Methyl Jasmonate Induces Genes Involved in Linalool Accumulation and Increases the Content of Phenolics in Two Iranian Coriander (Coriandrum sativum L.) Ecotypes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes in Linalool under MeJA Concentrations
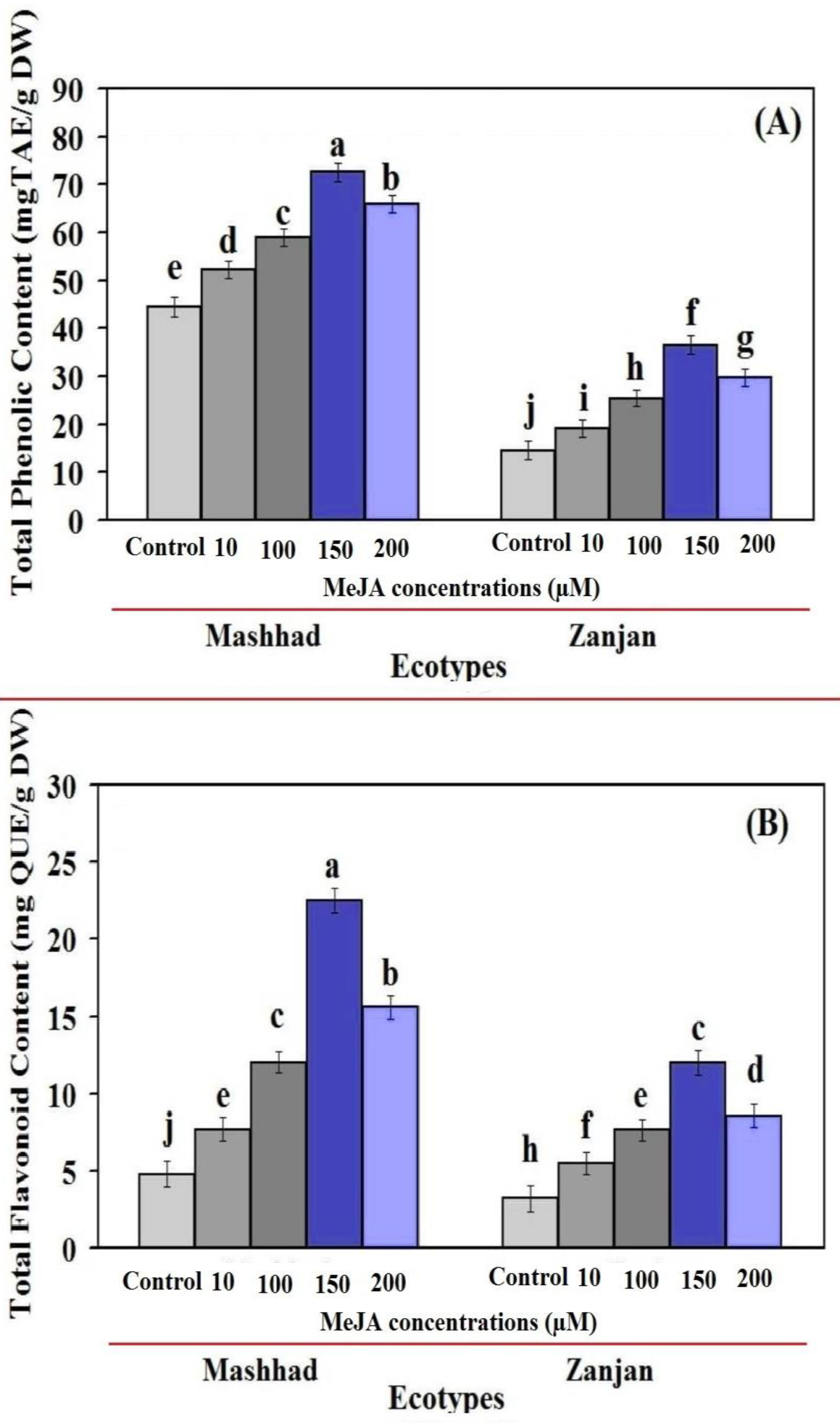
2.2. Total Flavonoid Content and Total Phenol Content (TPC) as a Function of MeJA Concentration (TFC)
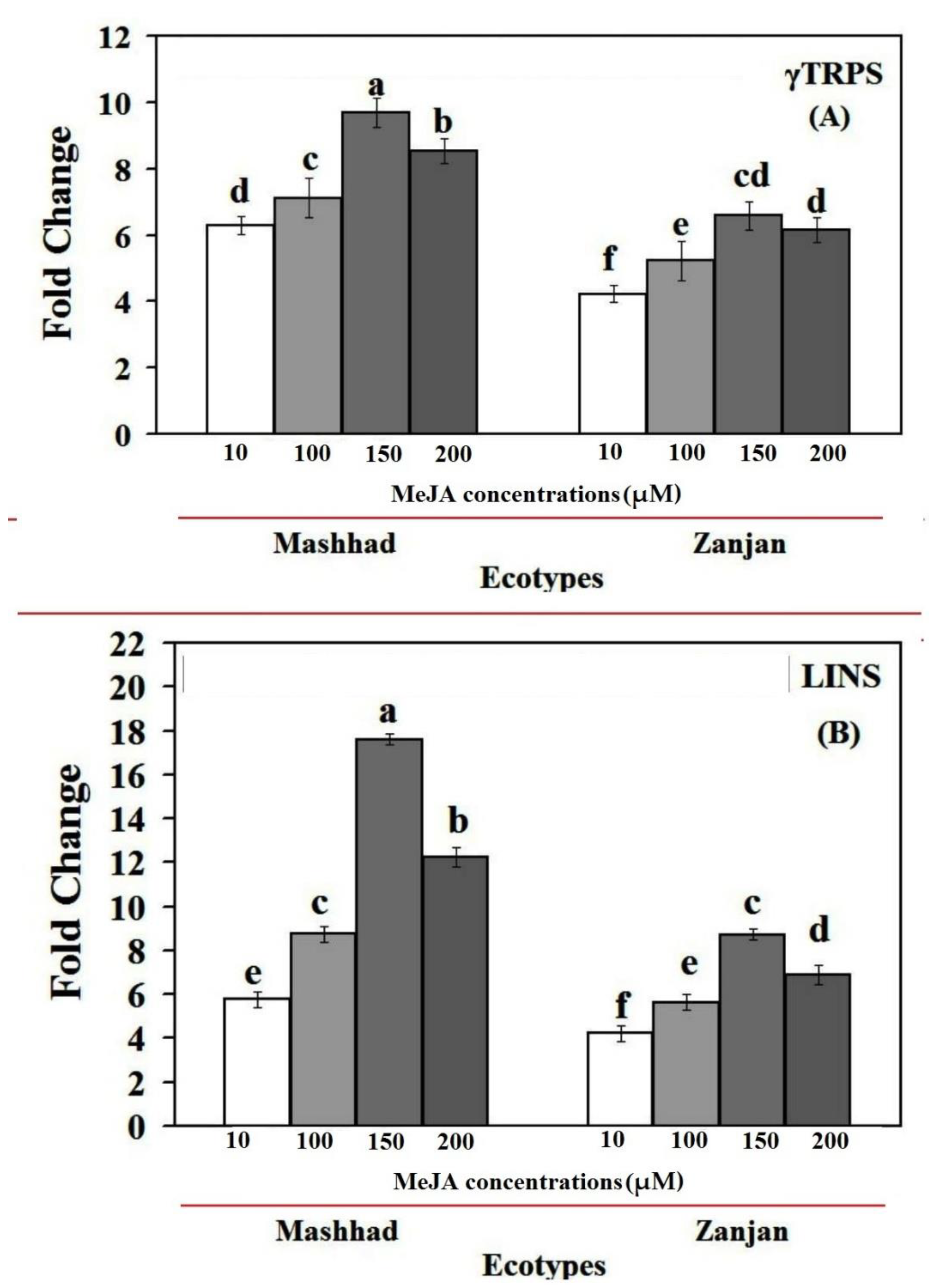
2.3. MeJA Effects on the Expression of the CsγTRPS and CsLINS Genes
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. MeJA Treatments
3.3. RNA Extraction, Complementary DNA (cDNA) Synthesis, and q-PCR Evaluation
3.4. Determination of Linalool
3.5. Flavonoid Contents and Total Phenolic Assay
3.6. Statistical Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, A.; Ciftci, V. Genetic relationships and diversity analysis in Turkish laurel (Laurus nobilis L.) germplasm using ISSR and SCoT markers. Mol. Biol. Rep. 2021, 48, 4537–4547. [Google Scholar] [CrossRef]
- Yilmaz, A.; Guler, E.; Soydemir, H.E.; Demirel, S.; Mollahaliloglu, S.; Karadeniz, T.; Ciftci, V. Miracle plant: Aronia (Aronia melanocarpa). MAS J. Appl. Sci. 2021, 6, 83–94. [Google Scholar]
- Yilmaz, A.; Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crops Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]
- Arif, M.; Khurshid, H.; Khan, S. Genetic structure and green leaf performance evaluation of geographically diverse population of coriander (Coriandrum sativum L.). Eur. J. Acad. Res. 2014, 2, 3269–3285. [Google Scholar]
- Bhat, S.; Kaushal, P.; Kaur, M.; Sharma, H.K. Coriander (Coriandrum sativum L.): Processing, nutritional and functional aspects. Afr. J. Plant Sci. 2014, 8, 25–33. [Google Scholar]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Anjum, F.M.; Khan, M.I.; Tehseen, S.; El-Ghorab, A.; Sultan, J.I. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.): A review. Br. Food J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- Teneva, D.; Denkova, Z.; Goranov, B.; Denkova, R.; Kostov, G.; Atanasova, T.; Merdzhanov, P. Chemical composition and antimicrobial activity of essential oils from black pepper, cumin, coriander and cardamom against some pathogenic microorganisms. Food Technol. 2016, 2, 39–52. [Google Scholar] [CrossRef]
- Beyzi, E.; Karamanb, K.; Gunesc, A.; Beyzid, S.B. Change in some biochemical and bioactive properties and essential oil composition of coriander seed (Coriandrum sativum L.) varieties from Turkey. Ind. Crops Prod. 2017, 109, 74–78. [Google Scholar] [CrossRef]
- Reuter, J.; Huyke, C.; Casetti, F.; Theek, C.; Frank, U.; Augustine, M.; Schempp, C. Anti-inflammatory potential of a lipolotion containing coriander oil in the ultraviolet erythema test. J. Dtsch. Dermatol. Ges. 2008, 6, 847–851. [Google Scholar] [CrossRef]
- Kiralan, M.; Calikoglu, E.; Ipek, A.; Bayrak, A.; Gurbuz, B. Fatty acid and volatile oil composition of different coriander (Coriandrum sativum) registered varieties cultivated in Turkey. Chem. Nat. Compd. 2009, 45, 100–102. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Gross, M.; Joel, D.M.; Cohen, Y.; Bar, E.; Friedman, J.; Lewinsohn, E. Ontogenesis of mericarps of bitter fennel (Foeniculum vulgare mill. var. vulgare) as related to t-anethole accumulation. Isr. J. Plant Sci. 2006, 54, 309–316. [Google Scholar] [CrossRef]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef]
- Hasunuma, T.; Takeno, S.; Hayashi, S.; Sendai, M.; Bambi, T.; Yoshimura, S.; Tomizawa, K.; Fukusaki, E.; Miyake, C. Overexpression of 1-deoxy-Dxylulose-5-phosphate reductoisomerase gene in chloroplast contributes to increment of isoprenoid production. J. Biosci. Bioeng. 2008, 105, 518–526. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M. Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 2010, 504, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ganjewala, D.; Kumar, S.; Luthra, R. An account of cloned genes of methylerythritol-4-phosphate pathway of isoprenoid biosynthesis in plants. Curr. Issues Mol. Biol. 2009, 11, 35–45. [Google Scholar]
- Mahmoud, S.S.; Croteau, R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.; Schimmel, J.; Lukas, B.; Novak, J.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G.; Degenhardt, J.; Trindade, H. Genomic characterization, molecular cloning and expression analysis of two terpene synthases from Thymus caespititius. Planta 2013, 238, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.; Parthier, C.; Egerer-Sieber, C.; Geiger, D.; Muller, Y.A.; Kreis, W.; Müller-Uri, F. Expression, crystallization and structure elucidation of γ-terpinene synthase from Thymus vulgaris. Acta Crystallogr. F Struct. Biol. Commun. 2019, 72, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Liao, P.; Crocoll, C.; Boachon, B.; Forster, C.; Leidecker, F.; Wiese, N.; Zhaoe, D.; Joshua, C.; Wood, J.C.; et al. The biosynthesis of thymol, carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, 52. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, F.; PourAboughadareh, A.; Majdi, M.; Poczai, P. Effect of Methyl Jasmonate on Thymol, Carvacrol, Phytochemical Accumulation, and Expression of Key Genes Involved in Thymol/Carvacrol Biosynthetic Pathway in Some Iranian Thyme Species. Int. J. Mol. Sci. 2021, 22, 11124. [Google Scholar] [CrossRef] [PubMed]
- Ashaari, N.S.; Ab Rahim, M.H.; Sabri, S.; Lai, K.S.; Song, A.A.; Abdul Rahim, R.; Wan Abdullah, W.M.A.N.; Ong Abdullah, J. Functional characterization of a new terpene synthase from Plectranthus amboinicus. PLoS ONE 2020, 15, e0235416. [Google Scholar] [CrossRef]
- Galata, M.; Sarker, L.S.; Mahmoud, S.S. Transcriptome profiling, and cloning and characterization of the main monoterpene synthases of Coriandrum sativum L. Phytochemistry 2014, 102, 64–73. [Google Scholar] [CrossRef]
- Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 1998, 95, 4126–4133. [Google Scholar] [CrossRef]
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.I.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef]
- Bao, T.; Shadrack, K.; Yang, S.; Xue, X.; Li, S.; Wang, N.; Cronk, Q. Functional characterization of terpene synthases accounting for the volatilized-terpene heterogeneity in Lathyrus odoratus cultivar flowers. Plant Cell Physiol. 2020, 61, 1733–1749. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, C.; Zhang, G.; Teixeira da Silva, J.A.; Duan, J. Genome-wide identification and expression profile of TPS gene family in Dendrobium officinale and the role of DoTPS10 in linalool biosynthesis. Int. J. Mol. Sci. 2020, 21, 5419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Deng, R.; Xu, X.; Yang, Z. Enzyme catalytic efficiencies and relative gene expression levels of (R)-linalool synthase and (S)-linalool synthase determine the proportion of linalool enantiomers in Camellia sinensis var. sinensis. J. Agric. Food Chem. 2020, 68, 10109–10117. [Google Scholar] [CrossRef] [PubMed]
- Hectors, K.; Van Oevelen, S.; Geuns, J.; Guisez, Y.; Jansen, M.A.; Prinsen, E. Dynamic changes in plant secondary metabolites during UV acclimation in Arabidopsis thaliana. Physiol. Plant. 2014, 152, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hojati, M.; Modarres-Sanavy, S.A.M.; Tahmasebi Enferadi, S.; Majdi, M.; Ghanati, F.; Farzadfar, S. Differential deployment of parthenolide and phenylpropanoids in feverfew plants subjected to divalent heavy metals and transcinnamic acid. Plant Soil. 2016, 399, 41–59. [Google Scholar] [CrossRef]
- Majdi, M.; Abdollahi, M.R.; Maroufi, A. Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant Cell Rep. 2015, 34, 1909–1918. [Google Scholar] [CrossRef]
- Elyasi, R.; Majdi, M.; Bahramnejad, B.; Mirzaghaderi, G. Spatial modulation and abiotic elicitors responses of the biosynthesis related genes of mono/triterpenes in blak cumin (Nigella sativa). Ind. Crops Prod. 2016, 79, 240–247. [Google Scholar] [CrossRef]
- Fatemi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Garagounis, C.; Papadopoulou, K. Identification and expression profiling of rosmarinic acid biosynthetic genes from Satureja khuzistanica under carbon nanotubes and methyl jasmonate elicitation. Plant Cell Tissue Organ Cult. 2019, 136, 561–573. [Google Scholar]
- Fatemi, F.; Abdollahi, M.R.; Mirzaie-Asl, A.; Dastan, D.; Papadopoulou, K. Phytochemical, antioxidant, enzyme activity and antifungal properties of Satureja khuzistanica in vitro and in vivo explants stimulated by some chemical elicitors. Pharm. Biol. 2020, 58, 286–296. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Identification and tissue-specific expression of rutin biosynthetic pathway genes in Capparis spinosa elicited with salicylic acid and methyl jasmonate. Sci. Rep. 2020, 10, 8884. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Biosynthesis of rutin changes in Capparis spinosa due to altered expression of its pathway genes under elicitors’ supplementation. Plant Cell Tissue Organ Cult. 2020, 141, 619–631. [Google Scholar] [CrossRef]
- Kianersi, F.; Amin Azarm, D.; Pour-Aboughadareh, A.; Poczai, P. Change in Secondary Metabolites and Expression Pattern of Key Rosmarinic Acid Related Genes in Iranian Lemon Balm (Melissa officinalis L.) Ecotypes Using Methyl Jasmonate Treatments. Molecules 2022, 27, 1715. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R.; Kianersi, F.; Moosavi, S.S.; Dastan, D.; Asadi, S. Identification and Expression Analysis of Two Genes Involved in the Biosynthesis of t-Anethole in Fennel (Foeniculum vulgare Mill.) and Their Up-Regulation in Leaves in Response to Methyl Jasmonate Treatments. J. Plant Growth Regul. 2022, 1–12. [Google Scholar] [CrossRef]
- Van Schie, C.C.N.; Haring, M.A.; Schuurink, R.C. Tomato Linalool Synthase Is Induced in Trichomes by Jasmonic Acid. Plant Mol. Biol. 2007, 64, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Park, W.T.; Arasu, M.V.; Al-Dhabi, N.A.; Yeo, S.K.; Jeon, J.; Park, J.S.; Lee, S.Y.; Park, S.U. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in agastache rugosa cell culture. Molecules 2016, 21, 426. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Sa, R.; Feng, K.; Zhao, M.; Yu, J.; Ji, Y.; Hou, M.; et al. Effect of Methyl Jasmonate on Phenolic Accumulation in Wounded Broccoli. Molecules 2019, 24, 3537. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wang, X.; Choi, J.H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.J.; Lim, J.H. Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J. Agric. Food Chem. 2011, 59, 5707–5713. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Chun, S.W.; Chung, Y.S.; Lee, S.Y.; Park, S.U. Influence of Chitosan, Salicylic Acid and Jasmonic Acid on Phenylpropanoid Accumulation in Germinated Buckwheat (Fagopyrum esculentum Moench). Foods 2019, 8, 153. [Google Scholar] [CrossRef]
- Jaafar, H.Z.; Ibrahim, M.H.; Mohamad-Fakri, N.F. Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 2012, 17, 7305–7322. [Google Scholar] [CrossRef]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 2016, 213, 196–205. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G. Comparison of essential oil composition, flavonoid content and antioxidant activity in eight Achillea species. J. Essent. Oil-Bear. Plants 2015, 18, 1382–1394. [Google Scholar] [CrossRef]
- Brouki Milan, E.; Mandoulakani, B.A.; Kheradmand, F. The effect of methyl jasmonate on the expression of phenylalanine ammonia lyase and eugenol-o-methyl transferase genes in basil. Philipp. Agric. Sci. 2017, 100, 163–167. [Google Scholar]
- Bechen, L.L.; Johnson, M.G.; Broadhead, G.T.; Levin, R.A.; Overson, R.P.; Jogesh, T.; Fant, J.B.; Raguso, R.A.; Skogen, K.A.; Wickett, N.J. Differential gene expression associated with a floral scent polymorphism in the evening primrose Oenothera harringtonii (Onagraceae). BMC Genom. 2022, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Galletti, G.C.; Bocchini, P. Carnacini, A. Essential oil chemical composition of wild populations of italian oregano spice (Origanum vulgare ssp. hirtum (Link) Ietswaart): A preliminary evaluation of their use in chemotaxonomy by cluster analysis. 1. Inflorescences. J. Agri. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant. Mol. Biol. 2010, 73, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Patricelli, D.; Barbero, F.; Occhipinti, A.; Bertea, C.M.; Bonelli, S.; Casacci, L.P.; Zebelo, S.A.; Crocoll, C.; Gershenzon, J.; Maffei, M.E.; et al. Plant defences against ants provide a pathway to social parasitism in butterflies. Proc. R. Soc. 2015, 282, 1811. [Google Scholar] [CrossRef]
- Sarabandi, M.; Farokhzad, A.; Mandoulakani, B.A.; Ghasemzadeh, R. Biochemical and gene expression responses of two Iranian grape cultivars to foliar application of methyl jasmonate under boron toxicity conditions. Sci. Hort. 2019, 249, 355–363. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef] [PubMed]
- Khakshour, A.; Karimzadeh, G.; Sabet, M.S.; Sayadi, V. Study of Morpho-phenological diversity and expression of genes involved in γ-Terpinene and Linalool biosynthesis in Iranian endemic populations of coriander (Coriandrum sativum L.). Iran. J. Rangel. For. Plant Breed. Genet. Res. 2021, 29, 51–63. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Msaada, K.; Hosni, K.; Ben Taarit, M.; Ouchikh, O.; Marzouk, B. Variations in essential oil composition during maturation of coriander (Coriandrum sativum L.) fruits. J. Food Biochem. 2009, 33, 603–612. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Yao, X.H.; Duan, M.H.; Wei, F.Y.; Wu, G.H.; Li, L. Variation of essential oil content and antioxidant activity of Lonicera species in different sites of China. Ind. Crops Prod. 2015, 77, 772–779. [Google Scholar] [CrossRef]


| Real-Time Primers | Sequences (5′ to 3′) |
|---|---|
| CsγTRPS F CsγTRPS R | CGAAATGGTGGAAGGACACAGA GTAATAGCAGCGAGCACCTT |
| CsLINS F CsLINS R | GAGAAGGACTTGCATGCTACTG GACATCTGCACGGATACCT |
| β-Actin β-Actin | GACGAGGATGAGGCAGAGTT GGAGCATCAGAAACAGAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kianersi, F.; Amin Azarm, D.; Fatemi, F.; Pour-Aboughadareh, A.; Poczai, P. Methyl Jasmonate Induces Genes Involved in Linalool Accumulation and Increases the Content of Phenolics in Two Iranian Coriander (Coriandrum sativum L.) Ecotypes. Genes 2022, 13, 1717. https://doi.org/10.3390/genes13101717
Kianersi F, Amin Azarm D, Fatemi F, Pour-Aboughadareh A, Poczai P. Methyl Jasmonate Induces Genes Involved in Linalool Accumulation and Increases the Content of Phenolics in Two Iranian Coriander (Coriandrum sativum L.) Ecotypes. Genes. 2022; 13(10):1717. https://doi.org/10.3390/genes13101717
Chicago/Turabian StyleKianersi, Farzad, Davood Amin Azarm, Farzaneh Fatemi, Alireza Pour-Aboughadareh, and Peter Poczai. 2022. "Methyl Jasmonate Induces Genes Involved in Linalool Accumulation and Increases the Content of Phenolics in Two Iranian Coriander (Coriandrum sativum L.) Ecotypes" Genes 13, no. 10: 1717. https://doi.org/10.3390/genes13101717
APA StyleKianersi, F., Amin Azarm, D., Fatemi, F., Pour-Aboughadareh, A., & Poczai, P. (2022). Methyl Jasmonate Induces Genes Involved in Linalool Accumulation and Increases the Content of Phenolics in Two Iranian Coriander (Coriandrum sativum L.) Ecotypes. Genes, 13(10), 1717. https://doi.org/10.3390/genes13101717








