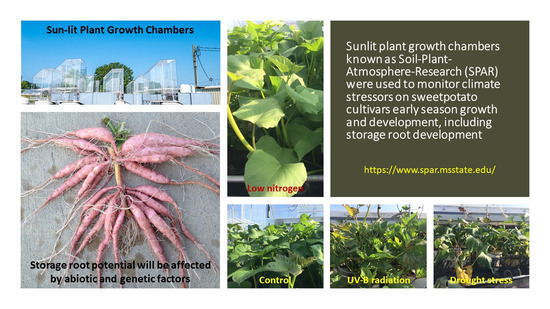
Drought, Low Nitrogen Stress, and Ultraviolet-B Radiation Effects on Growth, Development, and Physiology of Sweetpotato Cultivars during Early Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Crop Husbandry
2.2. Treatments
2.3. Photosynthesis, Fluorescence, and Leaf Pigment Parameters
2.4. Growth and Developmental Measurements
2.5. Root Sample Extraction and Processing
2.6. Stress-Tolerance Indices
2.7. Statistical Analysis
3. Results and Discussion
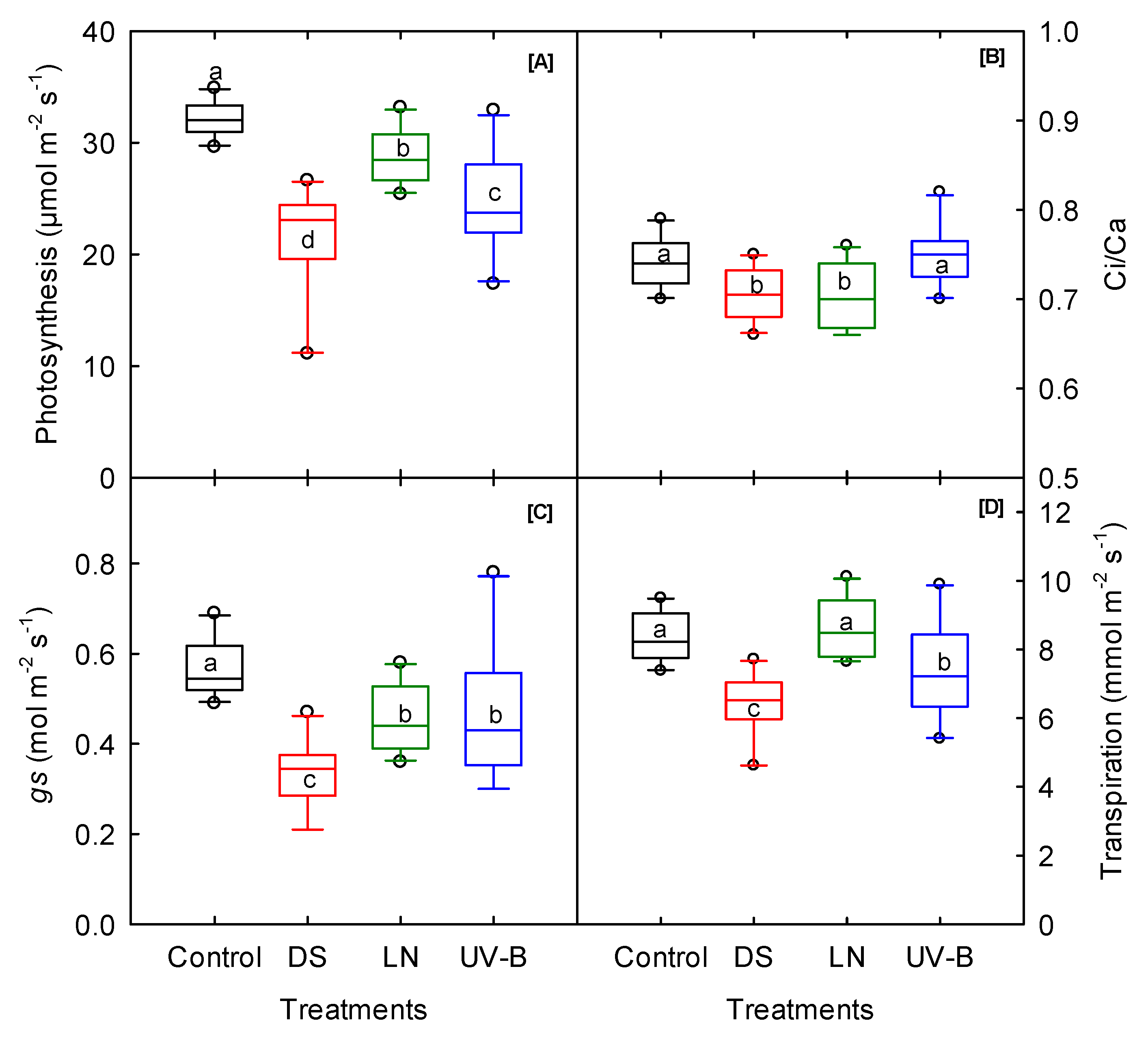
3.1. Photosynthesis and Fluorescence
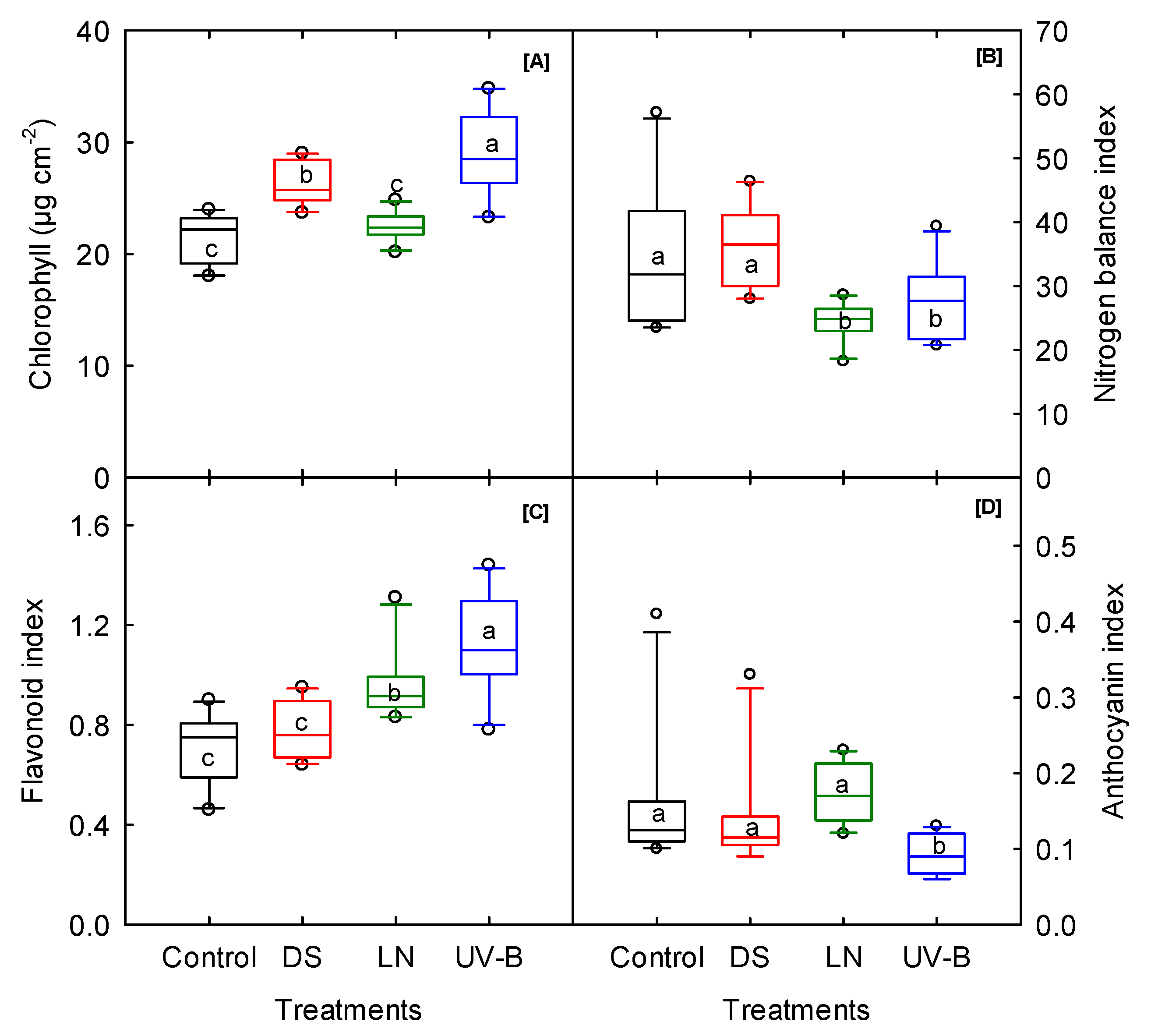
3.2. Leaf Pigments
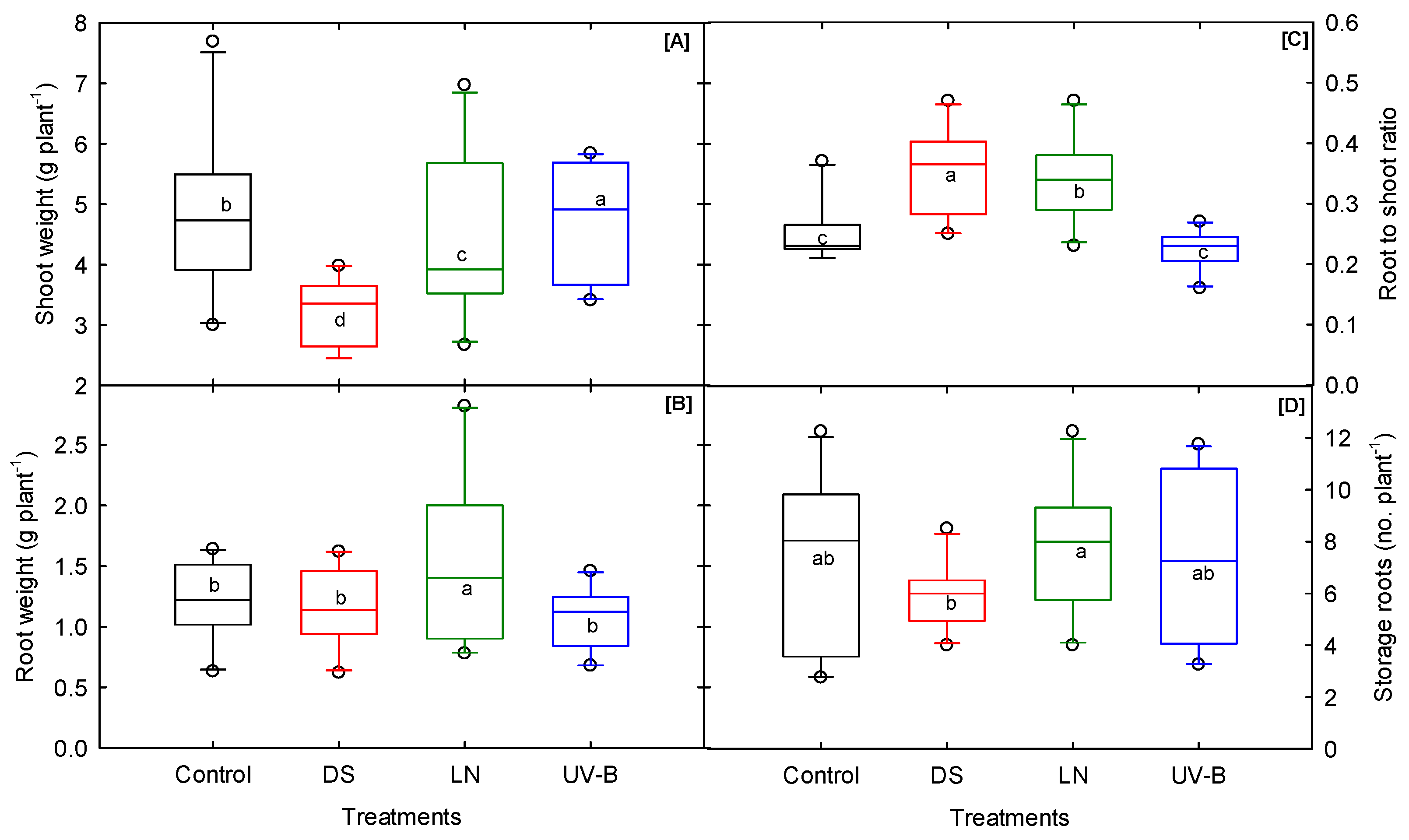
3.3. Shoot Morphological Traits
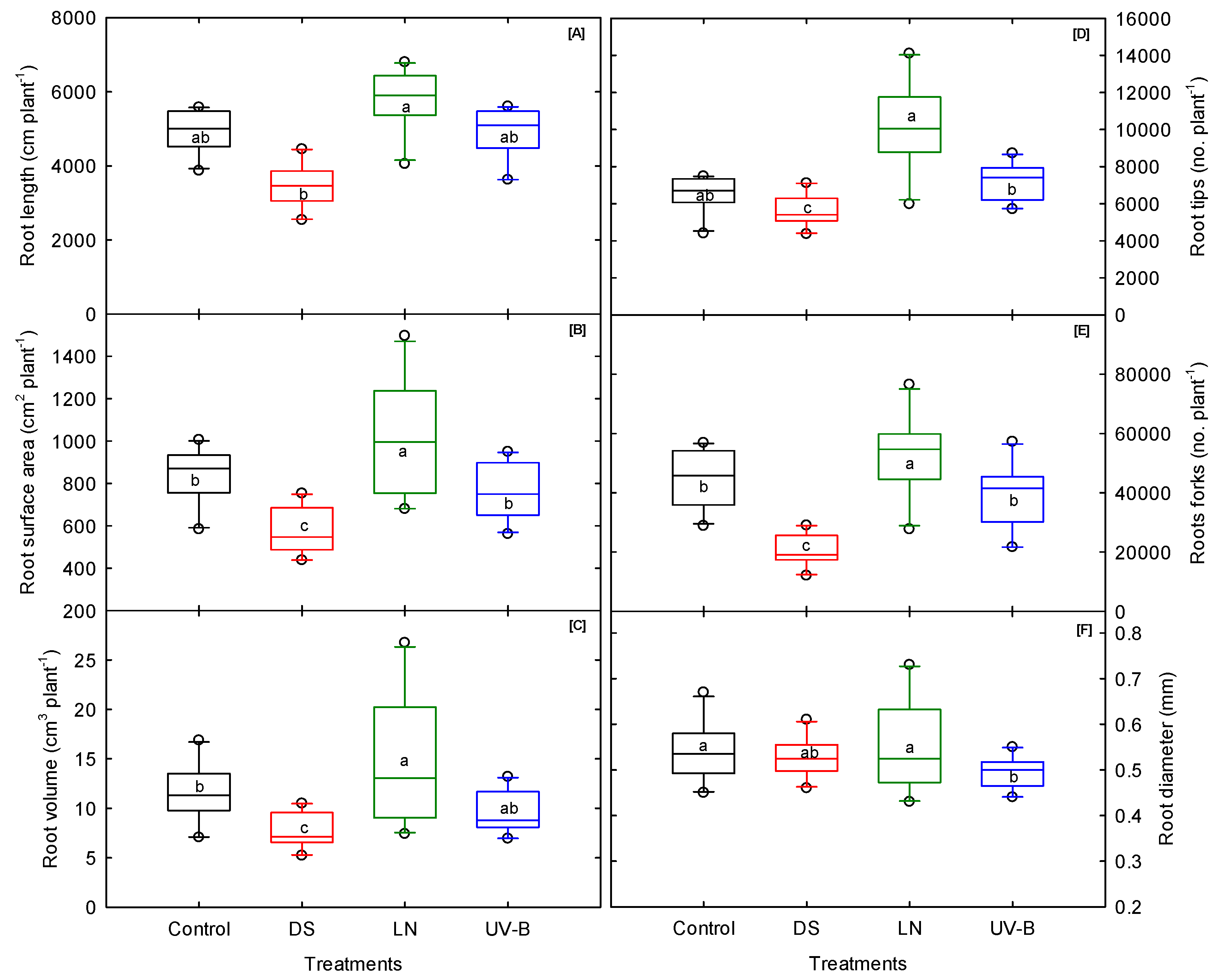
3.4. Root Morphological Traits
3.5. Biomass and Storage Root Number
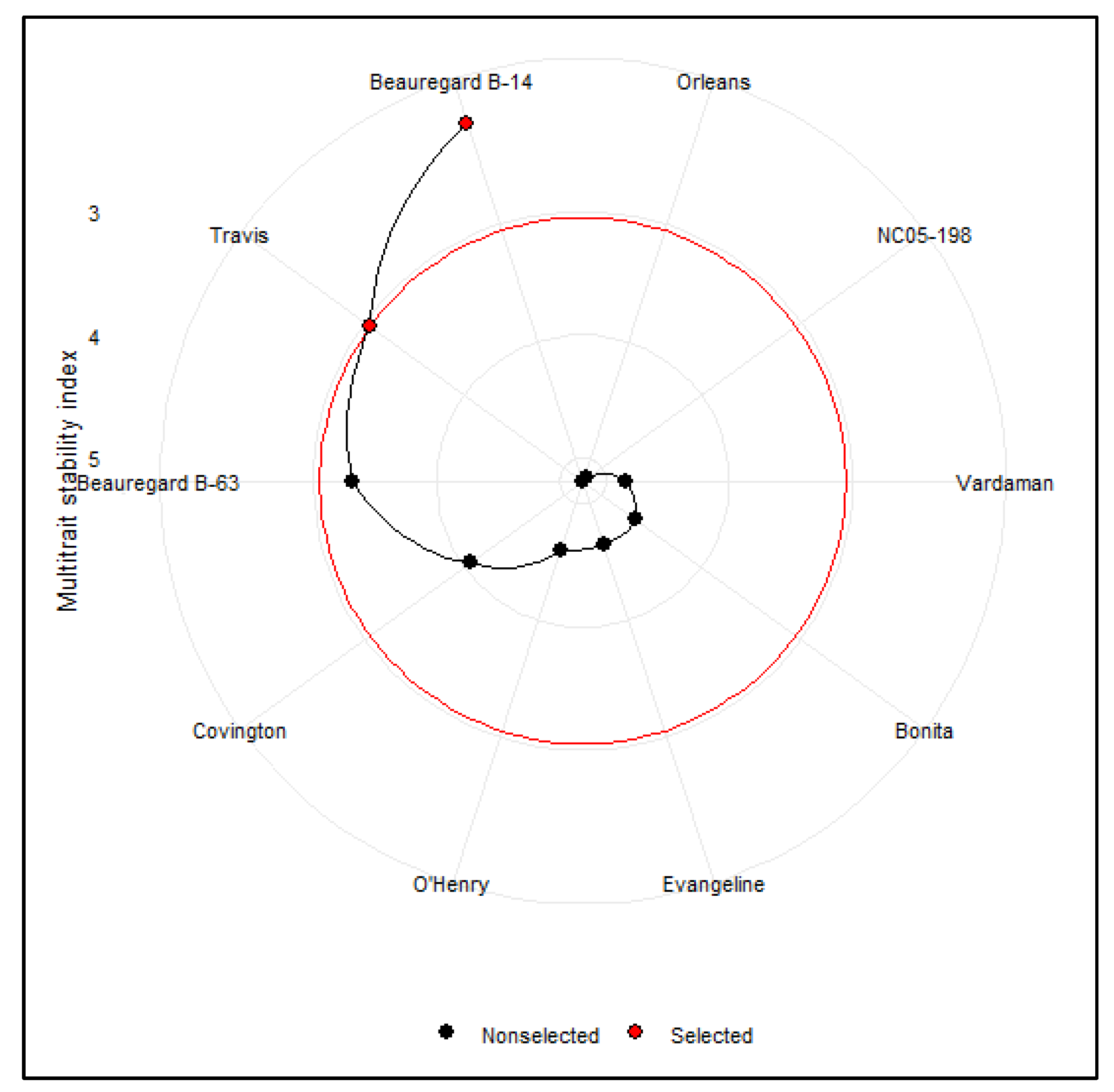
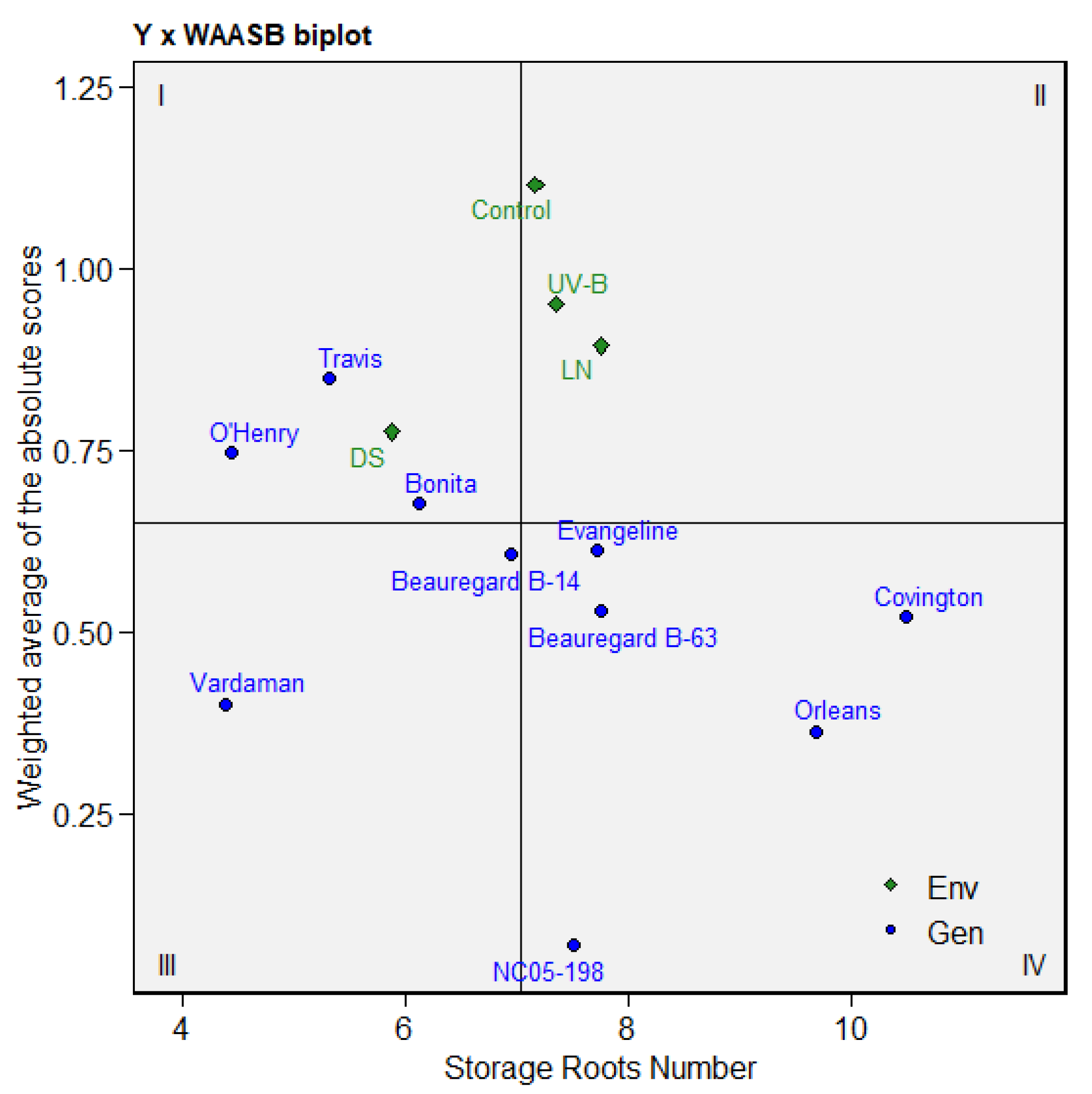
3.6. Multitrait Stability Analysis of Sweetpotato Cultivars
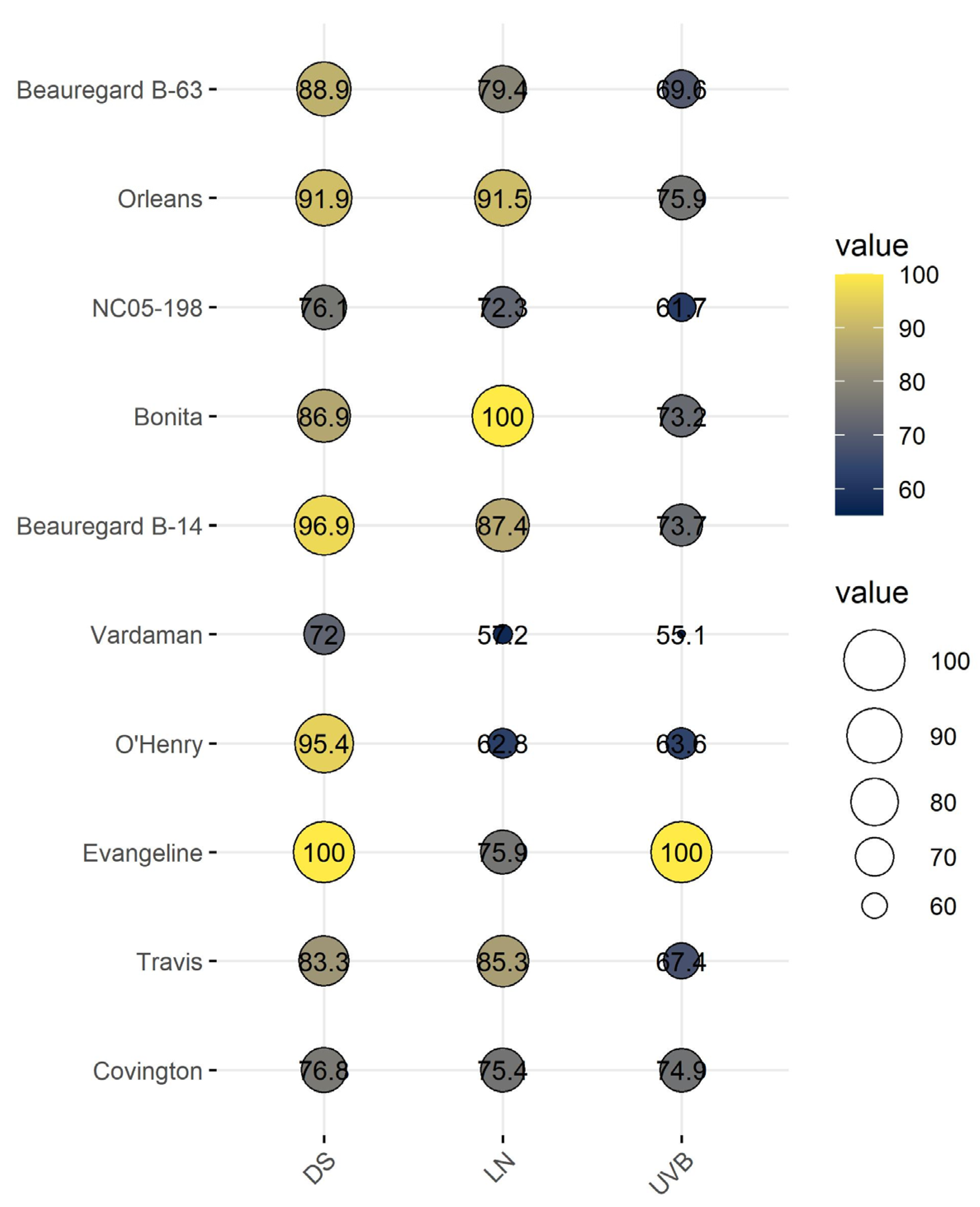
3.7. Cumulative Stress Response Index of Sweetpotato Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The US Sweetpotato Council. Sweet Potato Statistical Year Book: World Statistics; The US Sweetpotato Council: Dillsburg, PA, USA, 2020; pp. 1–62. [Google Scholar]
- Betty, J.B. Evaluating sweet potato as an intervention food to prevent vitamin A deficiency. Compr. Rev. Food Sci. Food Saf. 2011, 10, 118–130. [Google Scholar]
- Allen, J.C.; Corbitt, A.D.; Maloney, K.P.; Butt, M.S.; Truong, V.D. Glycemic index of sweet potato as affected by cooking methods. Open Nutr. J. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Fuglie, K.O. Priorities for sweetpotato research in developing countries: Results of a survey. Hortic. Sci. 2007, 42, 1200–1206. [Google Scholar] [CrossRef]
- Asiedu, R.; Sartie, A. Crops that feed the World. 1. Yams: Yams for income and food security. Food Secur. 2010, 2, 305–315. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, C.S.; Davis, R.M.; Cantwell, M. Sweetpotato Production in California; Vegetable Production Series, Publication 7237; University of California Vegetable Research and Information Center, Division of Agriculture and Natural Resources: Richmond, CA, USA, 2013. [Google Scholar]
- Valenzuela, H.; Fukuda, S.; Arakaki, A. Sweetpotato Production Guidelines for Hawaii-Crop Production Guidelines. 1994. Available online: http://www.extento.hawaii.edu/kbase/reports/sweetpot_prod.htm (accessed on 22 October 2021).
- Iamandei, M.; Draghici, R.; Diaconu, A.; Drăghici, I.; Dima, M.; Eun-Gi, C. Preliminary data on the arthropod biodiversity associated with sweet potato (Ipomoea batatas) crops under sandy soils conditions from southern Romania. Rom. J. Plant Prot. 2014, 7, 98–102. [Google Scholar]
- Diaconu, A.; Eun–Gi, C.; Draghici, R.; Croitoru, M.; Ploae, M.; Drăghici, I.; Dima, M. The behavior of sweet potato (Ipomoea batatas) in terms psamosoils in southern Romania. Sci. Pap. Ser. B. Hortic. 2016, LX, 167–174. [Google Scholar]
- Gajanayake, B.; Reddy, K.R.; Shankle, M.W.; Arancibia, R.A. Early–season Soil moisture deficit reduces sweetpotato storage root initiation and development. Hortic. Sci. 2013, 48, 1457–1462. [Google Scholar] [CrossRef]
- Yambao, E.B.; O’Toole, J.C. Effects of nitrogen nutrition and root medium water potential on growth, nitrogen uptake and osmotic adjustment of rice. Physiol. Plant 1984, 60, 507–515. [Google Scholar] [CrossRef]
- Gonzalez–Dugo, V.; Durand, J.L.; Gastal, F. Water deficit and nitrogen nutrition of crops. A review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef]
- Jeuffroy, M.H.; Ney, B.; Oury, A. Integrated physiological and agronomic modeling of N capture and use within the plant. J. Exp. Bot. 2002, 53, 809–823. [Google Scholar] [CrossRef]
- Ballare, C.L.; Caldwell, M.M.; Flint, S.D.; Robinson, S.A.; Bornman, J.F. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem. Photobiol. Sci. 2011, 10, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E.; Zepp, R.G.; Lucas, R.M.; Madronich, S.; Austin, A.T.; Ballare, A.L.; Norval, M.; Sulzberger, B.; Bais, A.F.; Mckenzie, R.L.; et al. Solar ultraviolet radiation in a changing climate. Nat. Clim. Chang. 2014, 4, 434–441. [Google Scholar] [CrossRef]
- González, R.; Mepsted, R.; Wellburn, A.R.; Paul, N.D. Non–photosynthetic mechanisms of growth reduction in pea (Pisum sativum L.) exposed to UV–B radiation. Plant Cell Environ. 1998, 21, 23–32. [Google Scholar] [CrossRef]
- Rozema, J.; Van De Staaij, J.; Bjorn, L.O.; De Bakker, N. Depletion of stratospheric ozone and solar UV–B radiation: Evolution of land plants, UV–screens and function of polyphenolics. In Stratospheric Ozone Depletion: The Effects of Enhanced UV–B Radiation on Terrestrial Ecosystems; Rozema, J., Ed.; Backhyn Publishers: Leiden, The Netherlands, 1999; pp. 1–19. [Google Scholar]
- Caldwell, M.M.; Ballare, C.L.; Bornman, J.F.; Flint, S.D.; Bjorn, L.O.; Teramura, A.H.; Kulandaivelu, G.; Tevini, M. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climate change factors. Photochem. Photobiol. Sci. 2003, 2, 29–38. [Google Scholar] [CrossRef]
- Olson, L.C.; Veit, M.; Borneman, J.F. Epidermal transmittance and phenolic composition in leaves of atrazine–tolerant and atrazine–sensitive cultivars of Brassica napus grown under enhanced UV–B radiation. Physiol. Plant 1999, 107, 259–266. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Osvald, J. Influence of UV–B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 2005, 43, 445–448. [Google Scholar] [CrossRef]
- Schumaker, M.A.; Bassman, J.H.; Robberecht, R.; Radamaker, G.K. Growth, leaf anatomy, and physiology of Populus clones in response to solar ultraviolet–B radiation. Tree Physiol. 1997, 17, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Victor, G.; Greenberg, B.M. Higher plants and UV–B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. Altitudinal differences in UV absorbance, UV reflectance and related morphological traits of Quercus ilex and Rhododendron ferrugineum in the Mediterranean region. Plant Ecol. 1999, 145, 157–165. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet–B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Allen, D.J.; Nogués, S.; Baker, N.R. Ozone depletion and increased UV–B radiation: Is there a real threat to photosynthesis? J. Exp. Bot. 1998, 49, 1775–1788. [Google Scholar]
- Bolink, E.M.; Van–Schalkwijk, I.; Posthumus, F.; Van–Hasselt, P.R. Growth under UV–B radiation increases tolerance to high–light stress in pea and bean plants. Plant Ecol. 2001, 154, 49–156. [Google Scholar] [CrossRef]
- Savitch, L.V.; Pocock, T.; Krol, M.; Wilson, K.E.; Greenberg, B.M.; Huner, N.P.A. Effects of growth under UV–A radiation on CO2 assimilation, carbon partitioning, PSII photochemistry and resistance to UV–B radiation in Brassica napus cv. Topas. Aust. J. Plant Physiol. 2001, 28, 203–212. [Google Scholar]
- Duque, L.O.; Setter, T.L. Cassava response to water deficit in deep pots: Root and shoot growth, ABA, and carbohydrate reserves in stems, leaves and storage roots. Trop. Plant Biol. 2013, 6, 199–209. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Fatima, T.; Handa, A.K.; Mattoo, A.K. Differential association of free, conjugated, and bound forms of polyamines and transcript abundance of their biosynthetic and catabolic genes during drought/salinity stress in tomato (Solanum lycopersicum L.) Leaves. Front. Plant Sci. 2021, 12, 743568. [Google Scholar] [CrossRef] [PubMed]
- Gajanayake, B.; Reddy, K.R.; Shankle, M.W.; Arancibia, R.A. Growth, developmental, and physiological responses of two sweet potato (Ipomoea batatas L. [Lam.]) cultivars to early season soil moisture deficit. Sci. Hortic. 2014, 168, 218–228. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Alsajri, F.A.; Irby, T.; Krutz, J.; Golden, B. Quantifying soil moisture deficit effects on soybean yield and yield component distribution patterns. Irrig. Sci. 2018, 36, 241–255. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, W.; Reddy, K.R.; Chen, M.; Taduri, S.; Meyers, S.L.; Shankle, M.W. Ultraviolet (UV)-B effects on growth and yield of three contrasting sweet potato cultivars. Photosynthetica 2020, 58, 37–44. [Google Scholar] [CrossRef]
- Gajanayake, B.; Reddy, K.R. Sweetpotato responses to mid– and late–season soil moisture deficits. Crop Sci. 2016, 56, 1865–1877. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Hodges, H.F.; Read, J.J.; McKinion, J.M.; Baker, J.T.; Tarpley, L.; Reddy, V.R. Soil–plant–atmosphere–research (SPAR) facility: A tool for plant research and modelling. Biotronics 2001, 30, 27–50. [Google Scholar]
- Reddy, A.R.; Reddy, K.R.; Padjung, R.; Hodges, H.F. Nitrogen nutrition and photosynthesis in leaves of Pima cotton. J. Plant Nutr. 1996, 19, 755–770. [Google Scholar] [CrossRef]
- Reddy, K.R.; Singh, S.K.; Koti, S.; Kakani, V.G.; Zhao, D.; Gao, W.; Reddy, V.R. Quantifying the effects of corn growth and physiological responses to ultraviolet–b radiation for modeling. J. Agron. 2013, 105, 1367–1377. [Google Scholar] [CrossRef]
- Wijewardana, C.; Henry, W.B.; Gao, W.; Reddy, K.R. Interactive effects on CO2, drought, and ultraviolet–B radiation on maize growth and development. J. Photochem. Photobiol. 2016, 160, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Wijewardana, C.; Henry, W.B.; Hock, M.W.; Reddy, K.R. Growth and physiological trait variation among corn hybrids for cold tolerance. Can. J. Plant Sci. 2016, 96, 639–656. [Google Scholar] [CrossRef]
- Reddy, K.R.; Seghal, A.; Jumaa, S.; Bheemanahalli, R.; Kakar, N.; Redoña, E.D.; Wijewardana, C.; Alsajri, F.A.; Chastain, D.; Gao, W.; et al. Morpho–physiological characterization of diverse rice genotypes for seedling stage high and low-temperature tolerance. Agronomy 2021, 11, 112. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.; Silva, J.A.G.; Marchioro, V.S.; Souza, V.Q.; Jost, E. Mean performance and stability in multi-environment trials i: Combining features of AMMI and BLUP techniques. J. Agron. 2019, 111, 249–260. [Google Scholar] [CrossRef]
- Frutos, E.; Galindo, M.P.; Leiva, V. An interactive biplot implementation in R for modeling genotype–by–environment interaction. Stoch. Environ. Res. Risk Assess. 2014, 28, 1629–1641. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A. Metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Biol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Beerling, D.J.; Chaloner, W.G. Evolutionary responses of stomatal density to global CO2 change. Biol. J. Linn. Soc. 1993, 48, 343–353. [Google Scholar] [CrossRef]
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 1995, 131, 311–327. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water–use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought–resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Krause, G.H. Photo–inhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Gao, W. Low and high–temperature effects on sweetpotato storage root initiation and early transplant establishment. Sci. Hortic. 2018, 240, 38–48. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Teramura, A.H. Effects of ultraviolet-B radiation on the growth and yield of crop plants. Physiol. Plant 1983, 58, 415–427. [Google Scholar] [CrossRef]
- Teramura, A.H.; Sullivan, J.H. Effects of UV–B radiation on photosynthesis and growth of terrestrial plants. Photosynth. Res. 1994, 39, 463–473. [Google Scholar] [CrossRef]
- Hidema, J.; Taguchi, T.; Ono, T.; Teranishi, M.; Yamamoto, K.; Kumagai, T. UV–B resistance and CPD photolyase activity in rice. Plant J. 2007, 50, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liang, Y.; Pei, K.; Ma, K. UV radiation–responsive proteins in rice leaves: A proteomic analysis. Plant Cell Physiol. 2011, 52, 306–316. [Google Scholar] [CrossRef]
- Lidon, F.C. Micronutrients’ accumulation in rice after supplemental UV–B irradiation. J. Plant Interact. 2012, 7, 19–28. [Google Scholar] [CrossRef][Green Version]
- Brosché, M.; Strid, A. Molecular events following perception of UV–B radiation by plants. Physiol. Plant 2003, 117, 1–10. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet–B radiation mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Fedina, I.; Velitchkova, M.; Georgieva, K.; Demirevska, K.; Simova, L. UV–B response of green and etiolated barley seedlings. Biol. Plant 2007, 51, 699–706. [Google Scholar] [CrossRef]
- Khan, M.A.; Gemenet, D.C.; Villordon, A. Root system architecture and abiotic stress tolerance: Current knowledge in root and tuber crops. Front. Plant Sci. 2016, 7, 1584. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E.; Wu, Y.; Voetberg, G.S.; Saab, I.N.; LeNoble, M.E. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J. Exp. Bot. 1994, 45, 1743–1751. [Google Scholar] [CrossRef]
- Goodger, J.Q.D.; Sharp, R.E.; Marsh, E.L.; Schachtman, D.P. Relationships between xylem sap constituents and leaf conductance of well–watered and water–stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 2005, 56, 2389–2400. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Okamoto, S.; Shinohara, H.; Mori, T.; Matsubayashi, Y.; Kawaguchi, M. Root–derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat. Commun. 2013, 4, 2191. [Google Scholar] [CrossRef]
- Tabata, R.; Sumida, K.; Yoshii, T.; Ohyama, K.; Shinohara, H.; Matsubayashi, Y. Perception of root–derived peptides by shoot LRR–RKs mediates systemic N–demand signaling. Science 2014, 346, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Indira, P. Crop physiology of sweetpotato. Hortic. Rev. 1999, 23, 277–338. [Google Scholar]
- Meyers, S.L.; Arancibia, R.A.; Shankle, M.W.; Main, J.; Gajanayake, B.; Reddy, K.R. Sweetpotato Storage Root Initiation; Publication No P2809; Mississippi State University Extension Service: Mississippi State, MS, USA, 2017; Available online: http://extension.msstate.edu/publications/sweetpotato-storage-root-initiation (accessed on 22 October 2021).
- Luo, J.; Zhou, J. Growth performance, photosynthesis, and root characteristics are associated with nitrogen use efficiency in six poplar species. Environ. Exp. Bot. 2019, 164, 40–51. [Google Scholar] [CrossRef]
- Zhang, J.X.; Nguyen, H.T.; Blum, A. Genetic analysis of osmotic adjustment in crop plants. J. Exp. Bot. 1999, 50, 291–302. [Google Scholar] [CrossRef]
- Cameron, R.W.F.; Harrison–Murray, R.S.; Scott, M.A. The use of controlled water stress to manipulate growth of container–grown Rhododendron cv Hoppy. J. Hort. Sci. Biotechnol. 1999, 74, 161–169. [Google Scholar] [CrossRef]
- Solis, J.; Villordon, A.; Baisakh, N.; LaBonte, D.; Firon, N. Effect of drought on storage root development and gene expression profile of sweetpotato under greenhouse and field conditions. J. Am. Soc. Hortic. Sci. 2014, 139, 317–324. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Kashiwagi, J.; Gaur, P.M.; Upadhyaya, H.D.; Vadez, V. Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crop Res. 2010, 119, 322–330. [Google Scholar] [CrossRef]
- Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Krishnamurthy, L. Kabuli and desi chickpeas differ in their requirement for reproductive duration. Field Crop Res. 2014, 163, 24–31. [Google Scholar] [CrossRef]
- Al-Oudat, M.; Baydoun, S.A.; Mohammad, A. Effects of enhanced UV–B on growth and yield of two Syrian crops wheat (Triticum durum var. Horani) and broad beans (Vicia faba) under field conditions. Environ. Exp. Bot. 1998, 40, 11–16. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.; Strid, A. UV–B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Koti, S.; Reddy, K.R.; Kakani, V.G.; Zhao, D.; Gao, W. Effects of carbon dioxide, temperature and ultraviolet-B radiation and their interactions on soybean (Glycine max L.) growth and development. Environ. Exp. Bot. 2007, 60, 1–10. [Google Scholar] [CrossRef]
- Wijewardana, C.; Henry, W.B.; Reddy, K.R. Evaluation of drought tolerant maize germplasm to induced drought stress. J. Miss. Acad. Sci. 2017, 62, 316–329. [Google Scholar]

| Trait | Unit | Drought | Low Nitrogen | Ultraviolet-B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Ta | C × Ta | C | Tb | C × Tb | C | Tc | C × Tc | ||
| Chlorophyll | µg cm−2 | ns | *** | ns | ns | ns | ns | * | *** | * |
| Flavonoids | unitless | ns | ns | ** | ns | *** | *** | * | *** | * |
| Anthocyanin | unitless | *** | ns | ns | ** | ns | ns | *** | *** | * |
| NBI | unitless | ns | ns | ** | ns | *** | *** | ** | ** | ** |
| Photosynthesis | µmol m−2 s−1 | ** | *** | ns | ns | *** | ns | ns | *** | ns |
| Stomatal conductance | mol m−2 s−1 | ns | *** | ns | ns | ** | ns | ** | ** | ** |
| Transpiration | H2O m−2 s−1 | ns | *** | ** | ns | ns | ns | * | ** | * |
| ETR | µmol m−2 s−1 | ** | *** | ns | * | *** | ns | ns | *** | * |
| Ci/Ca | ns | ** | ns | ns | ** | ns | ns | ns | ns | |
| Minimal fluorescence | ns | ns | ns | ns | *** | ns | ns | ** | ns | |
| Maximal fluorescence | ns | *** | ns | ns | *** | ns | ns | *** | ns | |
| Steady-state fluorescence | ns | ns | ns | * | *** | ** | ns | *** | ns | |
| Quantum efficiency | * | * | ns | * | ns | ns | ns | *** | ns | |
| Vine length | cm | *** | * | *** | *** | ns | ** | *** | ns | *** |
| Leaf number | no./plant | ns | *** | *** | ns | ** | *** | ** | * | ** |
| Leaf area | cm2 | *** | *** | * | *** | ** | ns | *** | ns | * |
| Leaf dry weight | g/plant | *** | ** | ** | * | ns | ns | *** | ns | ** |
| Total root length | cm | ** | *** | ns | ns | *** | ns | ns | ns | ns |
| Root surface area | cm2 | * | *** | ns | *** | ** | ns | ns | ns | ns |
| Root diameter | mm | *** | ns | ns | *** | ns | ** | *** | ** | ns |
| Root volume | cm3 | ** | *** | ns | *** | ** | * | * | ns | ns |
| Root tips | no./plant | ns | ns | ns | * | *** | ns | ns | ns | ns |
| Root forks | no./plant | ** | *** | ns | ** | * | ns | ** | ns | ns |
| Root crossings | no./plant | *** | ** | ns | ** | * | ns | *** | ns | ns |
| Stem dry weight | g/plant | *** | *** | ** | *** | ** | ns | *** | ns | * |
| Root dry weight | g/plant | *** | ns | ns | *** | ** | * | ** | ns | ns |
| Root to shoot ratio | ratio | *** | *** | ns | ** | *** | * | *** | * | ns |
| Storage root number | no./plant | ** | ns | ** | *** | ns | * | *** | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramamoorthy, P.; Bheemanahalli, R.; Meyers, S.L.; Shankle, M.W.; Reddy, K.R. Drought, Low Nitrogen Stress, and Ultraviolet-B Radiation Effects on Growth, Development, and Physiology of Sweetpotato Cultivars during Early Season. Genes 2022, 13, 156. https://doi.org/10.3390/genes13010156
Ramamoorthy P, Bheemanahalli R, Meyers SL, Shankle MW, Reddy KR. Drought, Low Nitrogen Stress, and Ultraviolet-B Radiation Effects on Growth, Development, and Physiology of Sweetpotato Cultivars during Early Season. Genes. 2022; 13(1):156. https://doi.org/10.3390/genes13010156
Chicago/Turabian StyleRamamoorthy, Purushothaman, Raju Bheemanahalli, Stephen L. Meyers, Mark W. Shankle, and Kambham Raja Reddy. 2022. "Drought, Low Nitrogen Stress, and Ultraviolet-B Radiation Effects on Growth, Development, and Physiology of Sweetpotato Cultivars during Early Season" Genes 13, no. 1: 156. https://doi.org/10.3390/genes13010156
APA StyleRamamoorthy, P., Bheemanahalli, R., Meyers, S. L., Shankle, M. W., & Reddy, K. R. (2022). Drought, Low Nitrogen Stress, and Ultraviolet-B Radiation Effects on Growth, Development, and Physiology of Sweetpotato Cultivars during Early Season. Genes, 13(1), 156. https://doi.org/10.3390/genes13010156