Identification of Reliable Reference Genes under Different Stresses and in Different Tissues of Toxicodendron succedaneum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Total RNA Isolation and cDNA Synthesis
2.3. Screening of Candidate RGs and Primers Design
2.4. RT-qPCR
2.5. Assessing the Stability of Candidate Genes Expression
3. Results
3.1. Specificity and Amplification Efficiency of Candidate RGs
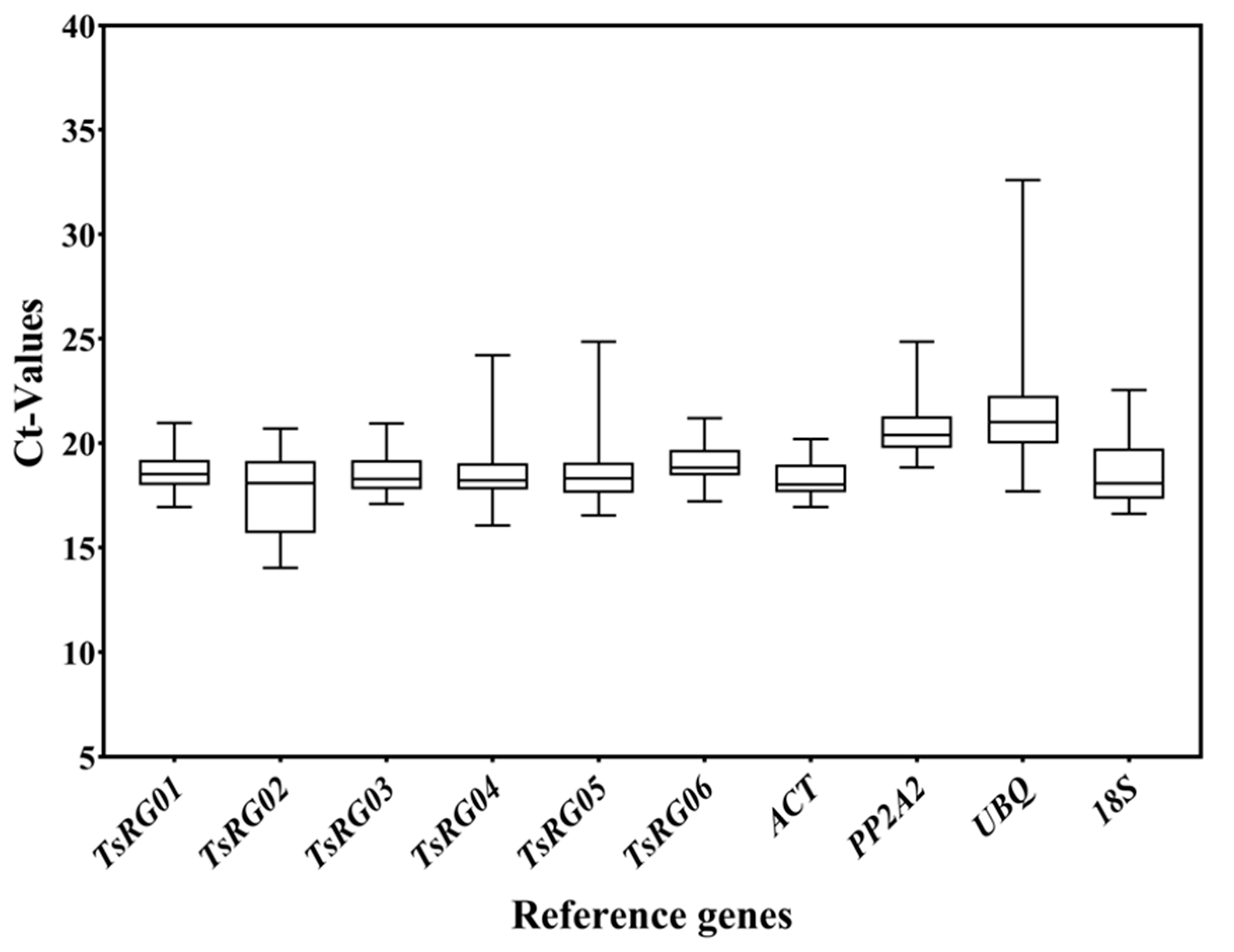
3.2. Expression Profiling of Candidate RGs
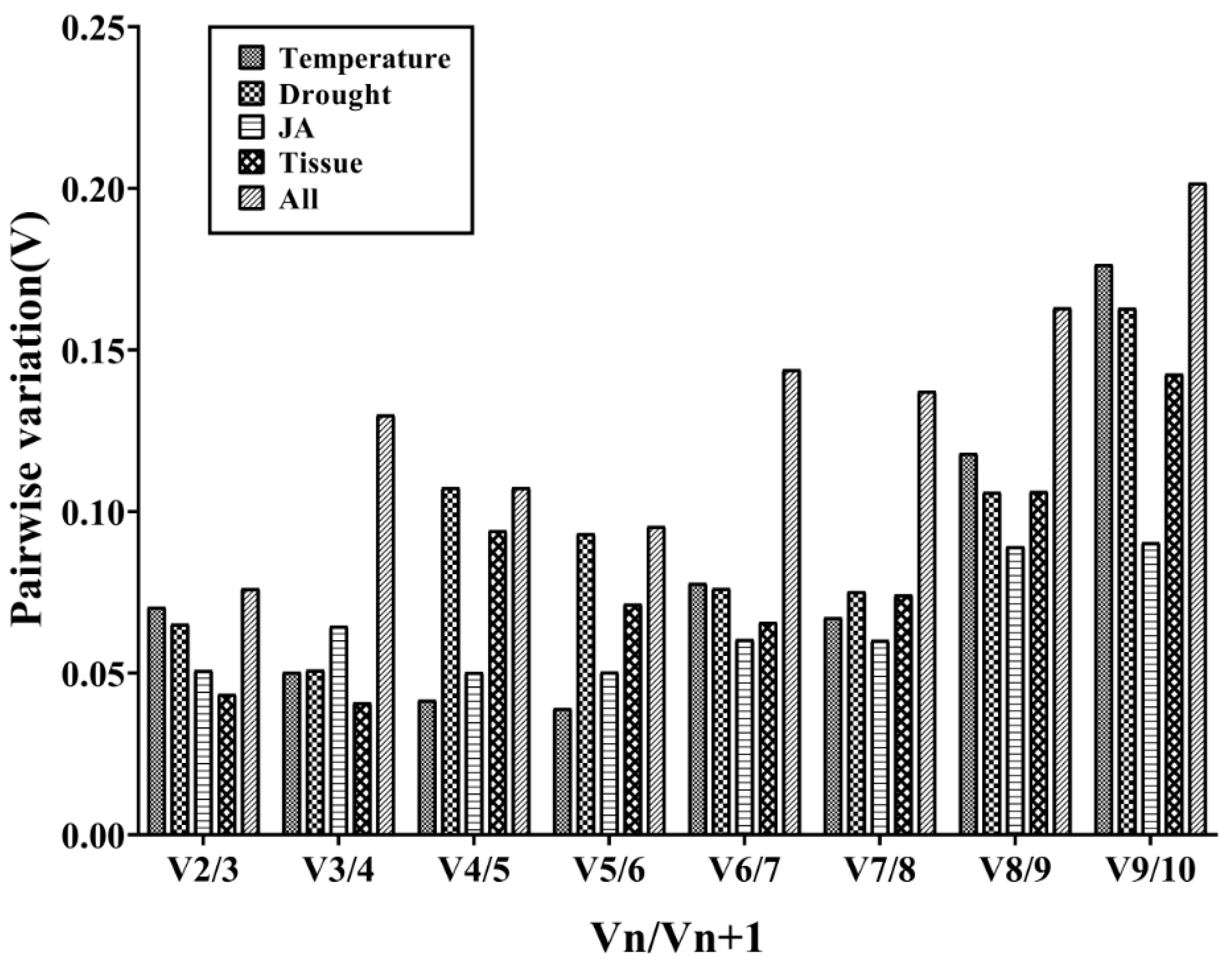
3.3. The Stability of Candidate RGs Was Analyzed by Genorm Software
3.4. The Stability of Candidate RGs Was Analyzed by Normfinder Software
3.5. The Stability of Candidate RGs Was Analyzed by Bestkeeper Software
3.6. The Stability of Candidate RGs Was Analyzed by ∆Ct Value Method
3.7. The Stability of Candidate RGs Was Comprehensive Analyzed by RefFinder Software
4. Discussion
4.1. Potential RGs Were Identified from the Transcriptome Data
4.2. Identification and Selection of RGs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciesielska, A.; Stączek, P. Selection and validation of RGs for qRT-PCR analysis of gene expression in Microsporum canis growing under different adhesion-inducing conditions. Sci. Rep. 2018, 8, 1197. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, X.; Yuan, S.; Dai, L.; Dong, S.; Liu, J.; Peng, L.; Wang, M.; Tang, Y.; Xiao, Y. Optimal RGs for gene expression analysis in polyploid of Cyprinus carpio and Carassius auratus. BMC Genet. 2020, 21, 107. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, R.; Zhou, Z. Identification and Validation of RGs for Gene Expression Analysis in Schima superba. Genes 2021, 12, 732. [Google Scholar] [CrossRef]
- Ahmed, U.; Xie, Q.; Shi, X.; Zheng, B. Development of RGs for Horticultural Plants. Crit. Rev. Plant Sci. 2022, 41, 190–208. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, F.; Shi, D.; Wang, Y.; Xu, F.; Zeng, S. Selection and validation of RGs for RT-qPCR analysis in Desmodium styracifolium Merr. 3 Biotech 2021, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Guénin, S.; Mauriat, M.; Pelloux, J.; Van Wuytswinkel, O.; Bellini, C.; Gutierrez, L. Normalization of qRT-PCR data: The necessity of adopting a systematic, experimental conditions-specific, validation of references. J. Exp. Bot. 2009, 60, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dong, Y.; Yu, Y.; Xing, Y.; Li, X.; Zhang, X.; Hou, X.; Sun, X. Identification and evaluation of reliable RGs for quantitative real-time PCR analysis in tea plants under differential biotic stresses. Sci. Rep. 2020, 10, 2429. [Google Scholar] [CrossRef]
- Ferradás, Y.; Rey, L.; Martínez, Ó.; Rey, M.; González, M.V. Identification and validation of RGs for accurate normalization of real-time quantitative PCR data in kiwifruit. Plant Physiol. Biochem. 2016, 102, 27–36. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Liu, L.; Li, W.; Wei, Y.; Shi, S. Validation of RGs for RT-qPCR Studies of Gene Expression in Preharvest and Postharvest Longan Fruits under Different Experimental Conditions. Front. Plant Sci. 2016, 7, 780. [Google Scholar] [CrossRef]
- Liu, D.; Shi, L.; Han, C.; Yu, J.; Li, D.; Zhang, Y. Validation of RGs for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 2012, 7, e46451. [Google Scholar] [CrossRef]
- Gantasala, N.P.; Papolu, P.K.; Thakur, P.K.; Kamaraju, D.; Sreevathsa, R.; Rao, U. Selection and validation of RGs for quantitative gene expression studies by real-time PCR in eggplant (Solanum melongena L). BMC Res. Notes 2013, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pardo, R.; Ruiz De Galarreta, J.I.; Ritter, E. Selection of housekeeping genes for qRT-PCR analysis in potato tubers under cold stress. Mol. Breed. 2013, 31, 39–45. [Google Scholar] [CrossRef]
- Chen, X.; Truksa, M.; Shah, S.; Weselake, R.J. A survey of quantitative real-time polymerase chain reaction internal RGs for expression studies in Brassica napus. Anal. Biochem. 2010, 405, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Artico, S.; Nardeli, S.M.; Brilhante, O.; Grossi-De-Sa, M.F.; Alves-Ferreira, M. Identification and evaluation of new RGs in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC Plant Biol. 2010, 10, 49. [Google Scholar] [CrossRef]
- Barsalobres-Cavallari, C.F.; Severino, F.E.; Maluf, M.P.; Maia, I.G. Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol. Biol. 2009, 10, 1. [Google Scholar] [CrossRef]
- Die, J.V.; Román, B.; Nadal, S.; González-Verdejo, C.I. Evaluation of candidate RGs for expression studies in Pisum sativum under different experimental conditions. Planta 2010, 232, 145–153. [Google Scholar] [CrossRef]
- Luo, H.; Chen, S.; Wan, H.; Chen, F.; Gu, C.; Liu, Z. Candidate RGs for gene expression studies in water lily. Anal. Biochem. 2010, 404, 100–102. [Google Scholar] [CrossRef]
- Joseph, J.T.; Poolakkalody, N.J.; Shah, J.M. Plant RGs for development and stress response studies. J. Biosci. 2018, 43, 173–187. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Zhao, L.; Shi, D.; She, Z.; Huang, X.; Priyadarshani, S.V.G.N.; Niu, X.; Qin, Y. Differential Expression Analysis of RGs in Pineapple (Ananas comosus L.) during Reproductive Development and Response to Abiotic Stress, Hormonal Stimuli. Trop. Plant Biol. 2019, 12, 67–77. [Google Scholar] [CrossRef]
- Fan, C.; Qiu, Z.; Zeng, B.; Liu, Y.; Li, X.; Guo, G. Selection of RGs for quantitative real-time PCR in Casuarina equisetifolia under salt stress. Biol. Plant. 2017, 61, 463–472. [Google Scholar] [CrossRef]
- Zheng, T.; Chen, Z.; Ju, Y.; Zhang, H.; Cai, M.; Pan, H.; Zhang, Q. Reference gene selection for qRT-PCR analysis of flower development in Lagerstroemia indica and L. speciosa. PLoS ONE 2018, 13, e195004. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F.; Jiang, Q.; Wang, G.; Tian, C.; Xiong, A. Validation and Comparison of RGs for qPCR Normalization of Celery (Apium graveolens) at Different Development Stages. Front. Plant Sci. 2016, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xu, S.; Zhao, Y.; Xia, B.; Wang, R. Selection and Validation of Appropriate RGs for Quantitative Real-Time PCR Analysis of Gene Expression in Lycoris aurea. Front. Plant Sci. 2016, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mauriat, M.; Guénin, S.; Pelloux, J.; Lefebvre, J.F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C.; et al. The lack of a systematic validation of RGs: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, M.; Cao, S.; Sun, Y.; Long, R.; Kang, J.; Yan, L.; Cui, H. Selection and validation of RGs for target gene analysis with quantitative real-time PCR in the leaves and roots of Carex rigescens under abiotic stress. Ecotoxicol. Environ. Saf. 2019, 168, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, Y.; Tamaki, I.; Watanabe, A. The origin of wild populations of Toxicodendron succedaneum on mainland Japan revealed by genetic variation in chloroplast and nuclear DNA. J. Plant Res. 2018, 131, 225–238. [Google Scholar] [CrossRef]
- Jun, T.; Kun, Y.S.; Zhi, C.F.; Wei, C.Z.; Hua, Z.J.; Yun, X.D. Research progress on Toxicodendron succedaneum. Hubei Agric. Sci. 2019, 58, 16–20. [Google Scholar] [CrossRef]
- Nitta, K.; Kataoka, K.; Sakurai, T. Primary structure of a Japanese lacquer tree laccase as a prototype enzyme of multicopper oxidases. J. Inorg. Biochem. 2002, 91, 125–131. [Google Scholar] [CrossRef]
- Wu, P.L.; Lin, S.B.; Huang, C.P.; Chiou, R.Y. Antioxidative and cytotoxic compounds extracted from the sap of Rhus succedanea. J. Nat. Prod. 2002, 65, 1719–1721. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, H34. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Howe, G.A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 2005, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xiong, Y.; Guo, W.; Du, H. ERgene: Python library for screening endogenous RGs. Sci. Rep. 2020, 10, 18557. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, M.; Meng, Y. Identification and Validation of RGs for RT-qPCR Analysis in Switchgrass under Heavy Metal Stresses. Genes 2020, 11, 502. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Morales, M.A.; Mendoza, B.M.; Lavine, L.C.; Lavine, M.D.; Walsh, D.B.; Zhu, F. Selection of RGs for Expression Studies of Xenobiotic Adaptation in Tetranychus urticae. Int. J. Biol. Sci. 2016, 12, 1129–1139. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Patankar, H.V.; Assaha, D.V.M.; Al-Yahyai, R.; Sunkar, R.; Yaish, M.W. Identification of RGs for Quantitative Real-Time PCR in Date Palm (Phoenix dactylifera L.) Subjected to Drought and Salinity. PLoS ONE 2016, 11, e166216. [Google Scholar] [CrossRef] [PubMed]
- Auler, P.A.; Benitez, L.C.; Do, A.M.; Vighi, I.L.; Dos, S.R.G.; Da, M.L.; Braga, E.J. Evaluation of stability and validation of RGs for RT-qPCR expression studies in rice plants under water deficit. J. Appl. Genet. 2017, 58, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, T.; Chen, L.; Wu, H.; Zhang, S. Selection and Validation of RGs for qRT-PCR in Cycas elongata. PLoS ONE 2016, 11, e154384. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Lan, H. Validation of RGs for quantitative RT-PCR normalization in Suaeda aralocaspica, an annual halophyte with heteromorphism and C4 pathway without Kranz anatomy. Peerj 2016, 4, e1697. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and evaluation of reliable RGs for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef]
- Burke, T.J.; Callis, J.; Vierstra, R.D. Characterization of a polyubiquitin gene from Arabidopsis thaliana. Mol. Gen. Genet. 1988, 213, 435–443. [Google Scholar] [CrossRef]
- Ammar, M.H.; Khan, A.M.; Migdadi, H.M.; Abdelkhalek, S.M.; Alghamdi, S.S. Faba bean drought responsive gene identification and validation. Saudi J. Biol. Sci. 2017, 24, 80–89. [Google Scholar] [CrossRef]

| RGs | Product Size (bp) | Primer Sequences (5′-3′) | R2 | Efficiency (%) |
|---|---|---|---|---|
| TsRG01 | 177 | F-TTGGGCAGCCGTTGATCTT R-GCTTAGGGTTTCTCCTCTCCTT | 0.99943 | 98.11 |
| TsRG02 | 228 | F-GCCTCAAAGCCAGGTAAG R-AGAGTGCCGAAATCATCG | 0.99908 | 102.32 |
| TsRG03 | 134 | F-TCTGCTGCCTTCTCATCCTC R-GTGGTACGAATGCGTGTCTT | 0.99931 | 105.47 |
| TsRG04 | 245 | F-CTGTCTCACTTGCTGCGGCTAG R-GCACCCAGGCGTACTTGAATGA | 0.99913 | 94.94 |
| TsRG05 | 237 | F-CCACAAGTCCAGGGAATGCT R-AGGGAGTGAAGACGGAAACG | 0.99898 | 107.71 |
| TsRG06 | 122 | F-TTCACAAAGCGGGTCTCCC R-TTCACTTCCTCACGTGGGTC | 0.99897 | 108.57 |
| ACT | 188 | F-AACTCTCCACCTCGTCCTCC R-TGGACCAGACTCGTCGTACT | 0.99958 | 103.29 |
| PP2A2 | 161 | F-CCCTGTGACAATTTGTGGCG R-TAAGGGCCACTAACAGCGTG | 0.99980 | 94.12 |
| UBQ | 200 | F-GGGTCCTCCCATCCTCAAGT R-AACTCTCCACCTCGTCCTCC | 0.99901 | 94.13 |
| 18S | 209 | F-GAAGAGCATCCAGTGCTT R-TGCATCGGTGAGATCACG | 0.99927 | 101.17 |
| Rank | Temperature | Drought | JA | Tissues | All | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | |
| 1 | PP2A2 | 0.12 | PP2A2 | 0.27 | TsRG03 | 0.05 | PP2A2 | 0.11 | TsRG03 | 0.08 |
| 2 | 18S | 0.22 | 18S | 0.38 | TsRG02 | 0.11 | TsRG04 | 0.29 | ACT | 0.13 |
| 3 | TsRG05 | 0.24 | ACT | 0.39 | ACT | 0.2 | TsRG05 | 0.34 | TsRG01 | 0.13 |
| 4 | TsRG04 | 0.26 | TsRG03 | 0.43 | TsRG06 | 0.20 | 18S | 0.35 | TsRG05 | 0.30 |
| 5 | TsRG03 | 0.34 | TsRG04 | 0.45 | TsRG01 | 0.21 | TsRG03 | 0.39 | PP2A2 | 0.36 |
| 6 | ACT | 0.42 | TsRG05 | 0.47 | 18S | 0.28 | ACT | 0.44 | 18S | 0.48 |
| 7 | TsRG06 | 0.45 | TsRG06 | 0.47 | TsRG04 | 0.38 | TsRG01 | 0.46 | TsRG06 | 1.01 |
| 8 | TsRG01 | 0.46 | TsRG01 | 0.49 | TsRG05 | 0.57 | TsRG06 | 0.46 | TsRG02 | 1.08 |
| 9 | TsRG02 | 0.99 | TsRG02 | 0.86 | PP2A2 | 0.76 | TsRG02 | 0.88 | UBQ | 1.59 |
| 10 | UBQ | 1.76 | UBQ | 1.62 | UBQ | 0.90 | UBQ | 1.42 | TsRG04 | 2.00 |
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | gene | TsRG06 | TsRG01 | ACT | TsRG03 | TsRG04 | TsRG05 | PP2A2 | 18S | TsRG02 | UBQ |
| SD | 0.72 | 0.74 | 0.83 | 0.86 | 0.93 | 0.94 | 1.27 | 1.40 | 1.65 | 2.05 | |
| CV | 3.72 | 3.92 | 4.52 | 4.57 | 5.01 | 5.03 | 6.09 | 7.42 | 9.15 | 9.35 | |
| Drought | gene | TsRG01 | TsRG06 | TsRG03 | TsRG04 | ACT | TsRG05 | PP2A2 | 18S | TsRG02 | UBQ |
| SD | 0.65 | 0.65 | 0.66 | 0.74 | 0.74 | 0.74 | 0.95 | 1.22 | 1.47 | 1.89 | |
| CV | 3.47 | 3.42 | 3.56 | 3.99 | 4.05 | 4.00 | 4.55 | 6.40 | 8.17 | 8.45 | |
| JA | gene | TsRG05 | TsRG04 | TsRG06 | TsRG01 | TsRG03 | ACT | TsRG02 | 18S | PP2A2 | UBQ |
| SD | 0.40 | 0.64 | 0.67 | 0.74 | 0.77 | 0.92 | 0.93 | 0.97 | 1.30 | 1.43 | |
| CV | 2.33 | 3.80 | 3.71 | 4.17 | 4.27 | 5.13 | 6.09 | 5.50 | 6.11 | 7.28 | |
| Tissues | gene | TsRG01 | TsRG06 | TsRG03 | ACT | TsRG05 | TsRG04 | PP2A2 | 18S | TsRG02 | UBQ |
| SD | 0.67 | 0.70 | 0.72 | 0.73 | 0.81 | 0.84 | 1.05 | 1.32 | 1.51 | 1.82 | |
| CV | 3.59 | 3.64 | 3.88 | 3.99 | 4.33 | 4.50 | 5.05 | 6.99 | 8.41 | 8.36 | |
| All | gene | TsRG01 | TsRG06 | TsRG03 | ACT | TsRG05 | TsRG04 | PP2A2 | 18S | TsRG02 | UBQ |
| SD | 0.72 | 0.73 | 0.75 | 0.76 | 0.85 | 0.88 | 1.09 | 0.33 | 1.71 | 1.79 | |
| CV | 3.88 | 3.83 | 4.04 | 4.19 | 4.60 | 4.80 | 5.23 | 7.12 | 9.70 | 8.31 | |
| Rank | Temperature | Drought | JA | Tissues | All | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | |
| 1 | TsRG05 | 0.57 | ACT | 0.66 | TsRG03 | 0.42 | TsRG03 | 0.59 | TsRG03 | 0.85 |
| 2 | TsRG04 | 0.59 | TsRG03 | 0.67 | TsRG02 | 0.44 | PP2A2 | 0.62 | ACT | 0.87 |
| 3 | TsRG03 | 0.61 | TsRG06 | 0.68 | TsRG01 | 0.44 | TsRG04 | 0.62 | TsRG01 | 0.87 |
| 4 | TsRG06 | 0.62 | PP2A2 | 0.70 | ACT | 0.44 | ACT | 0.62 | TsRG05 | 0.93 |
| 5 | ACT | 0.63 | TsRG01 | 0.70 | TsRG06 | 0.46 | TsRG06 | 0.62 | PP2A2 | 0.93 |
| 6 | TsRG01 | 0.65 | TsRG04 | 0.76 | 18S | 0.48 | TsRG01 | 0.62 | 18S | 0.97 |
| 7 | PP2A2 | 0.68 | 18S | 0.77 | TsRG04 | 0.54 | TsRG05 | 0.65 | TsRG06 | 1.32 |
| 8 | 18S | 0.71 | TsRG05 | 0.79 | TsRG05 | 0.66 | 18S | 0.71 | TsRG02 | 1.34 |
| 9 | TsRG02 | 1.17 | TsRG02 | 1.07 | PP2A2 | 0.84 | TsRG02 | 1.05 | UBQ | 1.72 |
| 10 | UBQ | 1.80 | UBQ | 1.68 | UBQ | 0.97 | UBQ | 1.47 | TsRG04 | 2.09 |
| A. Ranking Order under High/Low Temperature Stress (Better–Good–Average) | ||||||
| Ranking | ∆Ct | geNorm | Normfinder | BestKeeper | Comprehensive ranking | Ranking values |
| 1 | TsRG05 | TsRG06|ACT | PP2A2 | TsRG01 | TsRG05 | 2.59 |
| 2 | TsRG04 | - | 18S | TsRG06 | TsRG06 | 2.74 |
| 3 | TsRG03 | TsRG05 | TsRG05 | ACT | ACT | 3.08 |
| 4 | TsRG06 | TsRG04 | TsRG04 | TsRG04 | TsRG04 | 3.36 |
| 5 | ACT | TsRG01 | TsRG03 | TsRG05 | TsRG01 | 3.94 |
| 6 | TsRG01 | TsRG03 | ACT | TsRG03 | PP2A2 | 4.30 |
| 7 | PP2A2 | PP2A2 | TsRG06 | PP2A2 | TsRG03 | 4.82 |
| 8 | 18S | 18S | TsRG01 | 18S | 18S | 5.66 |
| 9 | TsRG02 | TsRG02 | TsRG02 | TsRG02 | TsRG02 | 9.00 |
| 10 | UBQ | UBQ | UBQ | UBQ | UBQ | 10.00 |
| B. Ranking Order under drought stress (Better–Good–Average) | ||||||
| Ranking | ∆Ct | geNorm | Normfinder | BestKeeper | Comprehensive ranking | Ranking values |
| 1 | ACT | TsRG06|TsRG01 | PP2A2 | TsRG01 | TsRG01 | 2.51 |
| 2 | TsRG03 | - | 18S | TsRG06 | TsRG06 | 2.55 |
| 3 | TsRG06 | TsRG03 | ACT | TsRG03 | ACT | 2.78 |
| 4 | PP2A2 | ACT | TsRG03 | TsRG04 | TsRG03 | 2.91 |
| 5 | TsRG01 | PP2A2 | TsRG04 | ACT | PP2A2 | 3.44 |
| 6 | TsRG04 | TsRG04 | TsRG05 | TsRG05 | TsRG04 | 5.18 |
| 7 | 18S | TsRG05 | TsRG06 | PP2A2 | 18S | 5.47 |
| 8 | TsRG05 | 18S | TsRG01 | 18S | TsRG05 | 6.70 |
| 9 | TsRG02 | TsRG02 | TsRG02 | TsRG02 | TsRG02 | 9.00 |
| 10 | UBQ | UBQ | UBQ | UBQ | UBQ | 10.00 |
| C. Ranking Order under JA stress (Better–Good–Average) | ||||||
| Ranking | ∆Ct | geNorm | Normfinder | BestKeeper | Comprehensive ranking | Ranking values |
| 1 | TsRG03 | ACT|18S | TsRG03 | TsRG05 | TsRG03 | 2.24 |
| 2 | TsRG02 | - | TsRG02 | TsRG04 | ACT | 2.91 |
| 3 | TsRG01 | TsRG02 | ACT | TsRG06 | TsRG02 | 3.03 |
| 4 | ACT | TsRG01 | TsRG06 | TsRG01 | TsRG01 | 3.94 |
| 5 | TsRG06 | TsRG03 | TsRG01 | TsRG03 | 18S | 4.12 |
| 6 | 18S | TsRG06 | 18S | ACT | TsRG06 | 4.36 |
| 7 | TsRG04 | TsRG04 | TsRG04 | TsRG02 | TsRG05 | 4.76 |
| 8 | TsRG05 | TsRG05 | TsRG05 | 18S | TsRG04 | 5.12 |
| 9 | PP2A2 | PP2A2 | PP2A2 | PP2A2 | PP2A2 | 9.00 |
| 10 | UBQ | UBQ | UBQ | UBQ | UBQ | 10.00 |
| D. Ranking Order under different tissues (Better–Good–Average) | ||||||
| Ranking | ∆Ct | geNorm | Normfinder | BestKeeper | Comprehensive ranking | Ranking values |
| 1 | TsRG03 | TsRG03|ACT | PP2A2 | TsRG01 | TsRG03 | 1.97 |
| 2 | PP2A2 | - | TsRG04 | TsRG06 | ACT | 3.13 |
| 3 | TsRG04 | TsRG06 | TsRG05 | TsRG03 | PP2A2 | 3.15 |
| 4 | ACT | TsRG01 | 18S | ACT | TsRG01 | 3.6 |
| 5 | TsRG06 | TsRG04 | TsRG03 | TsRG05 | TsRG04 | 3.66 |
| 6 | TsRG01 | TsRG05 | ACT | TsRG04 | TsRG06 | 3.94 |
| 7 | TsRG05 | PP2A2 | TsRG01 | PP2A2 | TsRG05 | 5.01 |
| 8 | 18S | 18S | TsRG06 | 18S | 18S | 6.73 |
| 9 | TsRG02 | TsRG02 | TsRG02 | TsRG02 | TsRG02 | 9.00 |
| 10 | UBQ | UBQ | UBQ | UBQ | UBQ | 10.00 |
| E. Ranking Order under all samples (Better–Good–Average) | ||||||
| Ranking | ∆Ct | geNorm | Normfinder | BestKeeper | Comprehensive ranking | Ranking values |
| 1 | TsRG03 | TsRG03|ACT | TsRG03 | TsRG01 | TsRG03 | 1.32 |
| 2 | ACT | - | ACT | TsRG06 | ACT | 2.00 |
| 3 | TsRG01 | TsRG01 | TsRG01 | TsRG03 | TsRG01 | 2.28 |
| 4 | TsRG05 | TsRG05 | TsRG05 | ACT | TsRG05 | 4.23 |
| 5 | PP2A2 | PP2A2 | PP2A2 | TsRG05 | TsRG06 | 5.12 |
| 6 | 18S | 18S | 18S | TsRG04 | PP2A2 | 5.44 |
| 7 | TsRG06 | TsRG06 | TsRG06 | PP2A2 | 18S | 6.45 |
| 8 | TsRG02 | TsRG02 | TsRG02 | 18S | TsRG02 | 8.24 |
| 9 | UBQ | UBQ | UBQ | TsRG02 | TsRG04 | 8.80 |
| 10 | TsRG04 | TsRG04 | TsRG04 | UBQ | UBQ | 9.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Zhang, Q.; Zhou, J.; Lu, Y.; Duan, X.; He, C.; Yu, J. Identification of Reliable Reference Genes under Different Stresses and in Different Tissues of Toxicodendron succedaneum. Genes 2022, 13, 2396. https://doi.org/10.3390/genes13122396
Ma D, Zhang Q, Zhou J, Lu Y, Duan X, He C, Yu J. Identification of Reliable Reference Genes under Different Stresses and in Different Tissues of Toxicodendron succedaneum. Genes. 2022; 13(12):2396. https://doi.org/10.3390/genes13122396
Chicago/Turabian StyleMa, Dongxiao, Qin Zhang, Jintao Zhou, Yu Lu, Xiaomeng Duan, Chengzhong He, and Jinde Yu. 2022. "Identification of Reliable Reference Genes under Different Stresses and in Different Tissues of Toxicodendron succedaneum" Genes 13, no. 12: 2396. https://doi.org/10.3390/genes13122396
APA StyleMa, D., Zhang, Q., Zhou, J., Lu, Y., Duan, X., He, C., & Yu, J. (2022). Identification of Reliable Reference Genes under Different Stresses and in Different Tissues of Toxicodendron succedaneum. Genes, 13(12), 2396. https://doi.org/10.3390/genes13122396





