Effects of β-1,6-Glucan Synthase Gene (FfGS6) Overexpression on Stress Response and Fruit Body Development in Flammulina filiformis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
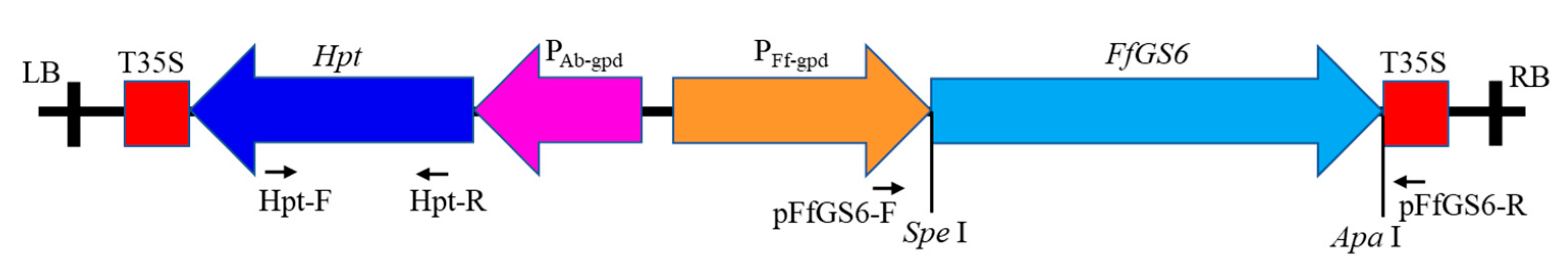
2.2. Construction of FfGS6 Overexpression Vector
2.3. Agrobacterium-Mediated Transformation of the FfGS6 Overexpression Vector into F. filiformis Mycelium
2.4. Validation of Putative Transformants
2.5. RNA Extraction and RT-qPCR Determination of Relative Gene Expression
2.6. Mycelial Growth Rate Assay
2.7. Microscopic Observation of Mycelial Cells
2.8. Scratching and Assessment of Mycelial Recovery
2.9. Hydrogen Peroxide Treatment and Mycelial Tolerance Test
2.10. F. filiformis Cultivation and Fruiting Body Phenotype Analysis
2.11. Statistical Analysis
3. Results
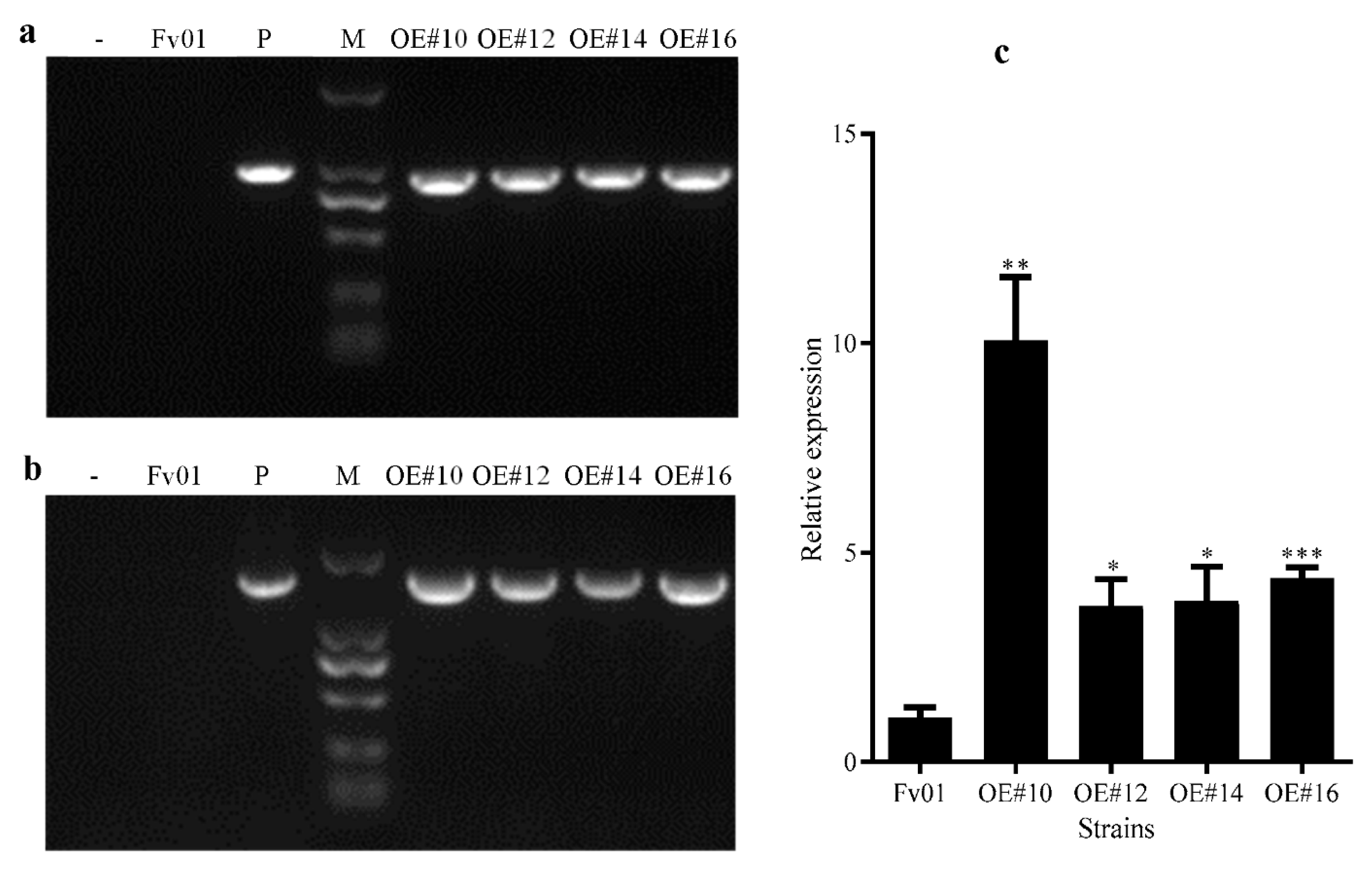
3.1. Acquisition and Validation of FfGS6 Overexpression Transformants
3.2. Effects of FfGS6 Overexpression on the Growth of F. filiformis Mycelium
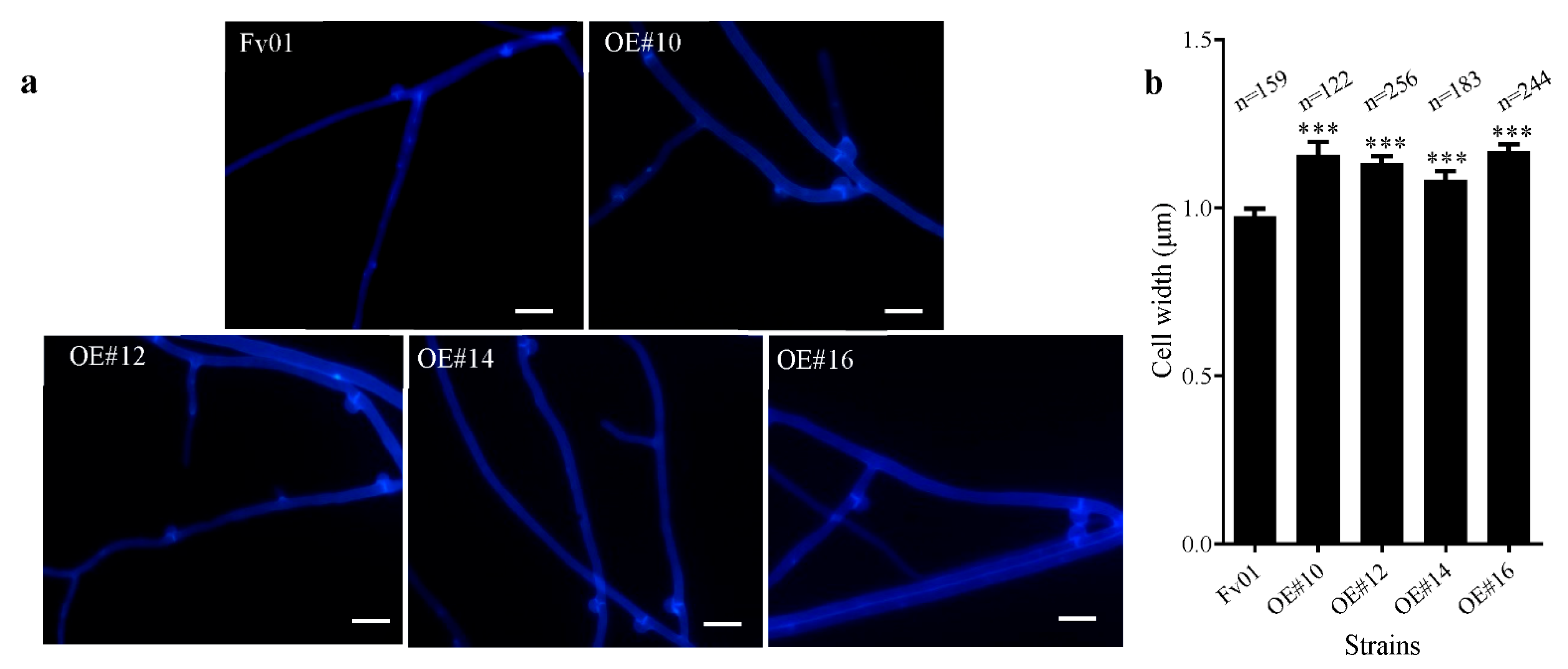
3.3. Impacts of FfGS6 Overexpression on the Microscopic Morphology of Mycelial Cells
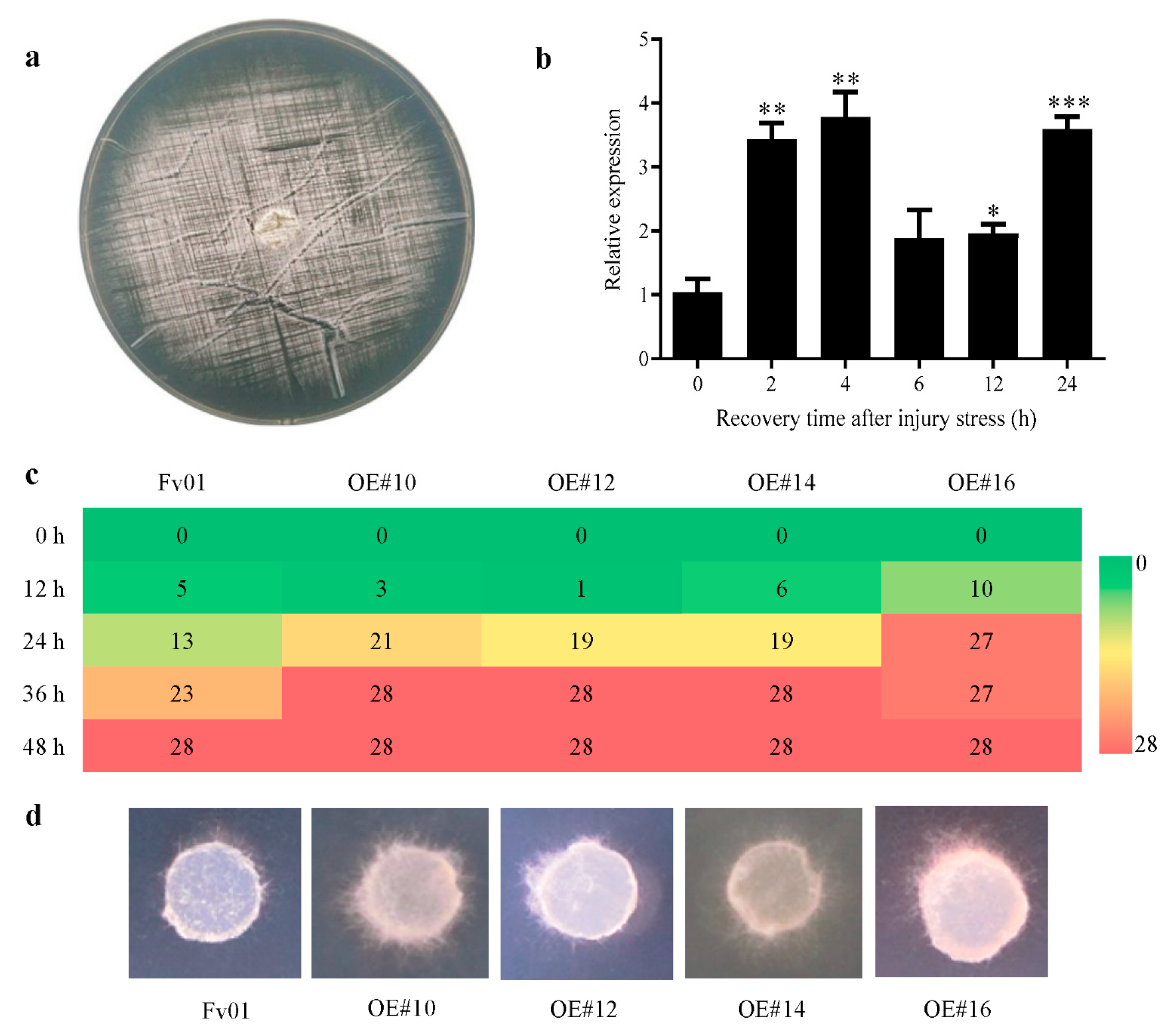
3.4. The Role of FfGS6 in the Injury Stress Response of Mycelium
3.5. The Action of FfGS6 in the Oxidative Stress Response of Mycelium
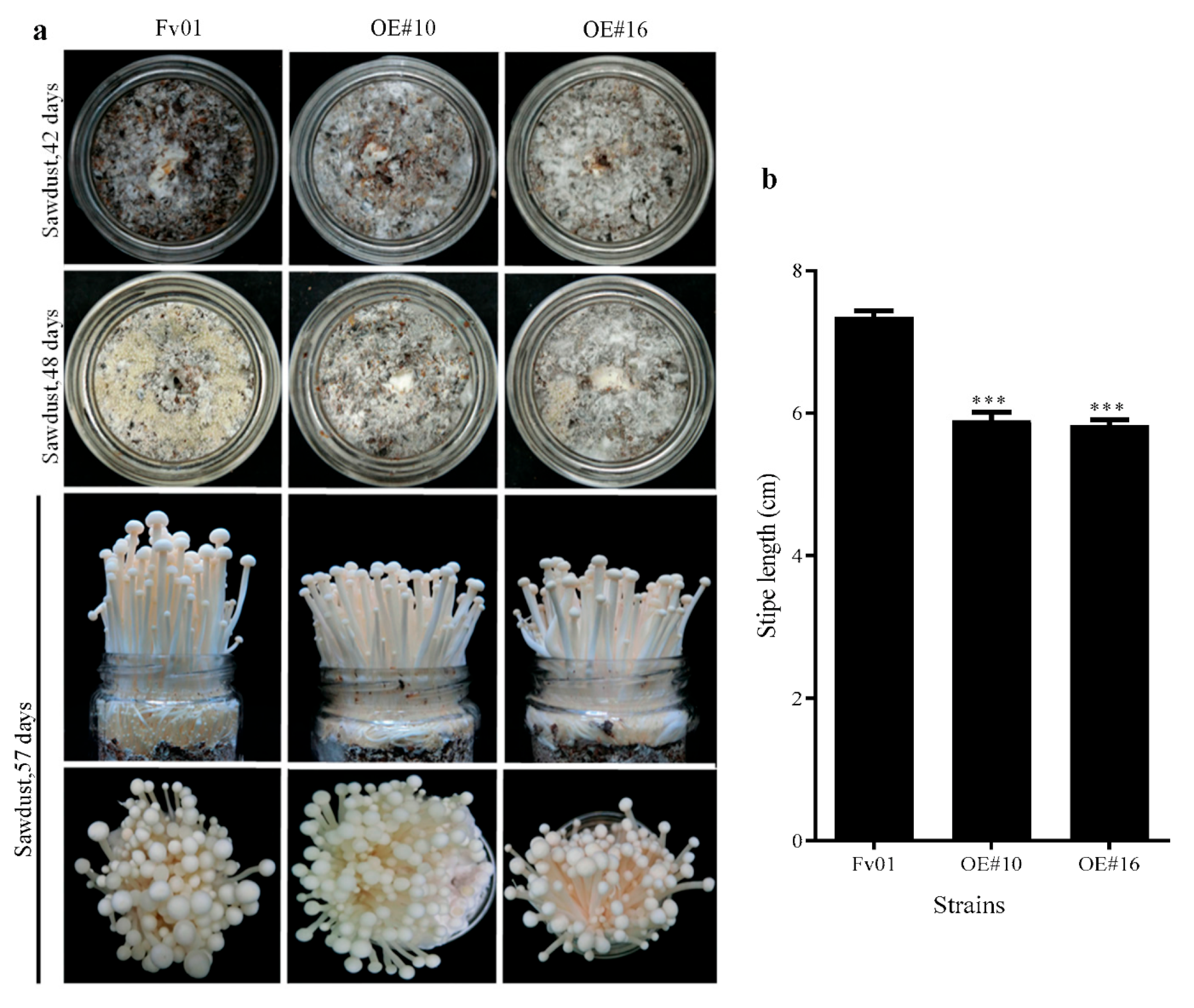
3.6. Impacts of FfGS6 Overexpression on F. filiformis Fruiting Body Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.M.; Liu, X.B.; Dai, Y.C.; Horak, E.; Steffen, K.; Yang, Z.L. Phylogeny and species delimitation of Flammulina: Taxonomic status of winter mushroom in East Asia and a new European species identified using an integrated approach. Mycol. Prog. 2018, 17, 1013–1030. [Google Scholar] [CrossRef]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Mario, A.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Attaran Dowom, S.; Rezaeian, S.; Pourianfar, H.R. Agronomic and environmental factors affecting cultivation of the winter mushroom or enokitake: Achievements and prospects. Appl. Microbiol. Biot. 2019, 103, 2469–2481. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ye, Z.; Guo, L.; Yang, X.; Lin, J. De novo transcriptome sequencing of Flammulina velutipes uncover candidate genes associated with cold-induced fruiting. J. Basic. Microb. 2018, 58, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Tong, Z.J.; Liu, Y.Y.; Li, Y.N.; Zhao, C.; Mukhtar, I.; Tao, Y.X.; Chen, B.Z.; Deng, Y.J.; Xie, B.G. Comparative transcriptomics of Flammulina filiformis suggests a high CO2 concentration inhibits early pileus expansion by decreasing cell division control pathways. Int. J. Mol. Sci. 2019, 20, 5923. [Google Scholar] [CrossRef]
- Liu, X.B.; Xia, E.H.; Li, M.; Cui, Y.Y.; Wang, P.M.; Zhang, J.X.; Xie, B.G.; Xu, J.P.; Yan, J.J.; Li, J.; et al. Transcriptome data reveal conserved patterns of fruiting body development and response to heat stress in the mushroom-forming fungus Flammulina filiformis. PLoS ONE 2020, 15, e0239890. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chang, M.C.; Meng, J.L.; Feng, C.P.; Wang, Y. A comparative proteome approach reveals metabolic changes associated with Flammulina velutipes mycelia in response to cold and light stress. J. Agr. Food Chem. 2018, 66, 3716–3725. [Google Scholar] [CrossRef]
- Liu, C.C.; Bi, J.J.; Kang, L.Q.; Zhou, J.S.; Liu, X.; Liu, Z.H.; Yuan, S. The molecular mechanism of stipe cell wall extension for mushroom stipe elongation growth. Fungal Biol. Rev. 2021, 35, 14–26. [Google Scholar] [CrossRef]
- Akira, Y.; Ken, M.; Keietsu, A. Cell wall structure and biogenesis in Aspergillus species. Biosci. Biotech. Bioch. 2016, 80, 1700–1711. [Google Scholar]
- Qian, B.; Liu, X.Y.; Jia, J.; Cai, Y.C.; Chen, C.; Zhang, H.F.; Zheng, X.B.; Wang, P.; Zhang, Z.G. MoPpe1 partners with MoSap1 to mediate TOR and cell wall integrity signalling in growth and pathogenicity of the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2018, 20, 3964–3979. [Google Scholar] [CrossRef]
- Patel, P.K.; Free, S.J. The genetics and biochemistry of cell wall structure and synthesis in Neurospora crassa, a model filamentous fungus. Front. Microbiol. 2019, 10, 2294–23122019. [Google Scholar] [CrossRef] [PubMed]
- Ramón, A.R.; Vanessa, N.; José, B. Morphological response to salinity, temperature, and pH changes by marine fungus Epicoccum nigrum. Environ. Monit. Assess. 2019, 191, 35. [Google Scholar]
- Aimanianda, V.; Clavaud, C.; Simenel, C.; Fontaine, T.; Delepierre, M.; Latgé, J.P. Cell Wall β-(1,6)-Glucan of Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 13401–13412. [Google Scholar] [CrossRef] [PubMed]
- Montijn, R.C.; Vink, E.; Müller, W.H.; Verkleij, A.J.; Van, D.E.H.; Henrissat, B.; Klis, F.M. Localization of synthesis of β-1,6-glucan in Saccharomyces cerevisiae. J. Bacteriol. 2000, 181, 7414–7420. [Google Scholar] [CrossRef] [PubMed]
- Humbel, B.M.; Konomi, M.; Takagi, T.; Kamasawa, N.; Ishijima, S.A.; Osumi, M. In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 2001, 18, 433–4442001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, J.; Wang, L.; Wang, Y.; Du, G.; Kang, Z. Metabolic engineering of Saccharomyces cerevisiae to improve glucan biosynthesis. J. Microbiol. Biotechnol. 2019, 29, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, N.; Yao, G.Y.; Mu, C.H.; Wang, Y.; Sang, J.L. Blocking β-1,6-glucan synthesis by deleting KRE6 and SKN1 attenuates the virulence of Candida albicans. Mol. Microbiol. 2019, 111, 604–620. [Google Scholar] [CrossRef]
- Oyama, S.; Inoue, H.; Yamagata, Y.; Nakajima, T.; Abe, K. Functional analysis of an endo-1,6-beta-D-glucanase gene (neg-1) from Neurospora crassa. Biosci. Biotech. Bioch. 2006, 70, 1773–1775. [Google Scholar] [CrossRef]
- Mol, P.C.; Jgh, W. Differences in wall structure between substrate hyphae and hyphae of fruit-body stipes in Agaricus bisporus. Mycol. Res. 1990, 94, 472–479. [Google Scholar] [CrossRef]
- Braaksma, A.; Van, D.A.A.; Kieft, H.; Van, A.A.C. Morphometric analysis of ageing mushrooms (Agaricus bisporus) during postharvest development. Postharvest Biol. Technol. 1997, 13, 71–79. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Kingsnorth, C.S.; Jones, H.E.; Burton, K.S. Genes with increased transcript levels following harvest of the sporophore of Agaricus bisporus, have multiple physiological roles. Mycol. Res. 2001, 105, 1223–1230. [Google Scholar] [CrossRef]
- Long, Y.; Yan, J.J.; Tong, Z.J.; Liu, Y.Y.; Miao, J.; Tao, Y.X.; Jiang, Y.J.; Xie, B.G. Fv-gs6: A glucan synthase gene associated with stipe elongation of Flammulina velutipes. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2018, 47, 698–704. [Google Scholar]
- Okamoto, T.; Yamada, M.; Sekiya, S.; Okuhara, T.; Taguchi, G.; Inatomi, S.; Shimosaka, M. Agrobacterium tumefaciens-mediated transformation of the vegetative dikaryotic mycelium of the cultivated mushroom Flammulina velutipes. Biosci. Biotech. Bioch. 2010, 74, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, C.; Xie, B.; Zhang, L.; Yan, S.; Wang, W.; Tao, Y.; Li, S. A single transcription factor PDD1 determines development and yield of Winter Mushroom Flammulina velutipes. Appl. Environ. Microb. 2019, 85, e01735-19. [Google Scholar] [CrossRef]
- Van Burik, J.A.H.; Schreckhise, R.W.; White, T.C.; Bowden, R.A.; Myerson, D. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Med. Mycol. 1998, 36, 299–303. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xie, L.Y.; Li, Y.N.; Ma, X.B.; Yang, H.; Wang, M.; Xie, B.G. Identification of exogenous DNA insertion site of transformants in Flammulina filiformis using matrix for sequencing. Mycosystema 2020, 39, 983–992. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, J.E.; Kim, B.B.; Kim, S.M.; Ro, H.S. Determination of Mineral Components in the Cultivation Substrates of Edible Mushrooms and Their Uptake into Fruiting Bodies. Mycobiology 2009, 37, 109–113. [Google Scholar] [CrossRef]
- Gong, W.; Zhou, Y.; Wang, R.; Wei, X.; Zhang, L.; Dai, W.; Zhu, Z. Analysis of T-DNA integration events in transgenic rice. J. Plant Physiol. 2021, 266, 153527. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive mamp in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jin, K.; Xia, Y. Mafks, a β-1,3-glucan synthase, is involved in cell wall integrity, hyperosmotic pressure tolerance and conidiation in Metarhizium acridum. Curr. Genet. 2011, 57, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Shuai, B. Rnai-mediated knockdown of β-1,3-glucan synthase suppresses growth of the phytopathogenic fungus Macrophomina phaseolina. Physiol. Mol. Plant Pathol. 2020, 110, 101486. [Google Scholar] [CrossRef]
- Jiang, L.H.; Li, X.F.; Zan, X.Y.; Fu, X.; Cui, F.J.; Zhu, H.A.; Sun, W.J.; Tao, T.L. The β-1,3-glucan synthase gene GFGLS2 plays major roles in mycelial growth and polysaccharide synthesis in Grifola frondose. Appl. Microbiol. Biot. 2022, 106, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Dallies, N.; Francois, J.; Paquet, V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast 1998, 14, 1297–1306. [Google Scholar] [CrossRef]
- Brown, J.L.; Bussey, H. The yeast KRE9 gene encodes an O glycoprotein involved in cell surface beta-glucan assembly. Mol. Cell. Biol. 1993, 13, 6346–6356. [Google Scholar]
- Pan, H.P.; Wang, N.; Tachikawa, H.; Nakanishi, H.; Gao, X.D. β-1,6-glucan synthesis-associated genes are required for proper spore wall formation in Saccharomyces cerevisiae. Yeast 2017, 34, 431–446. [Google Scholar] [CrossRef]
- Kuees, U.; Navarro-Gonzalez, M. How do Agaricomycetes shape their fruiting bodies? 1. Morphological aspects of development. Fungal Biol. Rev. 2015, 29, 63–97. [Google Scholar]
- Niu, X.; Liu, Z.H.; Zhou, Y.J.; Wang, J.; Zhang, W.M.; Yuan, S. Stipe cell wall architecture varies with the stipe elongation of the mushroom Coprinopsis cinerea. Fungal Biol. 2015, 119, 946–956. [Google Scholar] [CrossRef]
- Kang, L.Q.; Zhang, X.W.; Liu, X.; Wang, R.; Liu, C.C.; Zhou, J.S.; Liu, Z.H.; Yuan, S. Comparative study of β-glucan-degrading enzymes from Coprinopsis cinerea for their capacities to induce stipe cell wall extension. Int. J. Biol. Macromol. 2020, 152, 516–524. [Google Scholar] [CrossRef]
- Li, M.M.; Bi, J.J.; Bai, Y.; Kang, L.Q.; Duan, B.Y.; Liu, Z.H.; Yuan, S. Accumulation and cross-linkage of β-1,3/1,6-glucan lead to loss of basal stipe cell wall extensibility in mushroom Coprinopsis cinerea. Carbohyd. Polym. 2021, 259, 117743. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chekanova, J.; Liu, Y.; Gan, B.; Long, Y.; Han, X.; Tong, Z.; Miao, J.; Lian, L.; Xie, B.; et al. Reactive Oxygen Species Distribution Involved in Stipe Gradient Elongation in the Mushroom Flammulina filiformis. Cells 2022, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, J.; Chen, Y.; Wang, Y.; Chen, J.; Bi, J.; Lyu, L.; Yu, C.; Yuan, S.; Liu, Z. MAPK CcSakA of the HOG Pathway Is Involved in Stipe Elongation during Fruiting Body Development in Coprinopsis cinerea. J. Fungi 2022, 8, 534. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Primer Sequence (5′-3′) | Purpose |
|---|---|---|
| pFfGS6-F | CATCTGCTGTTTGCTGCTC | Verify target fragment |
| pFfGS6-R | CCCTTATCTGGGAACTACTCAC | |
| Hpt-F | CACATCCACCATCTCCGT | Amplify resistance gene |
| Hpt-R | AAATTGCCGTCAACCAAG | |
| qFfGAPDH-F | CCTCTGCTCACTTGAAGGGT | qRT-PCR |
| qFfGAPDH-R | GCGTTGGAGATGACTTTGAA | |
| qFfGS6-F | GAGTTGTGGTCGTTAAAGGGAA | qRT-PCR |
| qFfGS6-R | CGTCAATCATATCCACAGCGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ma, X.; Long, Y.; Yao, S.; Wei, C.; Han, X.; Gan, B.; Yan, J.; Xie, B. Effects of β-1,6-Glucan Synthase Gene (FfGS6) Overexpression on Stress Response and Fruit Body Development in Flammulina filiformis. Genes 2022, 13, 1753. https://doi.org/10.3390/genes13101753
Liu Y, Ma X, Long Y, Yao S, Wei C, Han X, Gan B, Yan J, Xie B. Effects of β-1,6-Glucan Synthase Gene (FfGS6) Overexpression on Stress Response and Fruit Body Development in Flammulina filiformis. Genes. 2022; 13(10):1753. https://doi.org/10.3390/genes13101753
Chicago/Turabian StyleLiu, Yuanyuan, Xinbin Ma, Ying Long, Sen Yao, Chuanzheng Wei, Xing Han, Bingcheng Gan, Junjie Yan, and Baogui Xie. 2022. "Effects of β-1,6-Glucan Synthase Gene (FfGS6) Overexpression on Stress Response and Fruit Body Development in Flammulina filiformis" Genes 13, no. 10: 1753. https://doi.org/10.3390/genes13101753
APA StyleLiu, Y., Ma, X., Long, Y., Yao, S., Wei, C., Han, X., Gan, B., Yan, J., & Xie, B. (2022). Effects of β-1,6-Glucan Synthase Gene (FfGS6) Overexpression on Stress Response and Fruit Body Development in Flammulina filiformis. Genes, 13(10), 1753. https://doi.org/10.3390/genes13101753






