Abstract
Many Camellia oleifera germplasm resources were collected from Guizhou Province, but the fruit morphological variation and genetic diversity of C. oleifera germplasm resources remain unclear. The genetic diversity of C. oleifera germplasms resources in Guizhou was studied based on fruit traits and simple sequence repeat (SSR) molecular markers to build a core collection. This paper aims to provide a scientific basis for the collection, management, development, and utilization of C. oleifera resources in Guizhou province. The variation coefficients among and within varieties of seven fruit phenotypic traits of C. oleifera ranged from 11.79% to 61.76% and from 8.15% to 42.31%, respectively, showing rich phenotypic variation. Furthermore, 12 SSR markers were used to analyze the genetic diversity. These primers generated 214 polymorphic bands, and the average number was 17.833. The average number of effective alleles (Ne), Shannon’s information index (I), observed heterozygosity (Ho), expected heterozygosity (He), polymorphic information content (PIC), and major allele frequency (MAF) were 8.999, 2.301, 0.965, 0.50, 0.836, and 0.238, respectively. The results showed that 12 SSR markers had high polymorphism, and the genetic diversity of 167 C. oleifera germplasm resources was high. Based on SSR molecular marker information and fruit traits clustering, 167 C. oleifera germplasm resources were divided into three groups. When constructing core collections based on fruit traits and molecular marker information, the PowerCore-25 of core collections greatly preserves fruit traits and improves genetic diversity. This paper can provide a reference for the genetic diversity and fruit traits variation of C. camellia germplasm resources in Guizhou Province. It is significant for establishing a core collection, thus promoting germplasm innovation and the development of the oil tea industry in Guizhou.
1. Introduction
C. oleifera, belongs to the genus Camellia of the Teaceae family [1]. C. camellia, olive (Olea europaea), oil palm (Elaeis guineensis Jacq.), and coconut (Cocos nucifera L.) are four important woody oil plants around the world [2]. C. oleifera has high genetic diversity and phenotypic variation, and the study of genetic diversity is the basis of breeding better varieties. Therefore, understanding the genetic diversity of C. oleifolia and constructing a core germplasm resources bank of C. oleifolia is an important link in the development of local C. oleifolia.
Genetic diversity is the basis of biological diversity and is the driving force for the stability and continuous evolution of species. The methods for studying genetic diversity include morphology, cytology, and molecular marker technology. Morphology, as the most simple and convenient labeling method, can reveal the degree of genetic variation to a certain extent and explore potential target traits [3]. With the development of science and technology, molecular marker technology has become one of the main ways to study genetic diversity, population structure, and kinship. SSR molecular markers, due to their high polymorphism and good stability, are currently considered one of the most important molecular markers. This method is widely used to study the genera Pyrus [4], Juglans mandshurica Maxim [5], Tunisian melon (Cucumis melo L.) [6], and pumpkins (Cucurbita spp.) [7]. It is also used to study camellia oil. He et al. [8] studied the germplasm resources of 150 oil teas; the Dice genetic similarity coefficients of 150 germplasm materials ranged from 0.05 to 0.91, which demonstrates rich genetic diversity. Both morphological and molecular markers can be used to construct a core collection. The construction of core collections based on morphological, physiological, and biochemical traits has been reported for pomegranate (Punica granatum L.) [9], Huangxinzimu (Catalpa fargesii f.duclouxii) [10], and Ziziphus mauritiana Lam. [11]. Based on molecular markers, core germplasm collection groups of Phaseolus lunatus [12], Myrtaceae (Eucalyptus cloeziana F. Muell.) [13], hazelnut (Corylus avellana L.) [14], Akebia trifoliata (Thunb.) Koidz [15], and Prunus sibirica [16] were constructed. In Pinus yunnanensis Franch. [17], Perilla [18], and Cymbopogon winterianus Jowitt [19], the diversity of phenotypes, agronomic traits, and molecular marker information of core collections has been verified. Furthermore, the degree of variation and genetic diversity of the core collection should be increased as far as possible.
This paper aims to establish the variation in fruit traits of C. oleifolia and evaluate the genetic diversity and relationships of C. oleifera germplasm resources in Guizhou Province. Constructing core collections of fruit traits and molecular marker information lays the foundation for the breeding of C. oleifolia and the protection of germplasm resources.
2. Materials and Methods
2.1. Plant Material
The experimental samples were collected from key oil tea distribution areas in Guizhou Province from 2008 to 2011. Information about the collection area is shown in Table 1. All of the samples were excellent families, clones, and superior varieties approved (recognized) by Guizhou Province and were over 10 years old (detailed information can be found in Attached Table 1). Leaves were quickly stored in dry ice, and promptly brought back to the laboratory in a −80 °C ultra-low-temperature refrigerator, as DNA extraction materials.

Table 1.
Information about the 167 C. oleifera germplasm samples collected.
2.2. Fruit Phenotype Determination
The index of phenotypic and economic traits was determined using the method described by Yang et al. [20]. In the ripening period, three plants of each variety were randomly selected to collect a sufficient amount of fruit in a mesh bag and marked. The fruits were spread on a shelf and measured immediately. A total of 20 fruits were randomly selected from each plant for determination, and vernier calipers were used to measure the fruit vertical diameter (FVD), fruit horizontal diameter (FHD), and peel thickness (PT). Single fresh fruit weight (FFW) and fresh seed weight (FSW) were determined using an electronic balance. Then, measured peel thickness (PT) was measured and the number of seeds (SGN) recorded. After the phenotypic data had been collected, the fresh weight of the seeds of 20 fruits from the same tree was measured and the seeds were then dried in an oven at 80 °C. The dry seed weight and dry kernel weight were determined using an electronic balance. The oil content of the dry kernels (DKOC) was determined by means of the cable extraction method, with petroleum ether (60–90 °C) as the solvent, and extracted for 6 h in the fat detector (temperature set at 80 °C). Before pumping, the kernel powder mass (m) was weighed. Kernel powder was put into the filter paper package to weigh the first mass (m1). After extraction, it was baked in an oven at 105 °C for 1 h to weigh the second mass (m2).
Fresh seed rate (FSR, %) = fresh seed weight/ fresh fruit weight × 100%.
Dry seed rate (DSR, %) = dry seed weight/fresh fruit weight × 100%.
Dry kernel yield (DKR, %) = dry kernel weight/dry seed weight × 100%.
DKOC (%) = (m1 − m2)/m
2.3. DNA Extraction and PCR Analysis
The SSR molecular marker test includes DNA extraction, primer screening, fluorescence quantitative PCR, and electrophoresis tube detection. DNA was extracted using a genomic kit from Beijing Botanical Biofet Plant. This DNA extraction kit was used to select primers as the 36 main reference pairs [21,22,23], and 12 pairs of primers with high and stable polymorphism were screened (Table 2) for fluorescence quantitative PCR and electrophoresis tube detection.

Table 2.
SSR primers and sequence information.
The fluorescence quantitative PCR consists of a 20 µL system, including 17 µL of gold mix (green), 1 µL of front primers, 1 µL of back primers, and 1 µL of DNA template. The amplification procedures are listed as follows. First, pre-denaturation was performed at 98 °C for 2 min. The second stage was the cycle stage. Samples were denatured at 98 °C for 10 s, annealing at 55–62 °C for 10 s, extended at 72 °C for 10 s, and cycled 35 times. The third stage was the extension stage. Samples were extended at 72 °C for 5 min. For the capillary test, the mixing plate was heated at 95 °C for 5 min with a metal bath heater and immediately put into an ice box at −20 °C. The mixing plate was removed after cooling, centrifuged at 4000 rpm, thawed, and mixed well. Then, it was placed in an ABI 3730xl sequencer for capillary electrophoresis.
2.4. Construction of Core Collection
The core collection was constructed with different sampling ratios (10%, 15%, 20%, 25%, and 30%). QGA software adopts Euclidean distance, multiple clustering priority sampling methods, and the shortest clustering method. PowerCore is an M strategy with a heuristic search for establishing core sets [26]. The core collections were evaluated using genetic diversity parameters and effective value parameters of fruit traits, and the difference significance was detected by t-test.
2.5. SSR Analysis and Statistical Analysis
The exact loci and statistic genotyping data were analyzed with Gene Mapper 4.1. The genetic diversity indexes of SSR loci, including the number of different alleles (Na), number of effective alleles (Ne), Shannon’s information index (I), observed heterozygosity (Ho), expected heterozygosity (He), polymorphism information content (PIC), and major allele frequency (MAF) were calculated using GenAlEx 6.51 and PowerMarker software. Genetic structure and phylogenetic trees were constructed by means of Structure 2.3.4 and MEGA v.6. The quantitative traits were divided into 10 levels according to mean value (X) and standard deviation (σ), and Xi < X − 2σ and Xi > X + 2σ increased from 1 to 10 [10]. The phenotypic differentiation coefficient was calculated as VST = δ2t/s/(δ2t/s + δ2s) × 100%, where δ2t/s and δ2s were variances between and within populations, respectively [27]. Other data analysis was completed using SPSS 26, and variance square calculation was completed using Minitab 19.
3. Results
3.1. Analysis of Fruit Phenotypic Variation
Variance analysis was conducted on seven phenotypic traits of 167 C. oleifera germplasm resources in Guizhou province. The F-value test showed that the fruit phenotypic traits exhibited significant differences within and among varieties (Table 3). These results indicate the rich diversity in the phenotypes of C. oleifera.

Table 3.
Analysis of phenotypic variance among different varieties and within varieties of germplasm resources of C. oleifera in Guizhou.
The phenotypic traits of C. oleifera differed greatly between and within varieties, and the variation between varieties was greater than that within varieties. The variation coefficients among different varieties and within the varieties were in the range of 11.79–61.76% and 8.15–42.31%, respectively. The highest variation among varieties was in FFW, and the lowest was the fruit shape index (FSI). The highest variation within varieties was SGN, and the lowest was in FSI. The variation ranges of variance components among different varieties and within the varieties were 0.006–15.108 and 0–2.294, respectively. The trait with the largest variance component among different varieties was PT (67.35%), and the one with the smallest was SGN (43.68%). The phenotypic differentiation coefficients ranged from 80.15% to 95.56%, and the phenotypic differentiation coefficients of fruit traits were greater than 70%. The results indicate that the fruit traits mainly vary among different varieties, which is consistent with the results of the coefficient of variation (Table 4).

Table 4.
Coefficient of variation, variance component, and phenotypic differentiation coefficient of C. oleifera in Guizhou.
3.2. Correlation and Classification of Fruit Phenotype and Economic Characters
The correlation of 12 fruit traits of 167 C. oleifera germplasm resources was highly significant. Most of the phenotypic traits were positively correlated, and the economic traits were positively correlated with each other. However, most phenotypic traits were negatively correlated with economic traits (Figure 1).
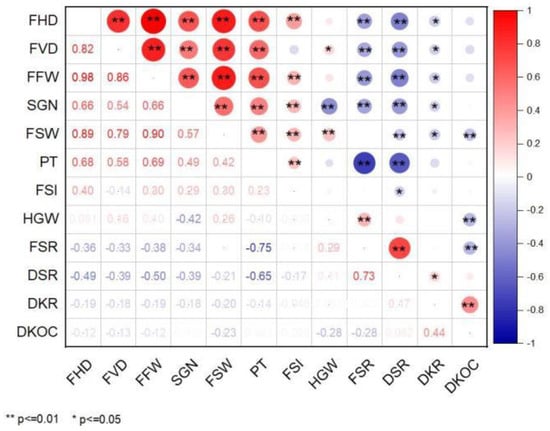
Figure 1.
Correlation analysis of fruit traits. Note: In Figure 1, the lower left corner is the Pearson correlation value; in the lower right corner, **, represents a highly significant correlation, p ≤ 0.01; * represents a significant correlation, p ≤ 0.05.
In the cluster heat map, the traits are divided into two groups. The first group consists of economic traits, including FSR, DSR, DKR, and DKOC. The second group comprises fruit phenotypic traits and HGW. In the second group, FVD and PT are divided into one class, FSW and HGW are divided into one class, and the rest are divided into one class (Figure 2).
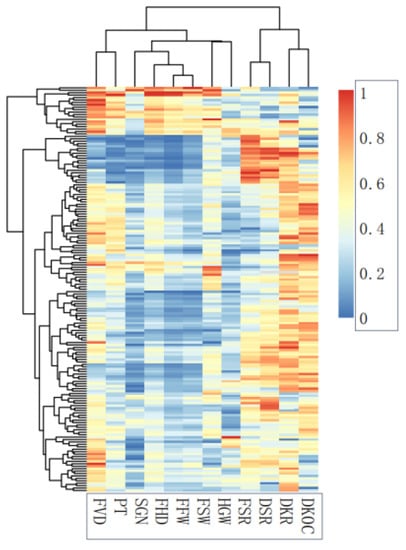
Figure 2.
Clustering heat map of fruit traits.
The 167 germplasm resources were divided into three groups according to 12 traits. There were 20 germplasm resources in group I, characterized by large fruit and thick peel. There were 20 germplasm resources in group II, characterized by a small fruit and thin pericarp and high FSR, DSR, and DSK. There were 127 germplasm resources in group III, which had medium fruit size, pericarp thickness, and high DSK and DKOC.
3.3. Genetic Diversity and Cluster Analysis
Genetic parameters based on 12 pairs of SSR primers are shown in Table 5. A total of 214 alleles were detected by 12 SSR primers, with an average of 17.833 alleles and 8.999 effective alleles. Shannon’s index ranged from 1.257 to 2.864, with an average of 2.302. The observed heterozygosity ranged from 0.867 to 1, with an average of 0.965. Expected heterozygosity ranged from 0.628 to 0.93, with an average of 0.850. The results for I, Ho, and He showed that the genetic diversity of 167 C. oleifera germplasm resources was high. The polymorphism information content (PIC) ranged from 0.592 to 0.927, with an average value of 0.836. The MAF ranged from 0.123 to 0.548, with an average of 0.238. Primers with a PIC higher than 0.5 indicated that that 12 pairs of primers had high polymorphism and could be used for diversity analysis and variety identification.

Table 5.
Genetic parameters based on 12 SSR in this study.
Based on phylogenetic tree analysis of Euclidean distance, the 167 C. oleifera germplasm resources were divided into three groups (Figure 3). Group I had four germplasm resources (GY86, GY137, GY88, and GY94); group II had four germplasm resources (GY124, GY117, GY110, and GY85); group III had 159 germplasm resources; and group III could be divided into four small groups. Group IIIa contained five germplasm resources (GY136, GY75, GY131, GY15, and GY9); Group IIIb only contained GY27; and Group IIIc contained 20 germplasm resources. Group IIId included the remaining 135 germplasm resources. The population structure of 167 germplasm resources was analyzed. The best classification population was two, and the Q values were higher than 0.6. Furthermore, the population results were relatively simple, but there were different classification combinations with the phylogenetic tree.
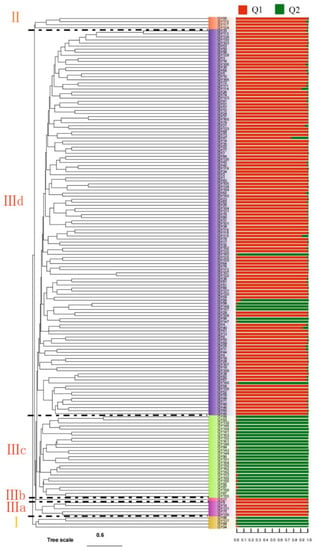
Figure 3.
Phylogenetic tree and genetic structure of 167 C. oleifera germplasm resources based on SSR Markers. Note: The stacked bar charts represent the Q value of different assigned groups. Q1 and Q2 represent two groups when the genetic structure of 167 C. oleifera germplasm resources is divided into two groups.
3.4. Construction of Core Collection
The core collections were constructed using PowerCore software and QGA software, respectively, according to different sampling proportions. The mean difference percentages (MDs) of the core collections were less than 20%, and the range coincidence rates (CRs) were greater than 80%, indicating that all core collections meet the conditions (Table 6). For core collections constructed by QGA software, MDs were in the range of 0–8.33%, with an average of 3.33%. CRs were in the range of 83.64–100%, with an average of 90.68%. The percentage differences of variance (VDs) were in the range of 8.33–75%, with an average of 38.33%. The change rates of coefficients of variation (VRs) were in the range of 116.87–128.66%, with an average of 121.92%. The trait retention rates (TRs) were in the range of 82.38–99.16%. The phenotypic diversity of the QGA-15, QGA-20, and QGA-25 core collections was rich. For core collections constructed by PowerCore software, MDs were in the range of 1.68–5.7%, with an average value of 3.57%. CRs were in the range of 84.61–92.18%, with an average value of 89.72%. VDs were in the range of 18.59–31.77%, with an average value of 25.87%. VRs were in the range of 109.43–116.03%, with an average value of 112.90%. TRs were in the range of 87.27–99.09%, and with an average value of 94.91%. The PowerCore-15, PowerCore-20, PowerCore-25, and PowerCore-30 core collections showed good phenotypic diversity.

Table 6.
Comparison of effective evaluation parameters of C. oleifera core collection based on traits.
Table 7 shows that for core collections constructed using PowerCore software, the Ne, I, Ho, He, and unbiased expected heterozygosity (uHe) of the core collections were all higher than in the original collection, and only Na was lower than in the original collection. We also constructed core collections using QGA software, only the Ho and uHe of QGA-15, QGA-10, and QGA-25 were slightly higher than in the original collection. In comparison, the PowerCore-25 can retain higher phenotypic traits of the original population and improve the variation and genetic diversity of the core collection. The mean value comparison and t-test between the PowerCore-25 and the original collection show no significant differences in 12 fruit traits (Table 8). The principal coordinate analysis of 167 C. oleifera germplasm resources showed that the core collection was evenly distributed in the original collection (Figure 4), indicating that the constructed core collection has a certain representativeness.

Table 7.
Comparison of genetic parameters of C. oleifera core collections based on SSR.

Table 8.
Comparison of character parameters between the original, reserved, and core collections.
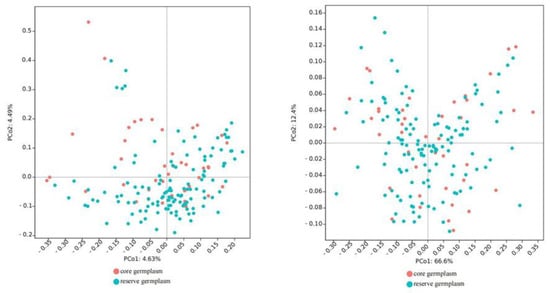
Figure 4.
Principal coordinate analysis based on SSR 167 germplasm resources (left) and principal coordinate analysis based on fruit traits of 167 germplasm resources (right).
4. Discussion
4.1. Fruit Phenotypic Variation
Genetic diversity is mainly studied at the morphological, cytological, biochemical, and molecular levels to understand the genetic diversity of species [28], thus providing a scientific reference for the germplasm, conservation, and utilization of species. Phenotypic variation is caused by the interaction between genes and the environment [29], resulting in relatively rich diversity within and between species and populations. Morphological markers are the simplest genetic markers that can, to a certain extent, reveal genetic variation within or between species [8,30]. In morphological study on pecan (Carya dabieshanensis M. C. Liu et Z. J. Li) [27], apricot (Prunus armeniaca L.) [31] and melon (C. melo L.) [32], substantial differences were found in phenotypic traits. In this study, seven phenotypic traits were measured in 167 germplasm resources from Guizhou Province. The coefficient of variation between and within cultivars ranged from 11.79% to 61.76% and from 8.15% to 42.31%, respectively, indicating that the phenotypic variation of 167 C. oleifera germplasm resources was also extremely abundant. He et al. [8] studied 150 oil tea germplasm resources, and found that the phenotypic variation range among four fruits was 13.24–39.60%. The results were consistent with this study, with relatively less variation in FHD and FVD and large variation in FFW, PT, and SGN. Gao et al. studied C. oleifera in 18 areas in China, and found that the coefficient of variation of FHD and FVD was less than 20% [33]. However, the variation in fruit traits was substantial in this study, and the average coefficient of variation of seven traits was 36.75%. The results were consistent with Xie et al.’s study on the phenotypic traits of four C. meiocarpa and one C. oleifera plants: the coefficients of variation of five phenotypic traits ranged from 23.86% to 56.94% in this study [34]. The germplasm resources of oil tea in this study may include part of C. oleifera and C. meiocarpa. C. meiocarpa has the characteristics of thin skin and small fruit, and its fruit phenotype was significantly different from that of C. oleifera [35]. In this study, FFW, PT, and SGN of oil tea had a large variation range, traits which could be used as reference for the selective breeding for oil tea.
4.2. Correlation Analysis and Cluster Analysis
Association analysis can reveal the relationship between different traits [36]. In this study, the 12 traits showed significant correlation, and the results were consistent with the results based on 150 oil tea plants provided by He et al. [8]. FSR and DSR were negatively correlated with six phenotypic traits. Liang et al. [20] studied 40 C. oleifera plants from Guizhou and found that only FSR and PT were negatively correlated, while DSR was negatively correlated with FHD. The result was probably due to the fact that the 40 C. oleifera plants came from a unique climate in low, hot valleys, exhibiting specific genotypic performance. Furthermore, Lu Yang studied 45 superior C. weiningensis YK Li., and found no significant correlation among PT and other fruit traits and economic traits. These results may be because the studied materials belong to the thin-skinned type, and the variation among the characteristics was small [37]. The results also indicated that C. oleifera has great potential for genetic improvement and is important for crossbreeding.
The 167 germplasm resources were divided into three groups by 12 phenotypic traits. Group I had 20 germplasm resources with large fruit and thick pericarp, and their FVD, FHD, FFW, and PT were 33.05%, 21.73%, 113.78%, and 41.49% higher than those in other groups. There were 20 germplasm resources in group II, with small and thick fruit pericarp and a high seed rate. The fresh and dry seed rates were 41.34% and 33.38% higher than those in other groups. There ware 127 germplasm resources in group III, and the oil content rate of dry kernels in this group was 27.22% higher than in other groups.
4.3. Genetic Diversity and Genetic Structure Analysis
In this study, 167 C. oleifera germplasm resources showed high genetic diversity. Jia et al. [25] studied 18 C. oleifera germplasm resources, and found the average values of Na, Ho, and He were 9.1, 0.741, and 0.746, respectively. The results were consistent with our results, indicating that C. oleifera has high genetic diversity due to geographical isolation and late self-incompatibility [38]. Zhao et al. [39] studied 50 genotypes of C. japonica and C. oleifera with 21 SSR pairs, and found that the H, I and PIC of C. oleifera were 0.2089, 0.3324, and 0.4014, respectively. The genetic parameters were smaller than those in this study, possibly because fewer research samples was used.
Based on SSR molecular marker information clustering, 167 C. oleifera germplasm resources were divided into three groups. The clustering of 12 fruit traits also demonstrated three groups, but the two methods differed greatly regarding the classification groups. Li et al. studied 89 C. camellia genotypes and found that the same species could cluster together well, and some accessions that were grouped in the same cluster or subcluster had similar flower colors, but these groups can also exist in different colors [40]. Jan et al. studied 105 barley (Hordeum vulgare L.) genotypes and found that the genotypes clustered in three sub-clusters did not show any trait-specific relationship with each other [41]. There were differences between the clustering based on molecular marker information and the clustering of fruit traits in this study. These differences may be because the samples used in this study were collected from different geographical origins, and the fruit traits were influenced by both genes and the environment, leading to natural variations in morphology, physiology, structure, and gene expression [42]. It may also be related to the number of SSR primers; Velázquez-Barrera et al. studied the population structure of 118 cultivated pear trees using 12 SSRs and 18 SSRs, they found different results of population structure when they eliminated the higher percentile of null alleles and linked loci [43]. The results showed that SSR primers have a substantial influence on classification.
4.4. Construction of Core Collections
Core collections can preserve the genetic diversity of the original collection to the maximum extent [44]. In this study, the effective value parameters of fruit traits and the genetic parameters of molecular marker information were comprehensively compared. The results suggest that core collections generated using PowerCore-25 are relatively better core collections which can retain the favorable fruit traits and high genetic diversity of the original collection. Combining phenotypic traits with molecular marker information, a core collection constructed using PowerCore software has been reported for walnuts (Juglans regia L.) [45], which could improve the effective value parameters and genetic parameters of the core collection. The results were consistent with this study. Kim et al. [46] used PowerCore software to build the core collection of Korean apple, and the parameters of Ne, I, and He of the core collection were greatly improved compared with the original collection. For olive (Olea europaea L.) [47] and Perilla [12], core collections constructed using PowerCore retained high allele loci and trait characteristics. In phenotypic and genetic parameters, PowerCore was used to establish a walnut core collection with the total coverage of traits in the entire collection [48]. PowerCore software is convenient and efficient for building core collections, and can be used to lay a foundation for the subsequent management of the germplasm bank.
5. Conclusions
In this study, 12 pairs of primers were found to show high polymorphism, which can provide a reference for subsequent studies on the genetic diversity of C. oleifera. Germplasm resources of C. oleifera in Guizhou were found to have a high genetic diversity and can be used to improve breeding. However, the cluster analysis showed that the environment had a substantial influence on the phenotypic and economic traits of C. oleifera. Therefore, the environment should be taken into consideration when introducing and breeding C. oleifera. In addition, the core collection of phenotype traits, economic traits, and molecular marker information was constructed in this study, and was found to be conducive to the preservation and management of C. oleifera.
Author Contributions
Data curation, Y.Z.; Formal analysis, Y.Z., D.L., Z.S., X.G. and Y.T.; Funding acquisition, D.W.; Writing—original draft, Y.Z.; Writing—review & editing, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Science and Technology Plan Project Guizhou Province of China (Qian Ke He Zhi Cheng [2021] General 220).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Science and Technology Plan Project Guizhou Province of China (Qian Ke He Zhi Cheng [2021] General 220). We are thankful for the support of the Guizhou Academy of Forestry and Qiandongnan Institute of Forestry for the resource collection of C. oleifera.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, P.; Wang, K.; Wang, Y.; Hu, Z.; Yan, C.; Huang, H.; Ma, X.; Cao, Y.; Long, W.; Liu, W.; et al. The genome of oil-Camellia and population genomics analysis provide insights into seed oil domestication. Genome Biol. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, X.; Liu, Z.; Lin, Q.; Zhang, L.; Yuan, J.; Zeng, Y.; Wu, L. In Vitro Propagation of Camellia oleifera Abel. Using Hypocotyl, Cotyledonary Node, and Radicle Explants. HortScience 2016, 51, 416–421. [Google Scholar] [CrossRef]
- Li, J.; Gao, G.; Li, B.; Li, B.; Lu, Q. Genetic Analysis of Prunus salicina L. by Random Amplified Polymorphic DNA (RAPD) and Intersimple Sequence Repeat (ISSR). Genet. Res. 2022, 2022, 2409324. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Shi, Y.Z.; Shoda, M.; Kotobuki, K.; Matsuta, N.; Hayashi, T.; Ban, Y.; Yamamoto, T. Identification of Asian Pear Varieties by SSR Analysis. Breed. Sci. 2002, 52, 115–121. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Tong, B.; Han, B.; Liu, D.; Zhang, P.; Hu, D. EST-SSR marker-based investigation on genetic diversity and genetic structure of Juglans mandshurica Maxim. in Shandong Province of China. Genet. Resour. Crop Evol. 2022, 1–11. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the genetic diversity and population structure of a tunisian melon (Cucumis melo L.) collection using phenotypic traits and SSR molecular markers. Agronomy 2021, 11, 1121. [Google Scholar] [CrossRef]
- Nyabera, L.A.; Nzuki, I.W.; Runo, S.M.; Amwayi, P.W. Assessment of genetic diversity of pumpkins (Cucurbita spp.) from western Kenya using SSR molecular markers. Mol. Biol. Rep. 2021, 48, 2253–2260. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Wang, X.; Wang, R.; Chen, Y.; Tian, Y. Assessment of genetic diversity in Camellia oleifera Abel. accessions using morphological traits and simple sequence repeat (SSR) markers. Breed. Sci. 2020, 70, 586–593. [Google Scholar] [CrossRef]
- Razi, S.; Soleimani, A.; Zeinalabedini, M.; Vazifeshenas, M.R.; Martinez-Gomez, P.; Kermani, A.M.; Raiszadeh, A.R.; Tayari, M.; Martinez-Garcia, P.J. Development of a Multipurpose Core Collection of New Promising Iranian Pomegranate (Punica granatum L.) Genotypes Based on Morphological and Pomological Traits. Horticulturae 2021, 7, 350. [Google Scholar] [CrossRef]
- Xue, H.F.; Yu, X.C.; Fu, P.Y.; Liu, B.Y.; Zhang, S.; Li, J.; Zhai, W.J.; Lu, N.; Zhao, X.Y.; Wang, J.H.; et al. Construction of the Core Collection of Catalpa fargesii f. duclouxii (Huangxinzimu) Based on Molecular Markers and Phenotypic Traits. Forests 2021, 12, 1518. [Google Scholar] [CrossRef]
- Sivalingam, P.N.; Singh, D.; Chauhan, S.; Changal, H.K.; Bhan, C.; Mohapatra, T.; More, T.A.; Sharma, S.K. Establishment of the core collection of Ziziphus mauritiana Lam. from India. Plant Genet. Resour.-Charact. Util. 2014, 12, 140–142. [Google Scholar] [CrossRef]
- Gomes, R.L.F.; Costa, M.F.; Alves-Pereira, A.; Bajay, M.M.; Viana, J.P.G.; Valente, S.E.d.S.; Lopes, Â.C.d.; Zucchi, M.I.; Pinheiro, J.B. A lima bean core collection based on molecular markers. Sci. Agric. 2019, 77. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Zhou, C.; Chen, J.; Li, F.; Weng, Q.; Li, M.; Wang, Y.; Chen, S.; Chen, J.; et al. Genetic diversity analysis of a breeding population of Eucalyptus cloeziana F. Muell. (Myrtaceae) and extraction of a core germplasm collection using microsatellite markers. Ind. Crops Prod. 2020, 145, 112157. [Google Scholar] [CrossRef]
- Boccacci, P.; Aramini, M.; Ordidge, M.; van Hintum, T.J.L.; Marinoni, D.T.; Valentini, N.; Sarraquigne, J.-P.; Solar, A.; Rovira, M.; Bacchetta, L.; et al. Comparison of selection methods for the establishment of a core collection using SSR markers for hazelnut (Corylus avellana L.) accessions from European germplasm repositories. Tree Genet. Genomes 2021, 17, 48. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Sun, Z.; Niu, J.; Shi, Y.; Huang, K.; Chen, J.; Chen, J.; Luan, M. Genetic Diversity of a Natural Population of Akebia trifoliata (Thunb.) Koidz and Extraction of a Core Collection Using Simple Sequence Repeat Markers. Front. Genet. 2021, 12, 716498. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, S.; Liu, Q.; Chen, J.; Pan, J.; Zhang, J. Selection of a core collection of Prunus sibirica L. germplasm by a stepwise clustering method using simple sequence repeat markers. PLoS ONE 2021, 16, e0260097. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Z.; Gao, C.; Li, K. Strategy for the construction of a core collection for Pinus yunnanensis Franch. to optimize timber based on combined phenotype and molecular marker data. Genet. Resour. Crop Evol. 2021, 68, 3219–3240. [Google Scholar] [CrossRef]
- Sa, K.J.; Kim, D.M.; Oh, J.S.; Park, H.; Hyun, D.Y.; Lee, S.; Rhee, J.H.; Lee, J.K. Construction of a core collection of native Perilla germplasm collected from South Korea based on SSR markers and morphological characteristics. Sci. Rep. 2021, 11, 23891. [Google Scholar] [CrossRef]
- Munda, S.; Saikia, R.J.; Begum, T.; Bhandari, S.; Gogoi, A.; Sarma, N.; Tamang, R.; Lal, M. Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm. Plants 2022, 11, 528. [Google Scholar] [CrossRef]
- Yang, L.; Gao, C.; Xie, J.J.; Qiu, J.; Deng, Q.E.; Zhou, Y.C.; Liao, D.S.; Deng, C.Y. Fruit economic characteristics and yields of 40 superior Camellia oleifera Abel plants in the low-hot valley area of Guizhou Province, China. Sci. Rep. 2022, 12, 7068. [Google Scholar] [CrossRef]
- Zhang, Z.J. Study on Germplasm Resources and Elite Germplasm Selection of Camellia oleifera in Sanjiang Area. Master’s Thesis, Beijing Forestry University, Beijing, China, 2018. [Google Scholar]
- Ren, W. Genetic diversity and cross compatibility of elite genotypes in Camellia oleifera. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- Chen, Y.N.; Dai, X.G.; Hou, J.; Guan, H.W.; Wang, Y.X.; Li, Y.; Yin, T.M. DNA fingerprinting of oil camellia cultivars with SSR markers. Tree Genet. Genomes 2016, 12, 7. [Google Scholar] [CrossRef]
- Jia, B.G.; Lin, Q.; Zhang, L.; Tan, X.F.; Lei, X.L.; Hu, X.Y.; Shao, F.G. Development of 15 genic-SSR markers in oil-tea tree (Camellia oleifera) based on transcriptome sequencing. Genetika 2014, 46, 789–797. [Google Scholar] [CrossRef]
- Jia, B.G.; Lin, Q.; Feng, Y.Z.; Hu, X.Y.; Tan, X.F.; Shao, F.G.; Zhang, L. Development and cross-species transferability of unigene-derived microsatellite markers in an edible oil woody plant, Camellia oleifera (Theaceae). Genet. Mol. Res. 2015, 14, 6906–6916. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Chung, H.-K.; Cho, G.-T.; Ma, K.-H.; Chandrabalan, D.; Gwag, J.-G.; Kim, T.-S.; Cho, E.-G.; Park, Y.-J. PowerCore: A program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics 2007, 23, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Xi, J.W.; Hong, J.Y.; Xia, G.H. Phenotypic diversity of fruit and leaf traits of Carya cathayensis in Dabie Mountains. For. Res. 2020, 33, 10. [Google Scholar]
- Li, B.; Gu, W.-F. Review on genetic diversity in Pinus. Yi Chuan = Hered. 2003, 25, 740–748. [Google Scholar]
- Aspinwall, M.J.; Loik, M.E.; Resco de Dios, V.; Tjoelker, M.G.; Payton, P.R.; Tissue, D.T. Utilizing intraspecific variation in phenotypic plasticity to bolster agricultural and forest productivity under climate change. Plant Cell Environ. 2015, 38, 1752–1764. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, Z.; Cao, X.; Jiang, F.L. Genetic diversity of cultivated and wild tomatoes revealed by morphological traits and SSR markers. Genet. Mol. Res. 2015, 14, 13868–13879. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Miao, X.J. Genetic variability of wild apricot (Prunus armeniaca L.) populations in the Ili Valley as revealed by ISSR markers. Genet. Resour. Crop Evol. 2013, 60, 2293–2302. [Google Scholar] [CrossRef]
- Guliyev, N.; Sharifova, S.; Ojaghi, J.; Abbasov, M.; Akparov, Z. Genetic diversity among melon (Cucumis melo L.) accessions revealed by morphological traits and ISSR markers. Turk. J. Agric. For. 2018, 42, 393–401. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.; Liu, F.; Zhao, J.; Yuan, J.; Xiao, S.; Masabni, J.; Zou, F.; Yuan, D. Variation in Fruit Morphology and Seed Oil Fatty Acid Composition of Camellia oleifera Collected from Diverse Regions in Southern China. Horticulturae 2022, 8, 818. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, X.; Li, Z.; Huang, Y. Analysis of Genetic Difference and Relationship of Camellia meiocarpa Native Varieties by Morphology and AFLP Markers. For. Res. 2014, 27, 201–207. [Google Scholar]
- Chen, K. Camellia meiocarpa and Camellia oleifera Phylogenetic Relationship and Fruit Size Differentiation Transcriptome Analysis. Masters’s Thesis, Nanchang University, Nanchang, China, 2020. [Google Scholar]
- Liang, D.Y.; Zhang, X.X.; Wang, C.; Wang, X.W.; Li, K.L.; Liu, G.F.; Zhao, X.Y.; Qu, G.Z. Evaluation of Betula platyphylla Families Based on Growth and Wood Property Traits. For. Sci. 2018, 64, 663–670. [Google Scholar] [CrossRef]
- Yang, L.; Gao, C.; Wei, H.L.; Long, L.; Qiu, J. Evaluation of the economic characteristics of the fruit of 45 superior Camellia weiningensis YK Li. trees. PLoS ONE 2022, 17, e0268802. [Google Scholar]
- Gong, W.; Xiao, S.; Wang, L.; Liao, Z.; Chang, Y.; Mo, W.; Hu, G.; Li, W.; Zhao, G.; Zhu, H. Chromosome-level genome of Camellia lanceoleosa provides a valuable resource for understanding genome evolution and self-incompatibility. Plant J. 2022, 110, 881–898. [Google Scholar] [CrossRef]
- Zhao, Y.; Ruan, C.J.; Ding, G.J.; Mopper, S. Genetic relationships in a germplasm collection of Camellia japonica and Camellia oleifera using SSR analysis. Genet. Mol. Res. GMR 2017, 16, 16019526. [Google Scholar] [CrossRef]
- Li, Q.; Su, X.; Ma, H.; Du, K.; Yang, M.; Chen, B.; Fu, S.; Fu, T.; Xiang, C.; Zhao, Q. Development of genic SSR marker resources from RNA-seq data in Camellia japonica and their application in the genus Camellia. Sci. Rep. 2021, 11, 9919. [Google Scholar] [CrossRef]
- Jan, S.; Khan, M.N.; Jan, S.; Zaffar, A.; Rashid, R.; Khan, M.A.; Sheikh, F.A.; Bhat, M.A.; Mir, R.R. Trait phenotyping and molecular marker characterization of barley (Hordeum vulgare L.) germplasm from Western Himalayas. Genet. Resour. Crop Evol. 2022, 69, 661–676. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.-T.; Wei, J.-T.; Li, Y.; Tigabu, M.; Zhao, X.-Y. Genetic Improvement of Pinus koraiensis in China: Current Situation and Future Prospects. Forests 2020, 11, 148. [Google Scholar] [CrossRef]
- Velázquez-Barrera, M.E.; Ramos-Cabrer, A.M.; Pereira-Lorenzo, S.; Ríos-Mesa, D.J. Genetic pool of the cultivated pear tree (Pyrus spp.) in the Canary Islands (Spain), studied using SSR molecular markers. Agronomy 2022, 12, 1711. [Google Scholar] [CrossRef]
- Han, P.; Tian, X.M.; Wang, Y.; Huang, C.; Ma, Y.Z.; Zhou, X.F.; Yu, Y.; Zhang, D.W.; Xu, H.J.; Cao, Y.; et al. Construction of a core germplasm bank of upland cotton (Gossypium hirsutum L.) based on phenotype, genotype and favorable alleles. Genet. Resour. Crop Evol. 2022, 69, 2399–2411. [Google Scholar] [CrossRef]
- Mahmoodi, R.; Dadpour, M.R.; Hassani, D.; Zeinalabedini, M.; Vendramin, E.; Leslie, C.A. Composite core set construction and diversity analysis of Iranian walnut germplasm using molecular markers and phenotypic traits. PLoS ONE 2021, 16, e0248623. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Oh, Y.; Lee, G.-A.; Kwon, Y.S.; Kim, S.A.; Kwon, S.-I.; Doi, Y.-S.; Choi, C. Genetic Diversity, Structure, and Core Collection of Korean Apple Germplasm Using Simple Sequence Repeat Markers. Hortic. J. 2019, 88, 329–337. [Google Scholar] [CrossRef]
- Belaj, A.; Dominguez-Garcia, M.D.; Atienza, S.G.; Urdiroz, N.M.; De la Rosa, R.; Satovic, Z.; Martin, A.; Kilian, A.; Trujillo, I.; Valpuesta, V.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- Mahmoodi, R.; Dadpour, M.R.; Hassani, D.; Zeinalabedini, M.; Vendramin, E.; Micali, S.; Nahandi, F.Z. Development of a core collection in Iranian walnut (Juglans regia L.) germplasm using the phenotypic diversity. Sci. Hortic. 2019, 249, 439–448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
