Insight into Glyproline Peptides’ Activity through the Modulation of the Inflammatory and Neurosignaling Genetic Response Following Cerebral Ischemia–Reperfusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Rat Transient Middle Cerebral Artery Occlusion Model
2.2.1. Operation
2.2.2. Peptide Administration
2.3. Sample Collection and RNA Isolation
2.4. cDNA Synthesis and Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Data Analysis of Real-Time RT-PCR and Statistics
2.6. Functional Analysis
3. Results
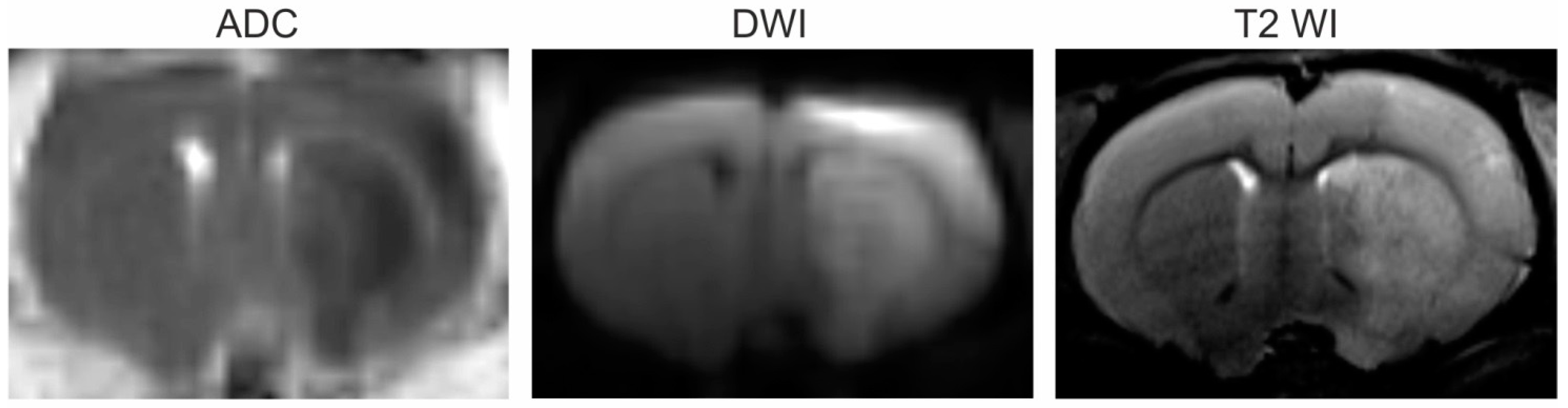
3.1. Magnetic Resonance Imaging (MRI)
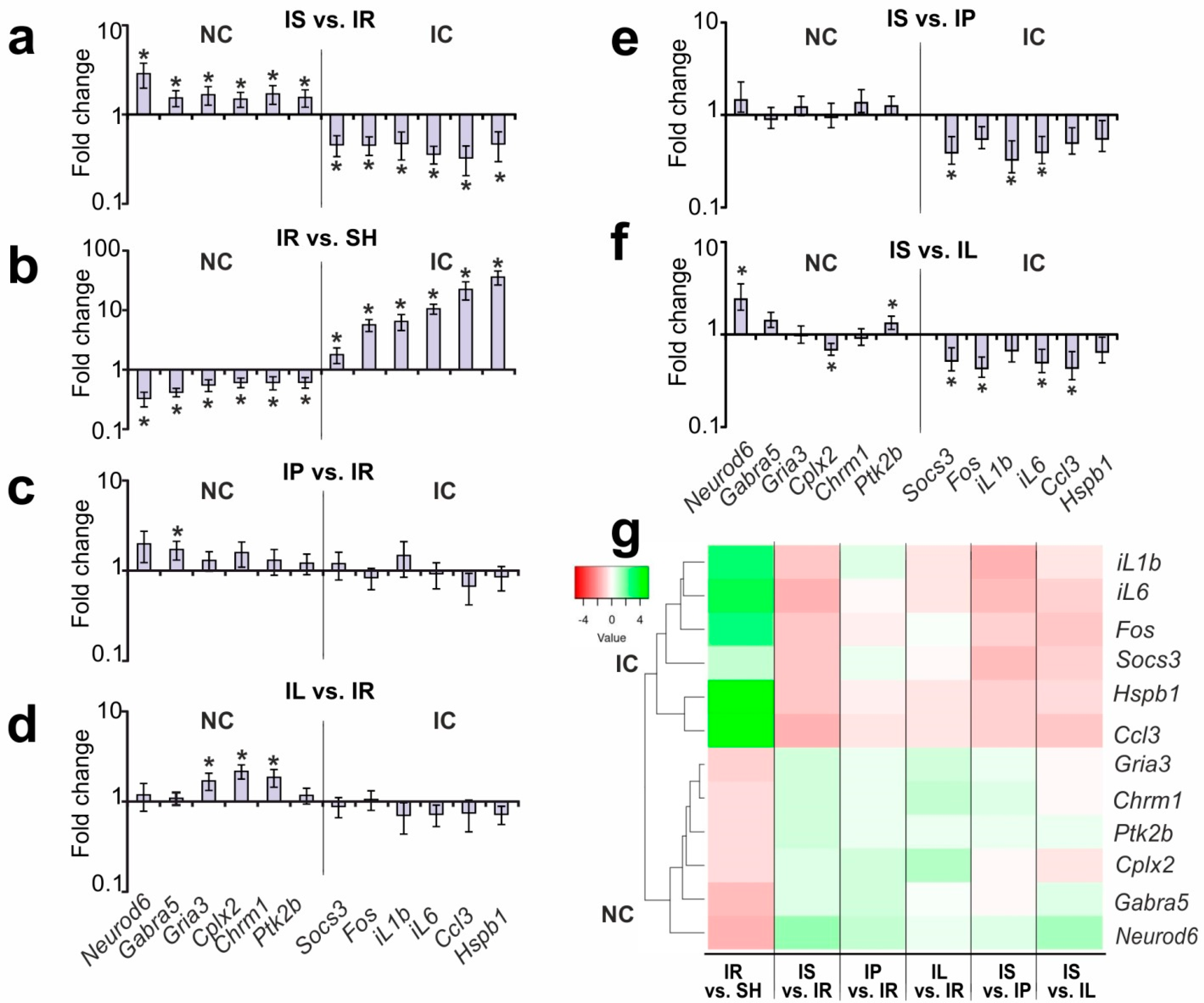
3.2. Selection of Genes for Studying PGP-Containing Peptides’ Action under Ischemia–Reperfusion (IR) Conditions Based on Our Previous Results
3.3. Analysis of the Effects of PGP and PGPL on the Expression of the IC and NC Genes versus IR’s Effect 24 h after tMCAO
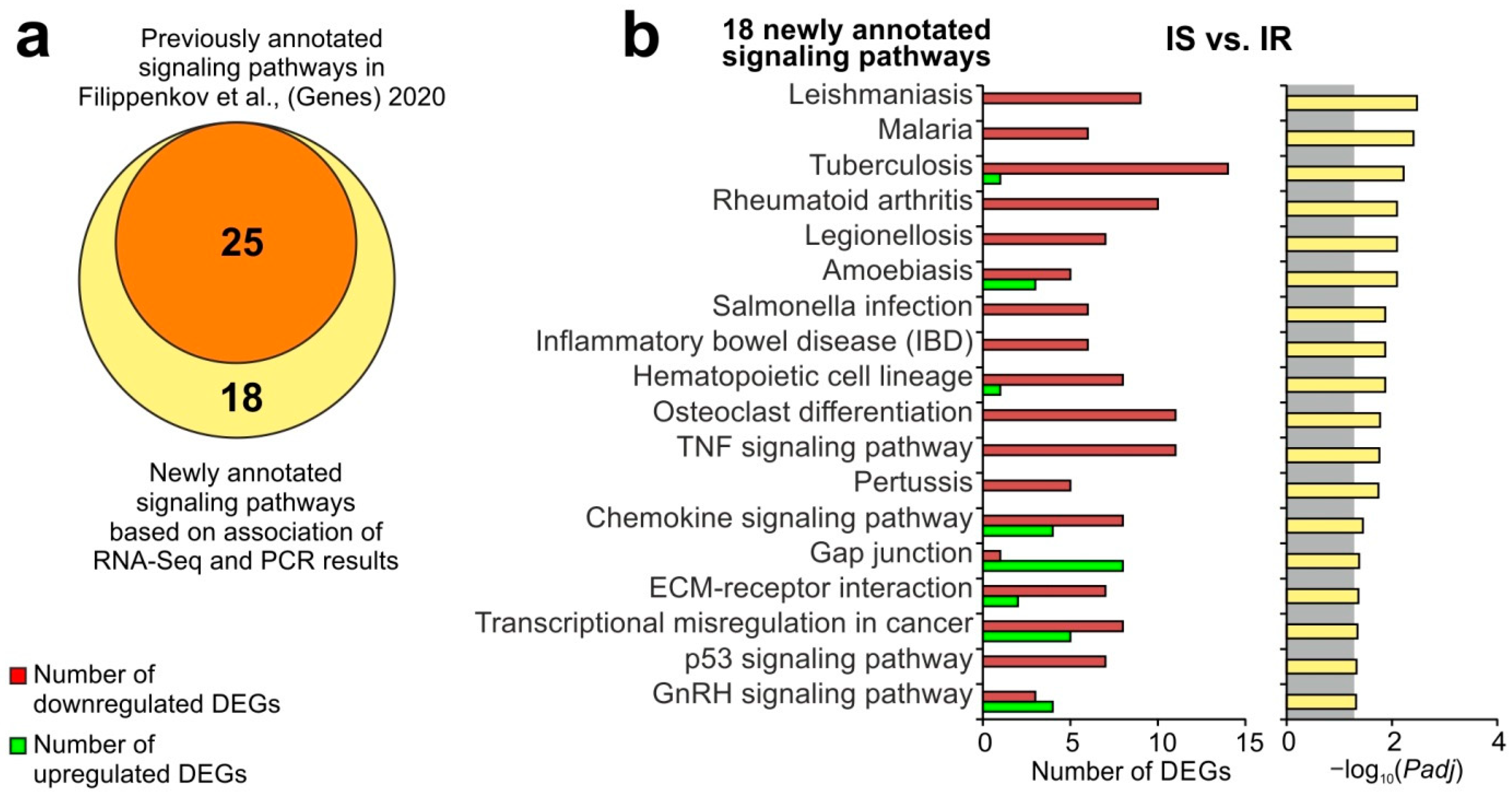
3.4. A Search for DEGs Associated with Semax Treatment Compared to PGP and PGPL Treatment under tMCAO Conditions
3.5. Update of Signaling Pathways Associated with Semax Treatment Based on the Combination of RNA-Seq and PCR Results under the tMCAO Conditions
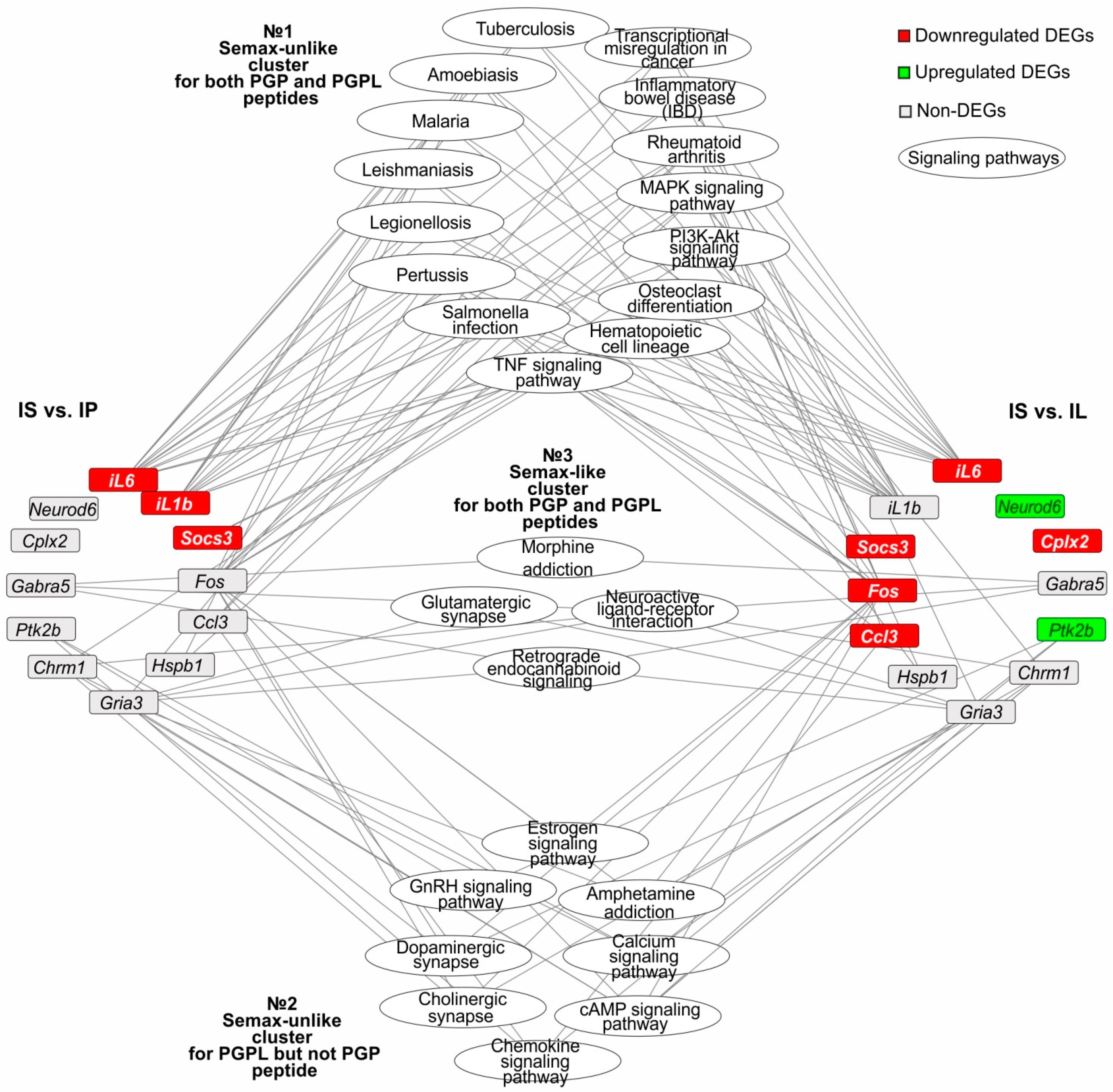
3.6. The Search for Signaling Pathways That Reflect Common and Unique Gene Expression Effects of PGP, PGPL, and Semax Peptides in IR Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samonina, G.; Ashmarin, I.; Lyapina, L. Glyproline peptide family: Review on bioactivity and possible origins. Pathophysiology 2002, 8, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ashmarin, I.P.; Karazeeva, E.P.; Lyapina, L.A.; Samonina, G.E. The simplest proline-containing peptides PG, GP, PGP, and GPGG: Regulatory activity and possible sources of biosynthesis. Biochemistry 1998, 63, 119–124. [Google Scholar] [PubMed]
- O’Reilly, P.; Jackson, P.L.; Noerager, B.; Parker, S.; Dransfield, M.; Gaggar, A.; Blalock, J.E. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir. Res. 2009, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaggar, A.; Jackson, P.L.; Noerager, B.D.; O’Reilly, P.J.; McQuaid, D.B.; Rowe, S.M.; Clancy, J.P.; Blalock, J.E. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 2008, 180, 5662–5669. [Google Scholar] [CrossRef] [Green Version]
- Misiura, M.; Miltyk, W. Proline-containing peptides-New insight and implications: A Review. Biofactors 2019, 45, 857–866. [Google Scholar] [CrossRef]
- Ashmarin, I.P.; Samonina, G.E.; Lyapina, L.A.; Kamenskii, A.A.; Levitskaya, N.G.; Grivennikov, I.A.; Dolotov, O.V.; Andreeva, L.A.; Myasoedov, N.F. Natural and hybrid (“chimeric”) stable regulatory glyproline peptides. Pathophysiology 2005, 11, 179–185. [Google Scholar] [CrossRef]
- Ashmarin, I.P. Glyprolines in regulatory tripeptides. Neurochem. J. 2007, 1, 173–175. [Google Scholar] [CrossRef]
- Asmarin, I.P.; Nezavibat’ko, V.N.; Miasoedov, N.F.; Kamenskiĭ, A.A.; Grivennikov, I.A.; Ponomareva-Stepnaia, M.A.; Andreeva, L.A.; Kaplan, A.I.; Koshelev, V.B.; Riasina, T. V [A nootropic adrenocorticotropin analog 4-10-semax (l5 years experience in its design and study)]. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 1997, 47, 420–430. [Google Scholar]
- de Wied, D. Neuropeptides in learning and memory processes. Behav. Brain Res. 1997, 83, 83–90. [Google Scholar] [CrossRef]
- Kaplan, A.I.; Koshelev, V.B.; Nezavibat’ko, V.N.; Ashmarin, I.P. Increased resistance to hypoxia effected by the neuropeptide preparation SEMAX. Fiziol. Cheloveka 1992, 18, 104–107. [Google Scholar]
- Storozhevykh, T.P.; Tukhbatova, G.R.; Senilova, Y.E.; Pinelis, V.G.; Andreeva, L.A.; Myasoyedov, N.F. Effects of semax and its Pro-Gly-Pro fragment on calcium homeostasis of neurons and their survival under conditions of glutamate toxicity. Bull. Exp. Biol. Med. 2007, 143, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Inozemtsev, A.N.; Bokieva, S.B.; Karpukhina, O.V.; Gumargalieva, K.Z.; Kamensky, A.A.; Myasoedov, N.F. Semax prevents learning and memory inhibition by heavy metals. Dokl. Biol. Sci. 2016, 468, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Bashkatova, V.G.; Koshelev, V.B.; Fadyukova, O.E.; Alexeev, A.A.; Vanin, A.F.; Rayevsky, K.S.; Ashmarin, I.P.; Armstrong, D.M. Novel synthetic analogue of ACTH 4–10 (Semax) but not glycine prevents the enhanced nitric oxide generation in cerebral cortex of rats with incomplete global ischemia. Brain Res. 2001, 894, 145–149. [Google Scholar] [CrossRef]
- Tabbì, G.; Magrì, A.; Giuffrida, A.; Lanza, V.; Pappalardo, G.; Naletova, I.; Nicoletti, V.G.; Attanasio, F.; Rizzarelli, E. Semax, an ACTH4–10 peptide analog with high affinity for copper(II) ion and protective ability against metal induced cell toxicity. J. Inorg. Biochem. 2015, 142, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel insights into the protective properties of acth(4–7)pgp (semax) peptide at the transcriptome level following cerebral ischaemia– reperfusion in rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Dmitrieva, V.G.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Valieva, L.V.; Sudarkina, O.Y.; Gubsky, L.V.; et al. The Peptide Drug ACTH(4–7)PGP (Semax) Suppresses mRNA Transcripts Encoding Proinflammatory Mediators Induced by Reversible Ischemia of the Rat Brain. Mol. Biol. 2021, 55, 402–411. [Google Scholar] [CrossRef]
- Sudarkina, O.Y.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Valieva, L.V.; Remizova, J.A.; Dmitrieva, V.G.; Gubsky, L.V.; et al. Brain Protein Expression Profile Confirms the Protective Effect of the ACTH(4–7)PGP Peptide (Semax) in a Rat Model of Cerebral Ischemia–Reperfusion. Int. J. Mol. Sci. 2021, 22, 6179. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L.V. Effect of semax and its C-terminal fragment Pro-Gly-Pro on the expression of VEGF family genes and their receptors in experimental focal ischemia of the rat brain. J. Mol. Neurosci. 2013, 49, 328–333. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L.V. The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: Genome-wide transcriptional analysis. BMC Genom. 2014, 15, 228. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, K.V.; Nagaev, I.Y.; Alfeeva, L.Y.; Andreeva, L.A.; Kamenskii, A.A.; Levitskaya, N.G.; Shevchenko, V.P.; Grivennikova, I.A.; Myasoedov, N.F. Kinetics of semax penetration into the brain and blood of rats after its intranasal administration. Russ. J. Bioorg. Chem. 2006, 32, 57–62. [Google Scholar] [CrossRef]
- Dmitrieva, V.G.; Dergunova, L.V.; Povarova, O.V.; Skvortsova, V.I.; Limborskaya, S.A.; Myasoedov, N.F. The effect of semax and the C-terminal peptide PGP on expression of growth factor genes and receptors in rats under conditions of experimental cerebral ischemia. Dokl. Biochem. Biophys. 2008, 422, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Dmitrieva, V.G.; Povarova, O.V.; Limborska, S.A.; Skvortsova, V.I.; Myasoedov, N.F.; Dergunova, L.V. Tripeptide Pro-Gly-Pro affects rat-brain transcriptome during focal ischemia. Mol. Biol. 2014, 48, 238–247. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Dmitrieva, V.G.; Limborska, S.A.; Myasoedov, N.F.; Dergunova, L.V. Semax, an analog of ACTH(4–7), regulates expression of immune response genes during ischemic brain injury in rats. Mol. Genet. Genom. 2017, 292, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Myasoedov, N.F.; Rochev, D.L.; Lyapina, L.A.; Obergan, T.Y.; Andreeva, L.A. Leucine-containing glyprolines (Pro-Gly-Pro-Leu and Leu-Pro-Gly-Pro): Participation in hemostatic reactions in vitro and in vivo in rats with blood coagulation and lipid metabolism disorders. Dokl. Biol. Sci. 2013, 453, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, L.A.; Myasoedov, N.F.; Lyapina, L.A.; Grigor’eva, M.E.; Obergan, T.Y.; Shubina, T.A. Effect of the PRO-GLY-PRO peptide on hemostasis and lipid metabolism in rats with hypercholesterolemia. Dokl. Biol. Sci. 2013, 453, 333–335. [Google Scholar] [CrossRef]
- Lyapina, L.A.; Pastorova, V.E.; Obergan, T.Y. Functional state of the anticoagulation system after the administration of complexes formed by natural heparin and the regulatory peptides: Pro-Gly, Pro-Gly-Pro, Semax and tuftsin. Neurochem. J. 2008, 2, 17–22. [Google Scholar]
- Dergunova, L.V.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Mozerov, S.A.; Gubsky, L.V.; Limborska, S.A. Genome-wide transcriptome analysis using RNA-Seq reveals a large number of differentially expressed genes in a transient MCAO rat model. BMC Genom. 2018, 19, 655. [Google Scholar] [CrossRef] [Green Version]
- Ashmarin, I.; Nezavibatko, V.; Levitskaya, N.; Koshelev, V.; Kamensky, A. Design and Investigation of an ACTH(4-10) Analog Lacking D-Amino Acids and Hydrophobic Radicals. Neurosci. Res. Commun. 1995, 16, 105–112. [Google Scholar]
- Miasoedova, N.F.; Skvortsova, V.I.; Nasonov, E.L.; Zhuravleva, E.I.; Grivennikov, I.A.; Arsen’eva, E.L.; Sukhanov, I.I. Investigation of mechanisms of neuro-protective effect of semax in acute period of ischemic stroke. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 1999, 99, 15–19. [Google Scholar]
- Shubina, T.A.; Grigor’eva, M.E.; Lyapina, L.A.; Obergan, T.Y.; Myasoedov, N.F.; Andreeva, L.A. Hypoglycemic and anticoagulant effects of tetrapeptide Pro-Gly-Pro-Leu in hypercholesterolemia. Bull. Exp. Biol. Med. 2014, 158, 30–33. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Stavchansky, V.V.; Glazova, N.Y.; Sebentsova, E.A.; Remizova, J.A.; Valieva, L.V.; Levitskaya, N.G.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L.V. Antistress action of melanocortin derivatives associated with correction of gene expression patterns in the hippocampus of male rats following acute stress. Int. J. Mol. Sci. 2021, 22, 34576218. [Google Scholar] [CrossRef] [PubMed]
- Masek, T.; Vopalensky, V.; Suchomelova, P.; Pospisek, M. Denaturing RNA electrophoresis in TAE agarose gels. Anal. Biochem. 2005, 336, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Myasoedov, N.F.; Lyapina, L.A.; Grigorjeva, M.E.; Obergan, T.Y.; Shubina, T.A.; Andreeva, L.A. Mechanisms for glyproline protection in hypercholesterolemia. Pathophysiology 2016, 23, 27–33. [Google Scholar] [CrossRef]
- Ceprian, M.; Fulton, D. Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease. Int. J. Mol. Sci. 2019, 20, 2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, A.J.; Robertson, G.; Zagnoni, M.; Connolly, C.N. Neuronal networks provide rapid neuroprotection against spreading toxicity. Sci. Rep. 2016, 6, 33746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wei, X.; Liu, K.; Zhang, X.; Yang, F.; Zhang, H.; He, Y.; Zhu, T.; Li, F.; Shi, W.; et al. NOX2 deficiency ameliorates cerebral injury through reduction of complexin II-mediated glutamate excitotoxicity in experimental stroke. Free Radic. Biol. Med. 2013, 65, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Uittenbogaard, M.; Baxter, K.K.; Chiaramello, A. NeuroD6 genomic signature bridging neuronal differentiation to survival via the molecular chaperone network. J. Neurosci. Res. 2010, 88, 33–54. [Google Scholar] [CrossRef] [Green Version]
- Halder, N.; Lal, G. Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front. Immunol. 2021, 12, 660342. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, L.; Tang, H. Vascular endothelial growth factor induces protein kinase D-dependent production of proinflammatory cytokines in endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C821–C827. [Google Scholar] [CrossRef]
- Wang, X.; Bao, X.; Pal, R.; Agbas, A.; Michaelis, E.K. Transcriptomic responses in mouse brain exposed to chronic excess of the neurotransmitter glutamate. BMC Genom. 2010, 11, 360. [Google Scholar] [CrossRef] [Green Version]
- Esenwa, C.C.; Elkind, M.S. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat. Rev. Neurol. 2016, 12, 594–604. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1677. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Liu, L.; Zhang, S.; Nanda, A.; Li, G. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res. 2013, 6, 834–851. [Google Scholar] [CrossRef] [Green Version]
- Rylski, M.; Kaczmarek, L. AP-1 targets in the brain. Front. Biosci. 2004, 9, 8–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.H.; Park, S.W.; Kapadia, R.; Vemuganti, R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem. Int. 2007, 50, 1014–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, B.J.; Akhtar, L.N.; Benveniste, E.N. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009, 30, 392. [Google Scholar] [CrossRef] [Green Version]
- Shimada, Y.; Tanaka, R.; Shimura, H.; Yamashiro, K.; Urabe, T.; Hattori, N. Phosphorylation enhances recombinant HSP27 neuroprotection against focal cerebral ischemia in mice. Neuroscience 2014, 278, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.F.; Snelgrove, R.J. The multifaceted roles of the matrikine Pro-Gly-Pro in pulmonary health and disease. Eur. Respir. Rev. 2018, 27, 180017. [Google Scholar] [CrossRef] [PubMed]
- Weathington, N.M.; Van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Zurek, A.A.; Lecker, I.; Yu, J.; Abramian, A.M.; Avramescu, S.; Davies, P.A.; Moss, S.J.; Lu, W.Y.; Orser, B.A. Memory deficits induced by inflammation are regulated by α5-subunit-containing GABAA receptors. Cell Rep. 2012, 2, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Schwab, C.; Mcgeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011, 59, 152–165. [Google Scholar] [CrossRef]
- Hazell, A.S.; Wang, D. Identification of complexin II in astrocytes: A possible regulator of glutamate release in these cells. Biochem. Biophys. Res. Commun. 2011, 404, 228–232. [Google Scholar] [CrossRef]
- Gamir-Morralla, A.; López-Menéndez, C.; Medina, M.; Iglesias, T. A Novel Neuroprotection Target With Distinct Regulation in Stroke and Alzheimer’s Disease. Neuroprot. Alzheimer’s Dis. 2017, 123–147. [Google Scholar]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Myasoedov, N.F. Synacton and individual activity of synthetic and natural corticotropins. J. Mol. Recognit. 2017, 30, e2597. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Shevchenko, V.P.; Bobrov, M.Y.; Bezuglov, V.V.; Myasoedov, N.F. Characteristic features of specific binding of pentapeptide HFPGP labeled at the C-terminal proline residue to rat forebrain plasma membranes. Dokl. Biochem. Biophys. 2014, 456, 101–103. [Google Scholar] [CrossRef]
- V’unova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Shevchenko, V.P.; Bobrov, M.Y.; Bezuglov, V.V.; Myasoedov, N.F. Binding of tripeptide Pro-Gly-Pro labeled at the C-terminal proline residue to plasma membranes of the rat forebrain. Dokl. Biol. Sci. 2008, 419, 95–96. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Filippenkov, I.B.; Limborska, S.A.; Myasoedov, N.F. Pharmacotranscriptomics of peptide drugs with neuroprotective properties. Med. Res. Rev. 2021, 41, 754–769. [Google Scholar] [CrossRef]
- de Pins, B.; Mendes, T.; Giralt, A.; Girault, J.A. The Non-receptor Tyrosine Kinase Pyk2 in Brain Function and Neurological and Psychiatric Diseases. Front. Synaptic Neurosci. 2021, 13, 53. [Google Scholar] [CrossRef]
- Shevchenko, K.V.; V’yunova, T.V.; Andreeva, L.A.; Nagaev, I.Y.; Shevchenko, V.P.; Myasoedov, N.F. Comparison of pharmacokinetics and metabolism of Pro-Gly-Pro-Leu administered intranasally and intravenously in the blood and brain of rats. Dokl. Biochem. Biophys. 2014, 456, 111–115. [Google Scholar] [CrossRef]
- Shevchenko, K.V.; V’yunova, T.V.; Nagaev, I.Y.; Andreeva, L.A.; Myasoedov, N.F. Proteolysis of simple glyprolines by leucine aminopeptidase and enzymes from nasal mucus, brain membranes, and blood of rats. Russ. J. Bioorg. Chem. 2013, 39, 283–287. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Myasoedov, N.F. An integrated approach to study the molecular aspects of regulatory peptides biological mechanism. J. Labelled Comp. Radiopharm. 2019, 62, 812–822. [Google Scholar] [CrossRef]
- Semple, B.D.; Kossmann, T.; Morganti-Kossmann, M.C. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010, 30, 459. [Google Scholar] [CrossRef] [Green Version]
- Mélik-Parsadaniantz, S.; Rostène, W. Chemokines and neuromodulation. J. Neuroimmunol. 2008, 198, 62–68. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/sra/PRJNA491404 (accessed on 18 September 2018).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavchansky, V.V.; Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Mozgovoy, I.V.; Gubsky, L.V.; Myasoedov, N.F.; Andreeva, L.A.; Limborska, S.A.; Dergunova, L.V. Insight into Glyproline Peptides’ Activity through the Modulation of the Inflammatory and Neurosignaling Genetic Response Following Cerebral Ischemia–Reperfusion. Genes 2022, 13, 2380. https://doi.org/10.3390/genes13122380
Stavchansky VV, Filippenkov IB, Remizova JA, Denisova AE, Mozgovoy IV, Gubsky LV, Myasoedov NF, Andreeva LA, Limborska SA, Dergunova LV. Insight into Glyproline Peptides’ Activity through the Modulation of the Inflammatory and Neurosignaling Genetic Response Following Cerebral Ischemia–Reperfusion. Genes. 2022; 13(12):2380. https://doi.org/10.3390/genes13122380
Chicago/Turabian StyleStavchansky, Vasily V., Ivan B. Filippenkov, Julia A. Remizova, Alina E. Denisova, Ivan V. Mozgovoy, Leonid V. Gubsky, Nikolay F. Myasoedov, Lyudmila A. Andreeva, Svetlana A. Limborska, and Lyudmila V. Dergunova. 2022. "Insight into Glyproline Peptides’ Activity through the Modulation of the Inflammatory and Neurosignaling Genetic Response Following Cerebral Ischemia–Reperfusion" Genes 13, no. 12: 2380. https://doi.org/10.3390/genes13122380
APA StyleStavchansky, V. V., Filippenkov, I. B., Remizova, J. A., Denisova, A. E., Mozgovoy, I. V., Gubsky, L. V., Myasoedov, N. F., Andreeva, L. A., Limborska, S. A., & Dergunova, L. V. (2022). Insight into Glyproline Peptides’ Activity through the Modulation of the Inflammatory and Neurosignaling Genetic Response Following Cerebral Ischemia–Reperfusion. Genes, 13(12), 2380. https://doi.org/10.3390/genes13122380






