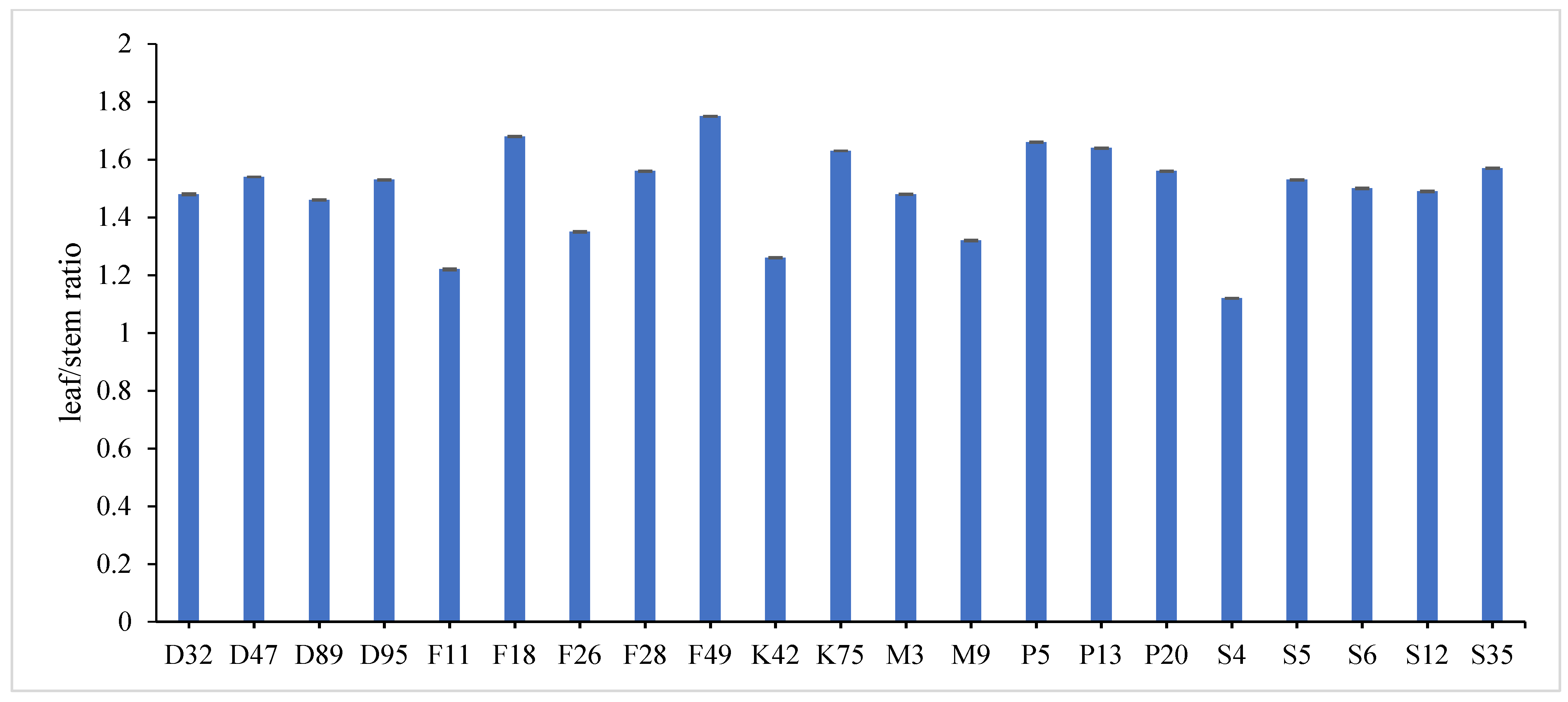1. Introduction
Forage legumes are important for farming systems, especially as protein sources, which are usually the most limiting nutrients in animal diets and can be grazed, harvested and fed fresh or stored as hay or silage. In general Alfalfa (
Medicago sativa L.) is already the most cultivated species in the world for forage production. Alfalfa is a perennial forage legume known for its high forage quality and positive effects on soil fertility [
1]. Its economic significance is based on its high potential for production of biomass, and it is reported to withstand long periods of water deficit by impeding its vegetative growth. In addition, it has access to water from the depths through its deeper root system [
2]. Alfalfa (
Medicago sativa L.) is a major forage crop grown in more than 80 countries on every continent of the world in an area of more than 35 million hectares. It is grown in a wide range of climatic and compositional conditions ranging from semi-arid to humid regions.
In Egypt, the regional varieties have been planted in oases for many years. Currently there are nearly 90,000 acres of alfalfa grown annually and the demand for the crop continues to rise [
3]. Yield data shown an approximate 18% increase in the area allocated to alfalfa recorded from the growing season 2014/2015 to 2016/2017, however, the increase in production was only 7%, indicating the need for improving crop productivity. Because alfalfa adapts to conditions of excessive heat, drought and salinity, it is preferred over the berseem reclamation in Nubaria, Ismailia, in the 1.5 million feddan reclamation project launched by the Egyptian government in 2014. The need for new varieties with higher yields and better feed quality is essential to deal with the increased demand for the crop. Regarding to its importance as a forage crops, scientific efforts have been and are being devoted annually in breeding program regarding the improvement of both yield and quality of alfalfa. Variability for agronomic and morphological characteristics of alfalfa are frequently used in breeding programs for developing cultivars with high forage production and quality [
4]. The success of a good breeding and selection program usually depends on the genetic variability present in the breeding materials and the variation in the population and helps to understand genomic composition, identify genes for vital traits and conserve and classify genetic variation in the plant germplasm.
Seiam and Mohamed (2020) [
5] found highly significant differences in years and seasons for fresh yield, dry yield and protein content of twenty five genotypes of alfalfa. In addition, phenotypic and genotypic coefficients of variation and heritability are very important indicators in improving traits [
6]. Hamd Alla et al. (2013) [
7] showed the values of phenotypic and genotypic coefficients of variation for yield and yield components and revealed relative variation among the tested varieties which were less influenced by the environment.
One of the main goals in alfalfa breeding is the improvement of forage quality through increasing protein and fiber contents that are closely associated with the relative production of the leaf and stem components as measured by leafiness percentage, but more often as leaf/stem ratio (LSR) and the nutritive value of the stem [
8]. Moreover, curd protein is considered an important indicator of forage quality [
9].
Several studies have shown the importance of selection and assessment varieties for quantitative and yield ability in any breeding program, so that the varieties can be introduced to a given local environment [
10]. In addition, multivariate statistical methods and numerical classifications are used, such as cluster analysis which is an effective tool aimed at determining the degree of genetic variation between tested genotypes based on their performance and their contributing characteristics [
11] and Principal Component Analysis (PCA), which has been widely used in the studies of variability in germplasm collections of many species [
12]. The current study was conducted to study and achieve the following objectives: (i) to evaluate twenty-one alfalfa genotypes by focusing on genetic diversity and heritability for yield components, forage yield and quality traits; (ii) to To determine genetic relationships among tested genotypes using cluster analysis and principal component analysis.
3. Results and Discussion
- A.
Agronomic Traits:
- 1.
Variability in Agronomic Traits
Data in
Table 3 and
Table 4 show the mean squares for plant height, number of tiller/m
2, leaf/stem ratio, total fresh and dry yields (ton/fed) of twenty-one alfalfa genotypes in two successive seasons (
Table 3) and the combined across the two years,
Table 4. The results indicated significant and highly significant differences among alfalfa genotypes for all traits under this study. Moreover, from the analysis of variance for combined the results showed significant and highly significant differences between seasons and among genotypes for all studied traits,
Table 4. In addition, the year × genotypes interaction was significant for all traits under this study. These results are confirmed with [
26,
27] who reported significant differences among genotypes (nine cultivars of alfalfa) as well as between genotypes and years for plant height, number of tillers, leaf/stem ratio, fresh and dry forage yields under the New Valley environment, Egypt. Significant differences among alfalfa cultivars and populations for plant height, leaf/stem ratio and green mass yield were detected by [
5,
28].
In addition, Jia et al. (2018) [
29] revealed that highly significant differences among 75 genotypes of alfalfa for different phenotypic traits were observed in both years. At the same time, there were highly significant variations among the genotypes for plant height, no. of tillers, leaf to stem ratio and fresh weight in ten genotypes of alfalfa, [
26]. These results reflect the different genetic backgrounds of the studied genotypes to help the breeder in selecting the germplasm collections for using in breeding programs. Therefore, it is recommended to use a broad genetic base from a variety of sources to include most of the genetic determinants of these traits [
26,
30].
The mean performance of forage yield and its components are tabulated in
Tables S1 and S2. The data revealed a high amount of variation in studied traits for the studied genotypes, where by the second year had higher values for agronomic traits. That is consistent with the results reported by Sabanci et al. (2013) [
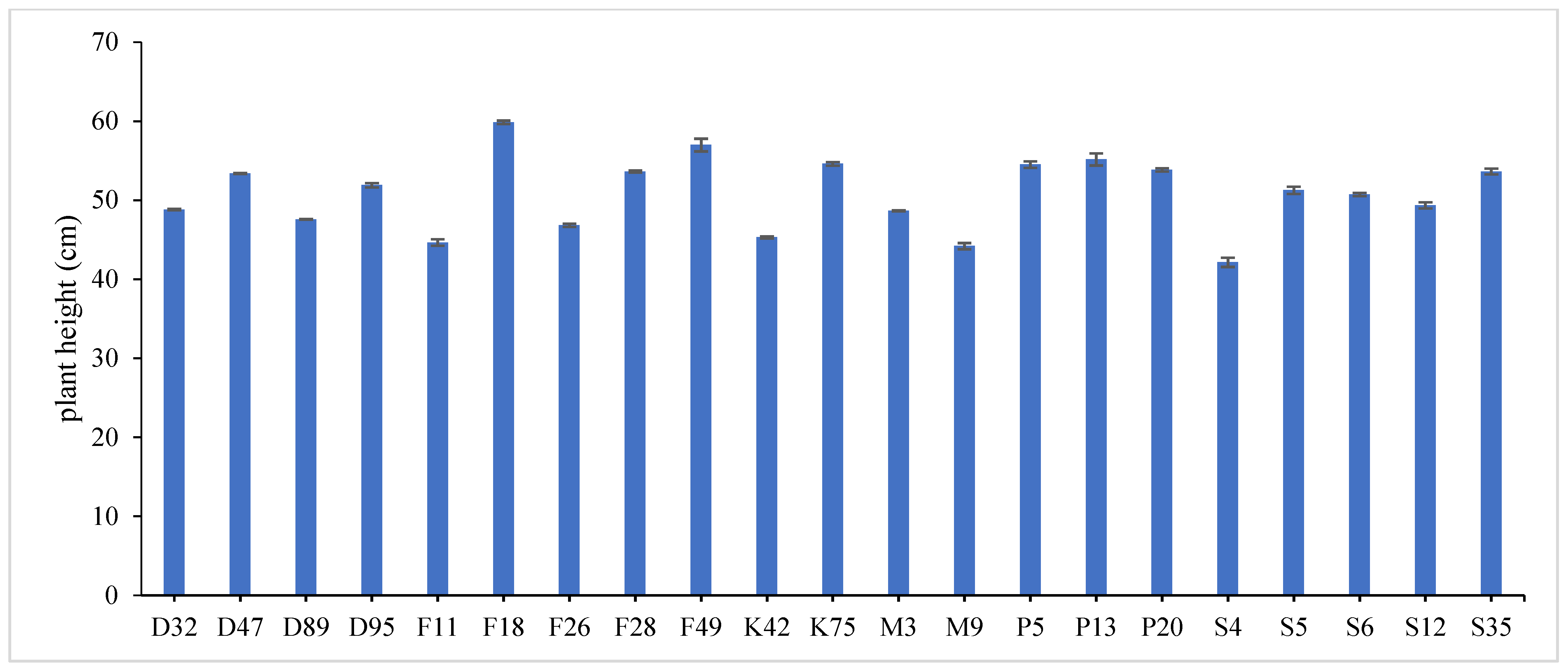
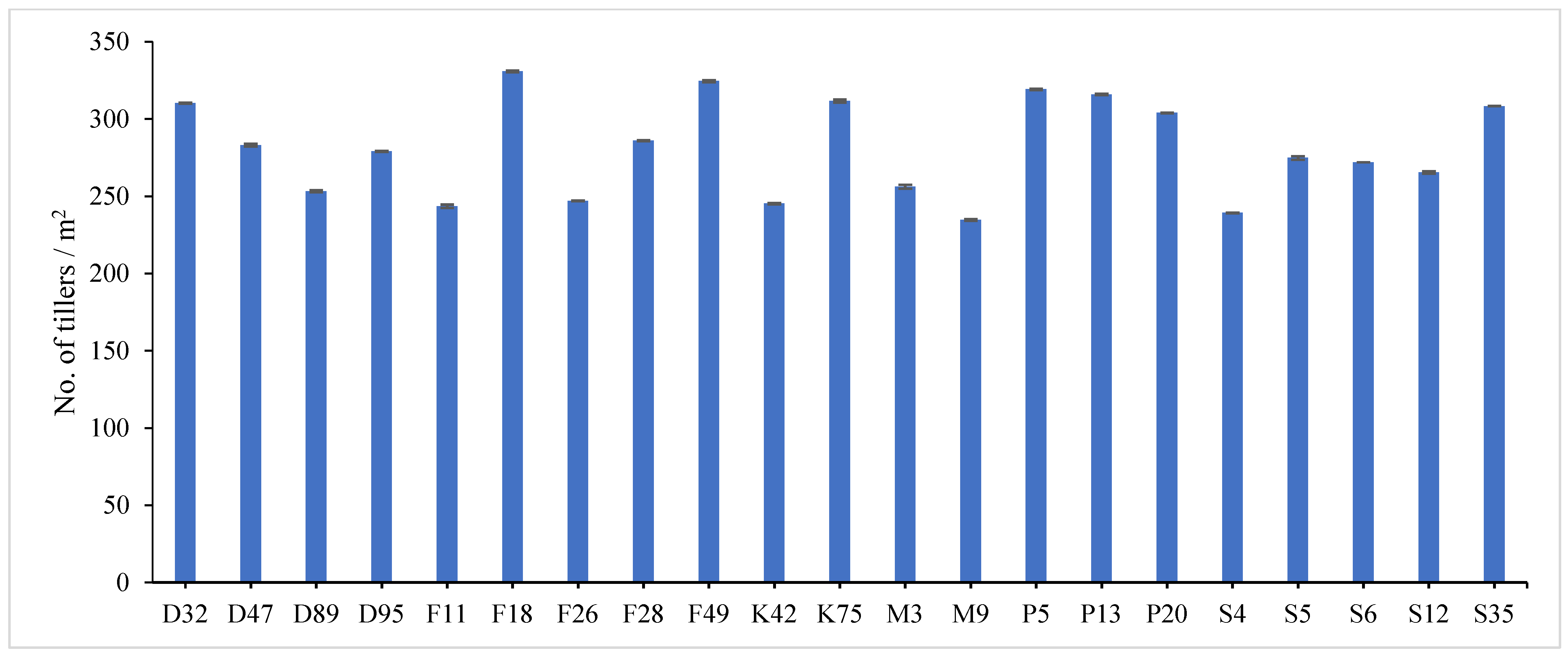
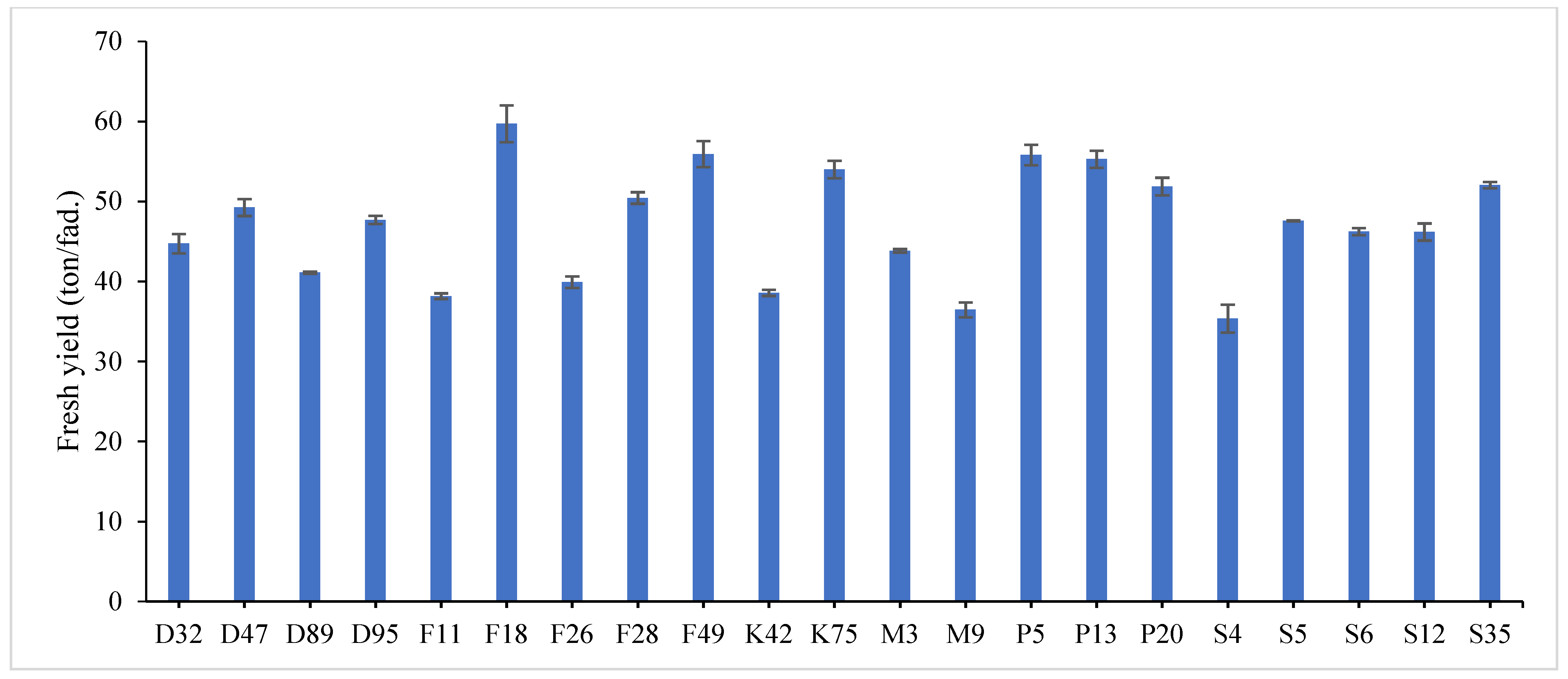
31]. Data revealed that F18 recorded the maximum value for plant height (60.78 and 58.62 cm), number of tiller/m
2 (335.23 and 326.34), total fresh yield (61.03 and 58.36 ton/fed) and total dry yield (15.26 and 13.58 ton/fed) in both years, respectively, while, F49 ranked first (1.859 and 1.644) followed by F18 (1.785 and 1.571) for leaf/stem ratio in both years, respectively. Otherwise, the genotype S4 had the lowest value for all studied traits except for number of tiller/m
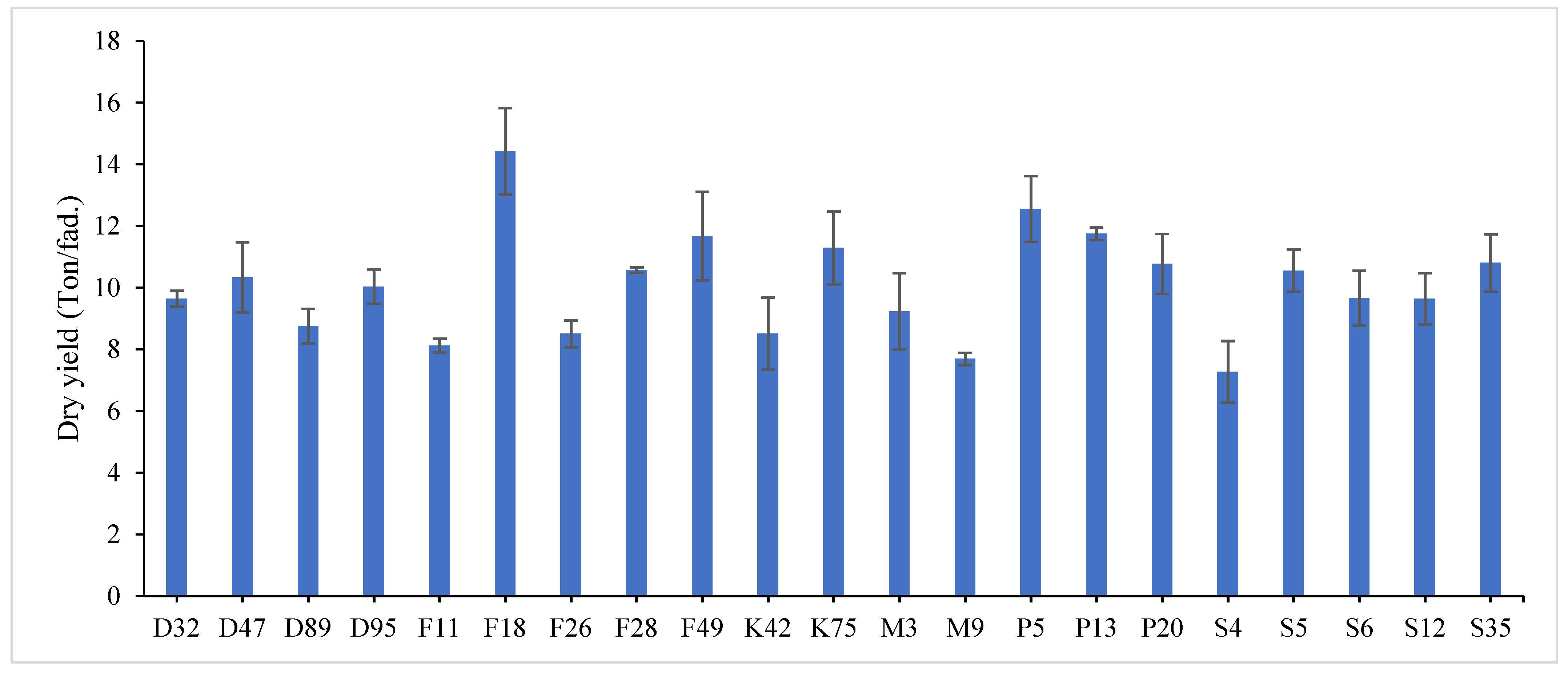
2, where it recorded (43.43 and 40.55 cm) for plant height, (1.016 and 1.231) for leaf/stem ratio, (36.26 and 34.45 ton/ fed) for total fresh yield and (7.91 and 6.62 ton/fed) for total dry yield in both years, respectively.
The combined data analysis over the two years revealed that F18 had the largest values for plant height (59.70 cm), number of tiller/m
2 (330.79), total fresh yield (59.70 ton/fed) and total dry yield (14.42 ton/fed) than the other genotypes, while, F49 ranked first for leaf/stem ratio (1.752), (
Tables S1 and S2) and
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5. The results showed the superiority of some lines and genotypes (F18, F49, K75, P5, P13, P20, S35 and F28) in the total dry yield (ton/fed) and the total fresh yield (ton/fed), as their production of total fresh yield reached 50 tons or more per feddan under the conditions of the experiment for these lines, although these genotypes were selected from different climatical and environmental regions (southern Egypt, where the temperature is very high and the conditions of the continental or desert climate), which indicates that they excel under a wide and varied range of climatic and environmental conditions. Thus, these lines (genotypes) can be used as commercial varieties in the event of their continued superiority after further evaluation and more study, in addition to that they can be used in programs to improve and develop new lines and varieties of alfalfa that are tolerant to climate change conditions in Egypt. The results showed the presence of diversity in alfalfa at the phenotypic level using morphological and yield related attributes, which conformed that the presence of genetic diversity among alfalfa genotypes can be exploited for future crop impartment breeding programs. These results are in harmony with those reported by [
32], that the investigated six alfalfa genotypes exhibited high total variability for plant height and forage yield. Some previous studies showed the broadest range of variation for some alfalfa cultivars in all morphological traits evaluated [
33,
34]. Generally, the alfalfa forage yield depends on plant height, and number of tillers. Therefore, these traits are the most considerably used selection standards in alfalfa breeding programs, [
35]. Previous studies showed that plant height positively correlated with production in alfalfa [
36]. Therefore, a standard is used when choosing the best genotypes for crossing and breeding programs as well as selected better genotypes and predicting high production during alfalfa growing and breeding [
37]. The leaf/stem ratio, the fresh yield and dry yield are an important indicator for forage palatability assessment and affects the forage production. The results of this study pointed to F18 and F49 as having higher plant height, leaf/stem ratio, fresh yield and dry yield. Therefore, these genotypes are suitable to improve promising new lines for high yield.
Noteworthly differences were observed for estimates of the agronomic the studied traits,
Table 5. The results revealed that the estimates of the phenotypic coefficient of variation (PCV) were higher than the genotypic coefficient of variation (GCV) for all studied traits, signifying that the apparent variation is not only due to the genetic effects, but also due to environmental effects. However, the differences between PCV and GCV for most of the traits were small, indicating high probabilities of genetic progress through selection under this study, whereas, the environmental impact on any trait is indicated by magnitude of the differences between the genotypic coefficients and the phenotypic coefficients of variance. Whereas large differences reflect a large environmental influence, while small differences show a high genetic influence, and these findings conformed with to the findings of [
5,
7,
38]. The results of the first year showed that, the highest genotypic and phenotypic coefficients of variation were recorded for leaf/stem ratio (22.26 and 23.03%), followed by total dry yield (15.48 and 16.29%), respectively. However, the lowest genotypic and phenotypic coefficients of variation were recorded for number of tiller/m
2 (0.56 and 0.62%), respectively. The heritability (H2%) of differences among genotypes were high. The values of heritability were ranged from 87.45 for number of tiller/m
2 to 95.22 for total fresh yield. Generally, the heritability estimates were high for all studied characters. The high heritability suggested that selection of these traits would be efficient, being less affected by environmental influences [
39]. Heritability considered a measurement for phenotypic variance attributable to genetic a cause that has prophetic function in plant breeding [
40].
On the other hand the results of the second year revealed that, the highest genotypic and phenotypic coefficients of variation were recorded for leaf/stem ratio (24.10 and 25.14%), followed by total dry yield (14.19 and 15.14%) and total fresh yield (10.35 and 10.63%) as moderate values. Plant height (8.06 and 8.36%) and leaf/stem ratio (0.50 and 0.54%), were recorded low values of genotypic and phenotypic coefficients of variation, while, the estimates of heritability in broad-sense were generally high for all studied characteristics and recorded values from 86.93% for number of tiller/m2 to 95.45 for total fresh yield.
The genetic estimates of the combined data across the two years showed large differences for all studied traits. The estimates of genotypic and phenotypic coefficients of variability were moderate for leaf /stem ratio (14.53 and 16.69%), total fresh yield (11.67 and 12.43%) and total dry yield (10.64 and 11.82%), while, the genotypic and phenotypic coefficients of variability were low for plant height (8.91 and 9.31%) and number of tiller/m
2 (1.43 and 1.51%). Heritability estimates were high for all studied characters and with the range values from (87.06%) for leaf/stem ratio to (95.70%) for plant height. These results are in accordance with the finding of [
41] who found low to moderate estimates of genotypic and phenotypic coefficients of variation (GCV and PCV) with high heritability for agro-morphological traits of alfalfa. However, the high estimates of heritability indicate the prevalence of genetic effects which are less affected by the environment. This finding is in agreement with results obtained by [
42]. Thus, estimating heritability helps breeders to determine resources efficaciously to select desirable traits and to maximize genetic gains with little time and resources [
43].
- B.
Chemical Composition:
- 1-
Variability in Agronomic Traits
The analysis of variance indicated significant and highly significant differences among the genotypes of alfalfa for crude protein (CP %), crude fiber (CF %) and ash % in the first growing year,
Table 6. These results are in line with those obtained by [
5,
44,
45] who found highly significant differences among the alfalfa population for crude protein, crude fiber and ash %.
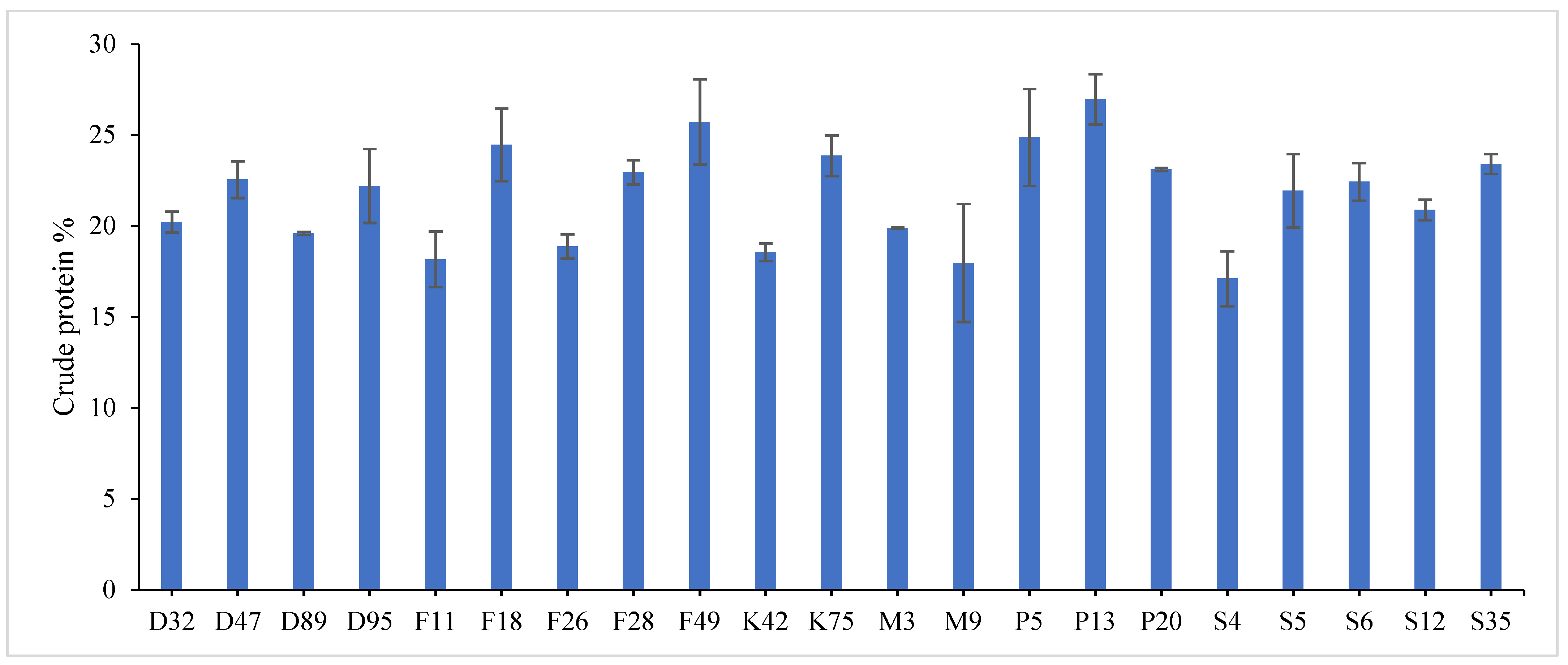
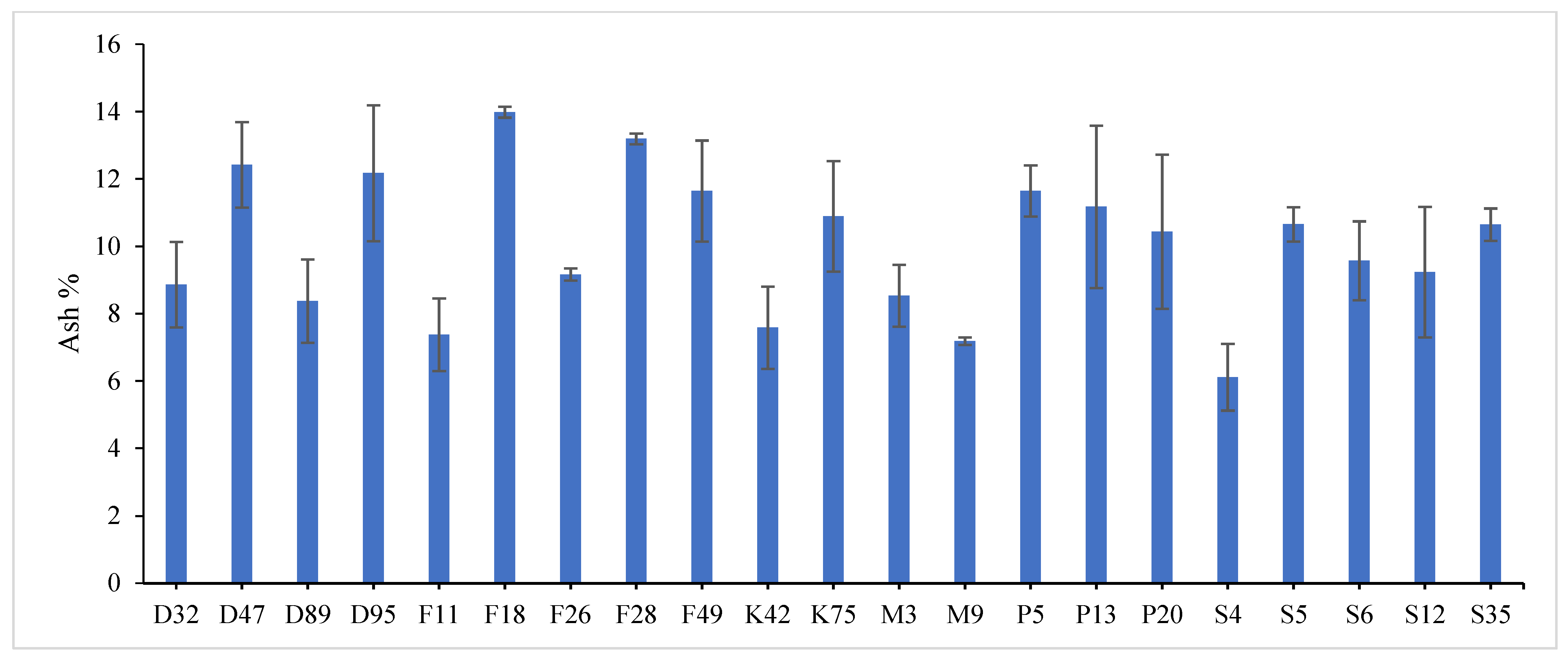
Highly significant differences were found among the investigated genotypes for different quality traits in the first year,
Figure 6,
Figure 7 and
Figure 8 and
Table S3. The value of crude protein content of alfalfa genotypes ranged from (17.11–26.96%), crude fiber content ranged from (21.64–31.46%) and ash% content ranged from (6.11–13.98%) in the first year. Data revealed that P13 ranked first (26.96%) followed by P5 (24.87%) for crude protein%, while, F18 recorded the highest values (31.46% and 13.98%) for crude fiber% and ash%, respectively. On the contrary, the lowest value was recorded with S4 in all quality traits under study in the first year. These results are in agreement with those reported by [
28,
46,
47]. The most useful forage quality traits include crude protein%, crude fiber% and ash% [
48] to raise the nutritional value of alfalfa for livestock [
49]. Therefore, the main goal in alfalfa breeding for the improvement of forage quality is increasing protein%, fiber% and ash content. Variations were found among eight alfalfa cultivars in CP% over two years, during a study of the nutritive value of alfalfa [
47]. The ratio of leaf/stem ratio directly reflects the nutritional value of the forage, as a large quantity of leaves in the forage indicates its good quality. In the same context, several studies showed the relationship between the leaf/stem ratio and quality. The forage quality can be improved by selection for increased leaf/stem ratio in alfalfa cultivars, [
7,
50]. In general, forage yield and quality are complex traits whose pressure is influenced by plant genetics as well as environmental factors [
51]. Therefore, it is necessary to understand the relationships between quality and agricultural traits that may help in breeding programs [
52].
- 2-
Genetic parameters:
Data in
Table 7 revealed that the phenotypic and genotypic coefficients of variability were high for crude fiber% (21.64 and 23.56%) and ash% (20.04 and 21.42%), while they were moderate for crude protein% (12.52 and 14.15%), respectively. This indicates the presence of exploitable genetic variability for these traits, similar results were obtained by [
53]. Therefore, the selection will be useful for the development of traits related to the degree of desired variance [
54]. Heritability (H2%) estimates were generally high for all quality traits and recorded values from 88.48% for crude protein% to 93.56% for ash%, (
Table 7). In general, all quality traits had high heritable variation. Hence, it can be assumed that mainly by phenotypes the genotypes of almost all traits are determined, [
44]. Therefore, the selection of traits with high heritability values accelerates the improvement of traits less influenced by environmental factors [
55].
Cluster analysis:
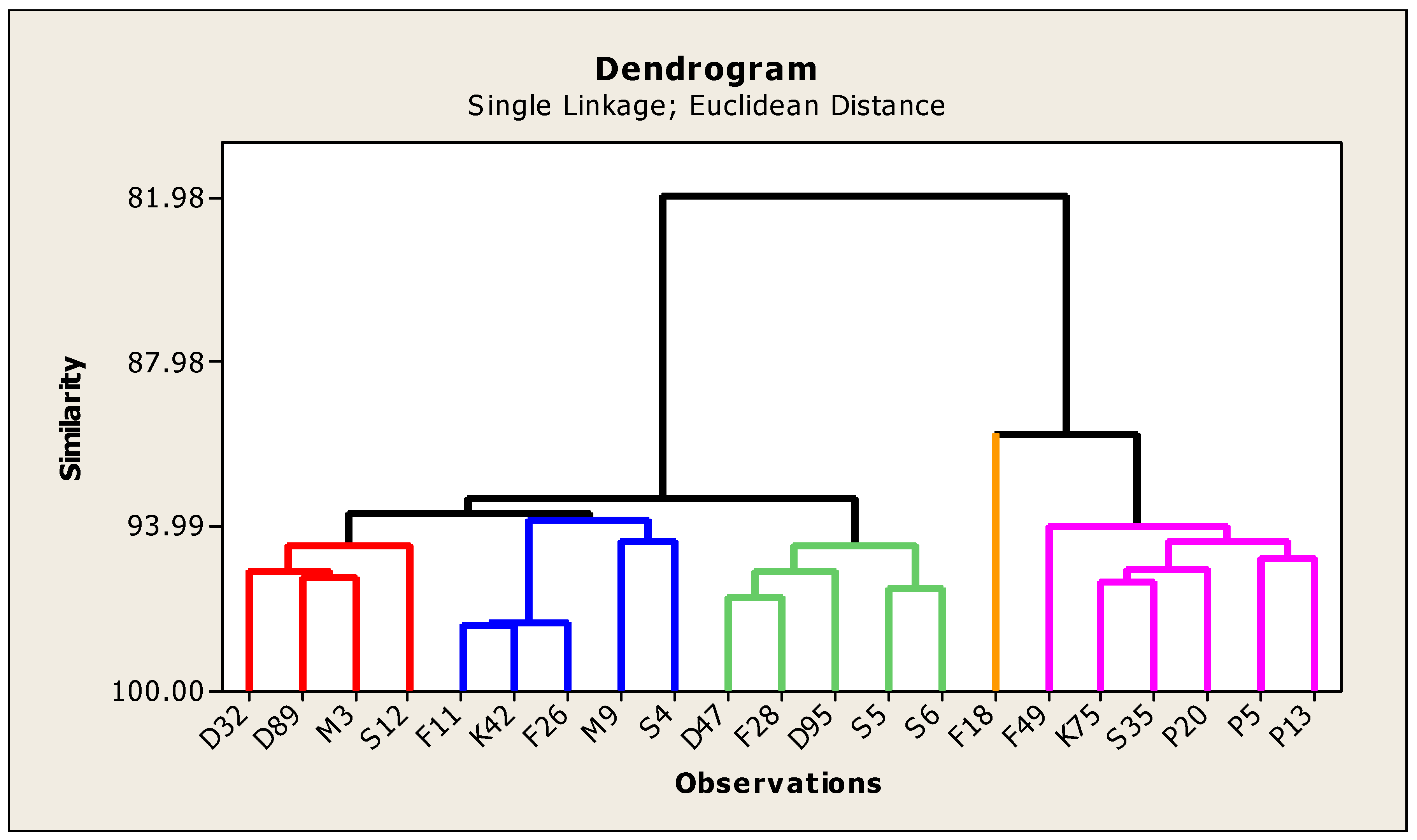
The neighbor-joining dendrogram is a tool for classifying objects, which has been widely used as an effective method to discover the structural associations among tested genotypes and provides a hierarchical classification of them. The genetic divergence can providing a visual idea about variability existing in the twenty–one genotypes, in addition to pledge the continued genetic improvement. In this study, based on Euclidean distance utilizing the UPGMA, the tested genotypes were estimated with agro-morphological and quality traits, and distance was achieved as revealed in dendrogram graph (
Figure 9). The studied genotypes were presented in groups based on the results of the cluster analysis to infer relationships among genotypes (
Table 8).
The genotypes of each cluster varied from 1 to 5. Cluster 1 contained 4 genotypes, Clusters 2 and 3 contained 5 genotypes, Cluster 4 contained one genotype only and Cluster 5 contained 6 genotypes. From the data in
Table 8 and dendrogram (
Figure 9), the aimed genotypes were grouped into two main clusters, that is; Cluster A and B. Nevertheless, the first main cluster was divided into three sub-clusters which could be named, 1, 2 and 3. The sub-cluster number one consisted of four genotypes (D32, D89, M3 and S12). The second sub-cluster included five genotypes (F11, K42, F26, M9 and S4). The third sub-cluster included five genotypes (D47, F28, D95, S5 and S6). The second main cluster consisted of two sub-clusters (4 and 5). The fourth sub cluster was comprised of one genotype (F18). While the fifth sub cluster included six genotypes (F49, K75, S35, P20, P5 and P13). These results are in agreement with [
26,
56]. The cluster analysis was used to distinguish diversity based on morphological and physiological characteristics among genotypes, [
56]. The genetic diversity between the genotypes of plant height, no. of tillers, leaf to stem ratio and fresh weight in ten genotypes of alfalfa were reported by Riasat et al. (2021) [
26], who, classified ten alfalfa genotypes into 4 clusters on the basis of morphology and yield by cluster analysis. Dendrograms were constructed using morpho-physiological and biochemical traits for
Medicago truncatula [
12] and with morphological traits for Pinus koraiensis [
57]. Generally, the genotypes S5 with S6 and P5 with P13 were more closely correlated to each other, whereas less similarity was found between D32 and P13 genotypes.
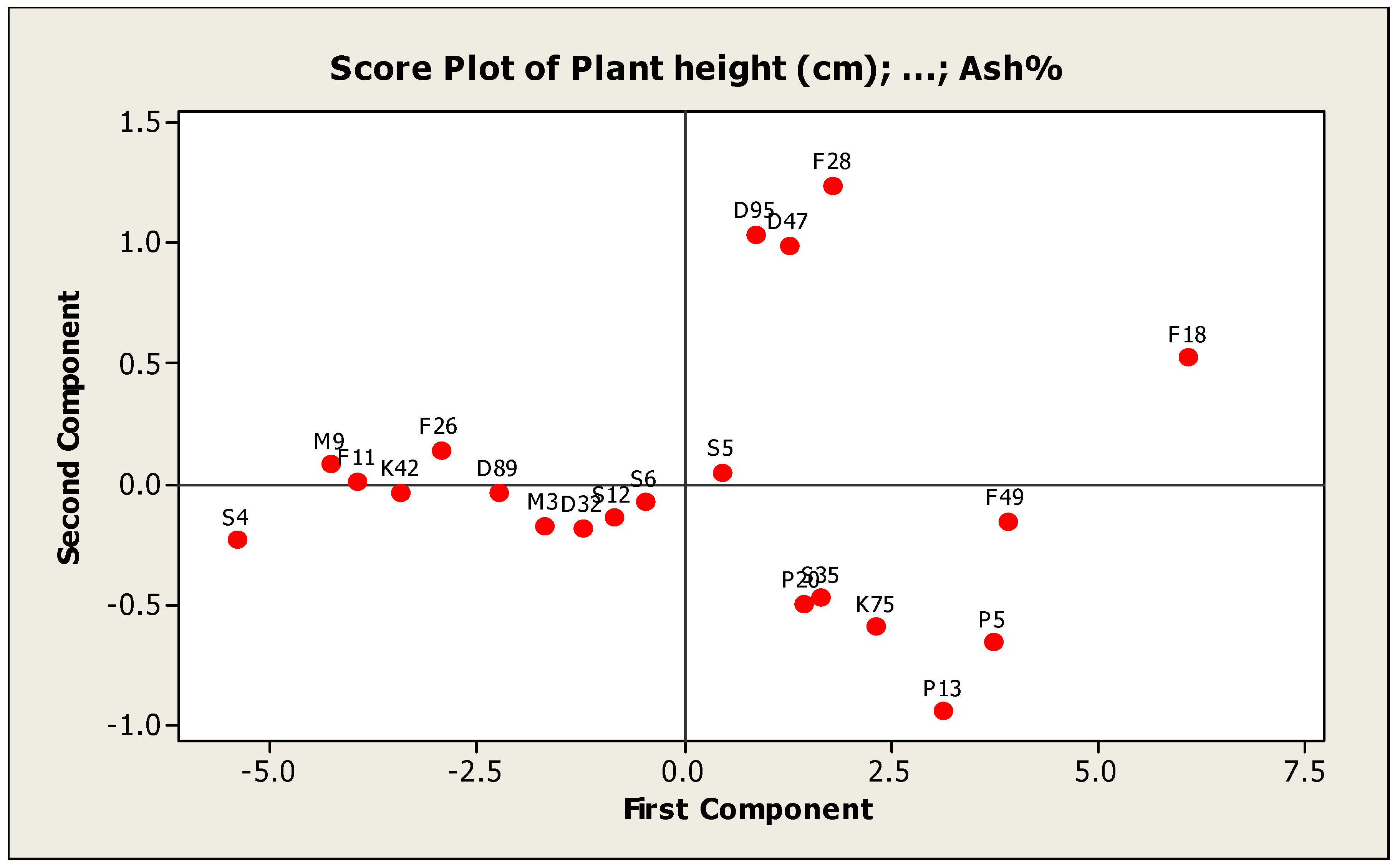
Principal Component Analysis:
The principal component analysis (PCA) grouped the studied genotypes into groups and remained spread in all four quadrants based on the yield components, forage yield and quality traits. The classification of groups depends on the material involved under study [
58]. The genotypes F28, F18, S4 and P13 were placed at extreme positions from the origin in the PCA biplot whereas the genotypes F11, K42 and D89 were concentrated around the origin on PC2. The results of the PCA analysis were presented in groups of genotypes to infer relationships among genotypes (
Figure 10).
These results are similar to the results obtained by [
59,
60] who studied genetic diversity and variability of forage crops by principal component analysis using agronomic characteristics.
The principal component analysis (PCA) was useful to identify the characters which were the main source of the variability and to clarify the genetic diversity in genotypes and could calculate each principal component contribution and the accumulative effect. In addition, it has been widely used in studies of variance in germplasm collections of many species, [
26,
61,
62]. The first five principal components gave eigenvalues greater than 1.0 and explained 88.50% of the total variability among the genotypes for all the studied traits, (
Table 9). Riasat et al., (2021) [
26] reported an assessment of genetic diversity for ten alfalfa genotypes at the phenotypic and genotypic level and found that the first five PCs contributed 90.19% of the entire variability.
The first PCA, which is the most important component, accounted for 5.76 of the eigen value and 53.24% of the total variability and various traits viz., plant height, number of tillers/m
2, leaf/stem ratio, total fresh yield, total dry yield and quality traits contributed significantly towards variation. Total fresh yield had the maximum contribution followed by plant height in PC1. Similar results were obtained by [
63] who studied morphological diversity of wild genetic resources of alfalfa and detected that the first PC explained 56.4% of the total variability in the measured traits and was associated with biomass production, which is approximately congruent with our results. However, Annicchiarico (2006) [
64] revealed that the first PC included only 35% of the total variability which is lower than our results. Perhaps this variance was linked to a higher level of homogeneity between the alfalfa materials under study. PC2 has an eigen value of 3.11 and variance contribution rate of 15.73%, and plant height, crude fiber% and ash% contributed positively but the other traits contributed negatively to variation in PC2. These results are line with [
65] revealed that the second PC included for 16.24% of the variability. PC3 contributed 13.21% in total variation. Leaf/stem ratio was a significantly positive contributor while total dry yield was a negative contributor for PC3. In the remaining PCs, number of tiller/m
2, crude protein% and ash% were the variables responsible for variation. Similar results were obtained by [
65], who reported that the PCA separated the majority of the twenty-seven cultivars and populations of alfalfa for thirteen traits into the first three principal components and the first PC ranked 58.21% of the total variability. In addition, Tlahig et al., (2017) [
66] reported that the PCA separated the majority of the thirty-nine genotypes of alfalfa for six traits into the first six principal components and the first PC ranked 34.45% of the total variability. The results obtained from the cluster analysis are similar to the results of PCA. Therefore, PCA is preferred to be used in conjunction with the dendrogram to gain a better understanding of the relationships between genotypes, [
67]. The crossing of parents with greater inter-cluster distances could produce desirable recombinants, while crossing parents from lower inter cluster distances seems to not produce desirable recombinants [
68].