; Zheng, C. Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J. Exp. Bot. 2009, 60, 339–349. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Long, X.; Liu, L.; Wang, J.; Zhu, J.; Ma, Y.; Qin, Y.; Qi, J.; Hu, X.; et al. Characterization of the rubber tree metallothionein family reveals a role in mitigating the effects of reactive oxygen species associated with physiological stress. Tree Physiol. 2018, 38, 911–924. [Google Scholar] [CrossRef]
- Janero, D.J. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nishimura, N.; Tohyama, C. Immunohistochemical localization of metallothionein in developing rat tissues. J. Histochem. Cytochem. 1989, 37, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Nartey, N.; Banerjee, D.; Cherian, M. Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of fetal human liver and kidney and its changes during development. Pathology 1987, 19, 233–238. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Suzuki, N.; Sagawa, K.; Itoh, M.; Sugiyama, T.; Kohama, Y.; Otaki, N.; Kimura, M.; Mimura, T. Induction and subcellular localization of metallothionein in regenerating rat liver. Eur. J. Cell Biol. 1994, 63, 240–246. [Google Scholar]
- Lei, G.J.; Yamaji, N.; Ma, J.F. Two metallothionein genes highly expressed in rice nodes are involved in distribution of Zn to the grain. New Phytol. 2021, 229, 1007–1020. [Google Scholar] [CrossRef]
- Ota, M.; Gonja, H.; Koike, R.; Fukuchi, S. Multiple-localization and hub proteins. PLoS ONE 2016, 11, e0156455. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Cui, M.; Ni, L.; Qin, Y.; Li, J.; Pan, Y.; Zhang, X. Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium. Genes 2022, 13, 2413. https://doi.org/10.3390/genes13122413
Zheng Y, Cui M, Ni L, Qin Y, Li J, Pan Y, Zhang X. Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium. Genes. 2022; 13(12):2413. https://doi.org/10.3390/genes13122413
Chicago/Turabian StyleZheng, Yilin, Meng Cui, Lei Ni, Yafei Qin, Jinhua Li, Yu Pan, and Xingguo Zhang. 2022. "Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium" Genes 13, no. 12: 2413. https://doi.org/10.3390/genes13122413
APA StyleZheng, Y., Cui, M., Ni, L., Qin, Y., Li, J., Pan, Y., & Zhang, X. (2022). Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium. Genes, 13(12), 2413. https://doi.org/10.3390/genes13122413






