Biomarkers for Monitoring Renal Damage Due to Fabry Disease in Patients Treated with Migalastat: A Review for Nephrologists
Abstract
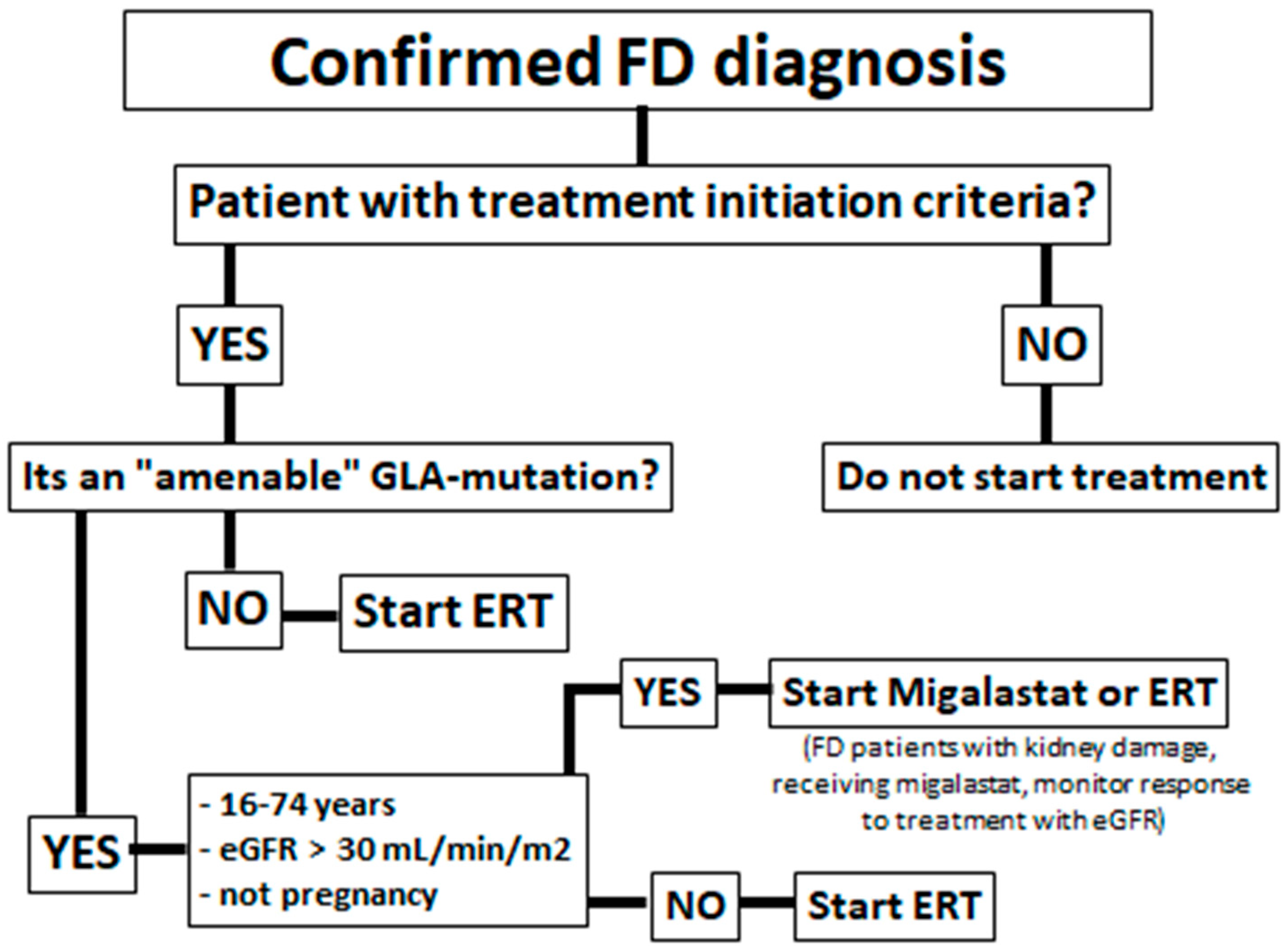
1. Introduction
2. Materials and Methods
3. Results
3.1. Serum Creatinine and Glomerular Filtration Rate
3.2. Albuminuria/Proteinuria
3.3. Plasma Lyso-Gb3
3.4. α-GalA Activity
3.5. Renal Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-GalA | α-galactosidase A |
| ERT | enzyme replacement therapy |
| ESRD | end-stage renal disease |
| FD | Fabry disease |
| Gb3 | globotriaosylceramide |
| GFR | glomerular filtration rate |
| KIC | kidney interstitial capillaries |
References
- Germain, D.P. Fabry disease. Orphanet. J. Rare Dis. 2010, 5, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Alroy, J.; Sabnis, S.; Kopp, J.B. Renal pathology in Fabry disease. J. Am. Soc. Nephrol. 2002, 13 (Suppl. 2), 134–138. [Google Scholar] [CrossRef]
- Ortiz, A.; Germain, D.P.; Desnick, R.J.; Politei, J.; Mauer, M.; Burlina, A.; Eng, C.; Hopkin, R.J.; Laney, D.; Linhart, A.; et al. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018, 123, 416–427. [Google Scholar] [CrossRef]
- Jaurretche, S.; Antongiovanni, N.; Perretta, F. Prevalence of chronic kidney disease in fabry disease patients: Multicenter cross sectional study in Argentina. Mol. Genet. Metab. Rep. 2017, 12, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Tøndel, C.; Bostad, L.; Larsen, K.K.; Hirth, A.; Vikse, B.E.; Houge, G.; Svarstad, E. Agalsidase benefits renal histology in young patients with Fabry disease. J. Am. Soc. Nephrol. 2013, 24, 137–148. [Google Scholar] [CrossRef]
- Politei, J.; Alberton, V.; Amoreo, O.; Antongiovanni, N.; Arán, M.N.; Barán, M.; Cabrera, G.; di Pietrantonio, S.; Durand, C.; Fainboim, A.; et al. Clinical parameters, LysoGb3, podocyturia, and kidney biopsy in children with Fabry disease: Is a correlation possible? Pediatr. Nephrol. 2018, 33, 2095–2101. [Google Scholar] [CrossRef]
- Perretta, F.; Antongiovanni, N.; Jaurretche, S. Early renal involvement in a girl with classic Fabry disease. Case Rep. Nephrol. 2017, 2017, 9543079. [Google Scholar] [CrossRef]
- Waldek, S.; Feriozzi, S. Fabry nephropathy: A review–how can we optimize the management of Fabry nephropathy? BMC Nephrol. 2014, 15, 1–13. [Google Scholar] [CrossRef]
- Branton, M.H.; Schiffmann, R.; Sabnis, S.G.; Murray, G.J.; Quirk, J.M.; Altarescu, G.; Lev, G.; Roscoe, B.; James, B.; Howard, A.; et al. Natural history of Fabry renal disease: Influence of α-galactosidase A activity and genetic mutations on clinical course. Medicine 2002, 81, 122–138. [Google Scholar] [CrossRef]
- Jaurretche, S.; Antongiovanni, N.; Perretta, F. Vascular disease in male patients with fabry disease on hemodialysis: A retrospective cohort study in Argentina. Rev. Nefrol. Dial. Traspl. 2019, 39, 101–107. [Google Scholar]
- Ishii, S.; Kase, R.; Sakuraba, H.; Suzuki, Y. Characterization of a mutant α-galactosidase gene product for the late-onset cardiac form of Fabry disease. Biochem. Biophys. Res. Commun. 1993, 197, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Jovanovic, A.; Herrmann, K.; Vardarli, I. Chaperone Therapy in Fabry Disease. Int. J. Mol. Sci. 2022, 23, 1887. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Q.; Ishii, S.; Asano, N.; Suzuki, Y. Accelerated transport and maturation of lysosomal α–galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 1999, 5, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.F.; Zuber, C.; Roth, J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005, 19, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Moran, N. FDA approves Galafold, a triumph for Amicus. Nat. Biotechnol. 2018, 36, 913. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource; Food and Drug Administration: Silver Spring, MD, USA; National Institutes of Health: Bethesda, MD, USA, 2016.
- Jaurretche, S. Pharmacological chaperones. New therapeutic alternative for Fabry disease nephropaty in Argentina. Rev. Nefrol. Dial. Traspl. 2020, 40, 51–61. [Google Scholar]
- Feldt-Rasmussen, U.; Hughes, D.; Sunder-Plassmann, G.; Shankar, S.; Nedd, K.; Olivotto, I.; Ortiz, D.; Ohashi, T.; Hamazaki, T.; Skuban, N.; et al. Long-term efficacy and safety of migalastat treatment in Fabry disease: 30-month results from the open-label extension of the randomized, phase 3 ATTRACT study. Mol. Genet. Metab. 2020, 131, 219–228. [Google Scholar] [CrossRef]
- Lenders, M.; Nordbeck, P.; Kurschat, C.; Eveslage, M.; Karabul, N.; Kaufeld, J.; Hennermann, J.B.; Patten, M.; Cybulla, M.; Müntze, J.; et al. Treatment of Fabry Disease management with migalastat—outcome from a prospective 24 months observational multicenter study (FAMOUS). Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 272–281. [Google Scholar] [CrossRef]
- Riccio, E.; Zanfardino, M.; Ferreri, L.; Santoro, C.; Cocozza, S.; Capuano, I.; Imbriaco, M.; Feriozzi, S.; Pisani, A.; AFFIINITY Group. Switch from enzyme replacement therapy to oral chaperone migalastat for treating fabry disease: Real-life data. Eur. J. Hum. Genet. 2020, 28, 1662–1668. [Google Scholar] [CrossRef]
- Müntze, J.; Gensler, D.; Maniuc, O.; Liu, D.; Cairns, T.; Oder, D.; Hu, K.; Lorenz, K.; Frantz, S.; Wanner, C.; et al. Oral chaperone therapy migalastat for treating Fabry disease: Enzymatic response and serum biomarker changes after 1 year. Clin. Pharm. Ther. 2019, 105, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; Feliciani, C.; Shankar, S.P.; Ezgu, F.; Amartino, H.; et al. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N. Engl. J. Med. 2016, 375, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Mauer, M.; Sokolovskiy, A.; Barth, J.A.; Castelli, J.P.; Williams, H.N.; Benjamin, E.R.; Najafian, B. Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment. J. Med. Genet. 2017, 54, 781–786. [Google Scholar] [CrossRef]
- Hughes, D.A.; Nicholls, K.; Shankar, S.P.; Sunder-Plassmann, G.; Koeller, D.; Nedd, K.; Vockley, G.; Hamazaki, T.; Lachmann, R.; Ohashi, T.; et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J. Med. Genet. 2017, 54, 288–296. [Google Scholar] [CrossRef]
- Lenders, M.; Nordbeck, P.; Kurschat, C.; Karabul, N.; Kaufeld, J.; Hennermann, J.B.; Patten, M.; Cybulla, M.; Müntze, J.; Üçeyler, N.; et al. Treatment of Fabry’s disease with migalastat: Outcome from a prospective observational multicenter study (FAMOUS). Clin. Pharm. Ther. 2020, 108, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Nicholls, K.; Giugliani, R.; Bichet, D.G.; Hughes, D.A.; Barisoni, L.M.; Colvin, R.B.; Jennette, J.C.; Skuban, N.; Castelli, J.P.; et al. Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: Data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study. Genet. Med. 2019, 21, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Nicholls, K.; Sunder-Plassmann, G.; Jovanovic, A.; Feldt-Rasmussen, U.; Schiffmann, R.; Giugliani, R.; Jain, V.; Viereck, C.; Castelli, J.P.; et al. Safety of switching to Migalastat from enzyme replacement therapy in Fabry disease: Experience from the Phase 3 ATTRACT study. Am. J. Med. Genet. 2019, 179, 1069. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; Bichet, D.; Germain, D.; Giugliani, R.; Hughes, D.; Nicholls, K.; Wilcox, W.; Williams, H.; Yu, J.; Castelli, J.; et al. SP004 effects of long-term migalastat treatment on renal function by baseline proteinuria in patients (PTS) with fabry disease. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 1), 347–348. [Google Scholar] [CrossRef]
- Mignani, R.; Dialisi, U.N. The Fabry nephropathy: New insight in diagnosis, monitoring and treatment. G. Ital. Nefrol. 2019, 36. [Google Scholar]
- Kusano, E.; Saito, O.; Akimoto, T.; Asano, Y. Fabry disease: Experience of screening dialysis patients for Fabry disease. Clin. Exp. Nephrol. 2014, 18, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Huynh-Do, U.; Krayenbuehl, P.A.; Beuschlein, F.; Schiffmann, R.; Barbey, F. Fabry disease genotype, phenotype, and migalastat amenability: Insights from a national cohort. J. Inherit. Metab. Dis. 2020, 43, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Narita, I.; Ohashi, T.; Sakai, N.; Hamazaki, T.; Skuban, N.; Castelli, J.P.; Lagast, H.; Barth, J.A. Efficacy and safety of migalastat in a Japanese population: A subgroup analysis of the ATTRACT study. Clin. Exp. Nephrol. 2020, 24, 157–166. [Google Scholar] [CrossRef]
- Di Stefano, V.; Mancarella, M.; Camporeale, A.; Regalia, A.; Ferraresi, M.; Pisaniello, M.; Cassinerio, E.; Pieruzzi, F.; Motta, I. Migalastat treatment in a kidney-transplanted patient with fabry disease and n215s mutation: The first case report. Pharmaceuticals 2021, 14, 1304. [Google Scholar] [CrossRef]
- Trimarchi, H.; Canzonieri, R.; Schiel, A.; Costales-Collaguazo, C.; Politei, J.; Stern, A.; Paulero, M.; Rengel, T.; Andrews, J.; Forrester, M.; et al. Increased urinary CD80 excretion and podocyturia in Fabry disease. J. Transl. Med. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Jaurretche, S.; Perez, G.; Antongiovanni, N.; Perretta, F.; Venera, G. Variables associated with a urinary MicroRNAs excretion profile indicative of renal fibrosis in Fabry disease patients. Int. J. Chronic Dis. 2019, 2019, 4027606. [Google Scholar] [CrossRef]

| Author (year) | Uni/ Multi-Center | Follow-Up | N | Included Both Genders | Included Classic and Late-Onset | Renal Biomarker | Other Biomarkers |
|---|---|---|---|---|---|---|---|
| Feldt-Rasmussen, et al. (2020) [18] | Multicenter (10 countries) * | 30-months | 48 | Yes | Yes | eGFR proteinuria | -cardiac function -stroke/TIA -α-GalA -Lyso-Gb3 |
| Müntze et al. (2019) [21] | Unicenter (Germany) | 12-months | 14 | Yes | Yes | eGFR proteinuria | -cardiac function - α-GalA -Lyso-Gb3 |
| Lenders et al. (2022) [19] | Multicenter (Germany) | 24-months | 54 | Yes | Yes | eGFR proteinuria | -LVMi -stroke/TIA -MSSI -DS3 -Lyso-Gb3 |
| Mauer et al. (2017) [23] | Unicenter (United States) | 6-months | 8 | Not (only males) | Not (only classic) | eGFR proteinuria renal histology | -Lyso-Gb3 |
| Germain et al. (2016) [22] | Multicenter (11 countries) ** | 12-months | 50 | Yes | Yes | eGFR proteinuria renal histology | -cardiac function - α-GalA -Lyso-Gb3 -GSRS -SF-36 -PS-SF |
| Riccio et al. (2020) [20] | Unicenter (Italy) | 12-months | 7 | Not (only males) | Yes | eGFR proteinuria | -cardiac function -neurologic changes -MSSI -SF-36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaurretche, S.; Conde, H.; Gonzalez Schain, A.; Ruiz, F.; Sgro, M.V.; Venera, G. Biomarkers for Monitoring Renal Damage Due to Fabry Disease in Patients Treated with Migalastat: A Review for Nephrologists. Genes 2022, 13, 1751. https://doi.org/10.3390/genes13101751
Jaurretche S, Conde H, Gonzalez Schain A, Ruiz F, Sgro MV, Venera G. Biomarkers for Monitoring Renal Damage Due to Fabry Disease in Patients Treated with Migalastat: A Review for Nephrologists. Genes. 2022; 13(10):1751. https://doi.org/10.3390/genes13101751
Chicago/Turabian StyleJaurretche, Sebastián, Hernan Conde, Ana Gonzalez Schain, Franco Ruiz, Maria Victoria Sgro, and Graciela Venera. 2022. "Biomarkers for Monitoring Renal Damage Due to Fabry Disease in Patients Treated with Migalastat: A Review for Nephrologists" Genes 13, no. 10: 1751. https://doi.org/10.3390/genes13101751
APA StyleJaurretche, S., Conde, H., Gonzalez Schain, A., Ruiz, F., Sgro, M. V., & Venera, G. (2022). Biomarkers for Monitoring Renal Damage Due to Fabry Disease in Patients Treated with Migalastat: A Review for Nephrologists. Genes, 13(10), 1751. https://doi.org/10.3390/genes13101751





