Structural and Functional Characterization of the FGF Signaling Pathway in Regeneration of the Polychaete Worm Alitta virens (Annelida, Errantia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. SU5402 and U0126 Treatments
2.3. EdU Labeling and Fluorescent Stainings
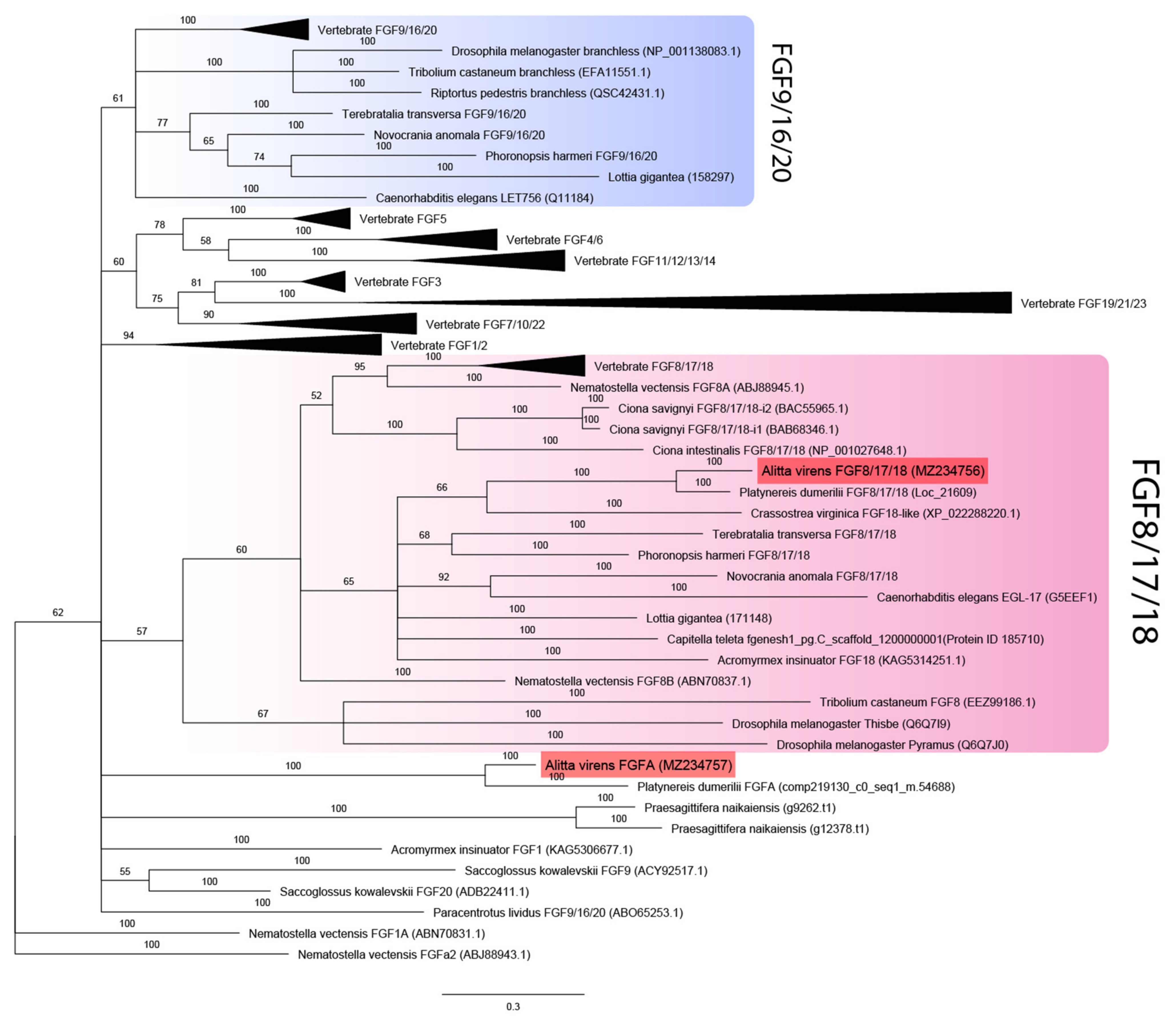
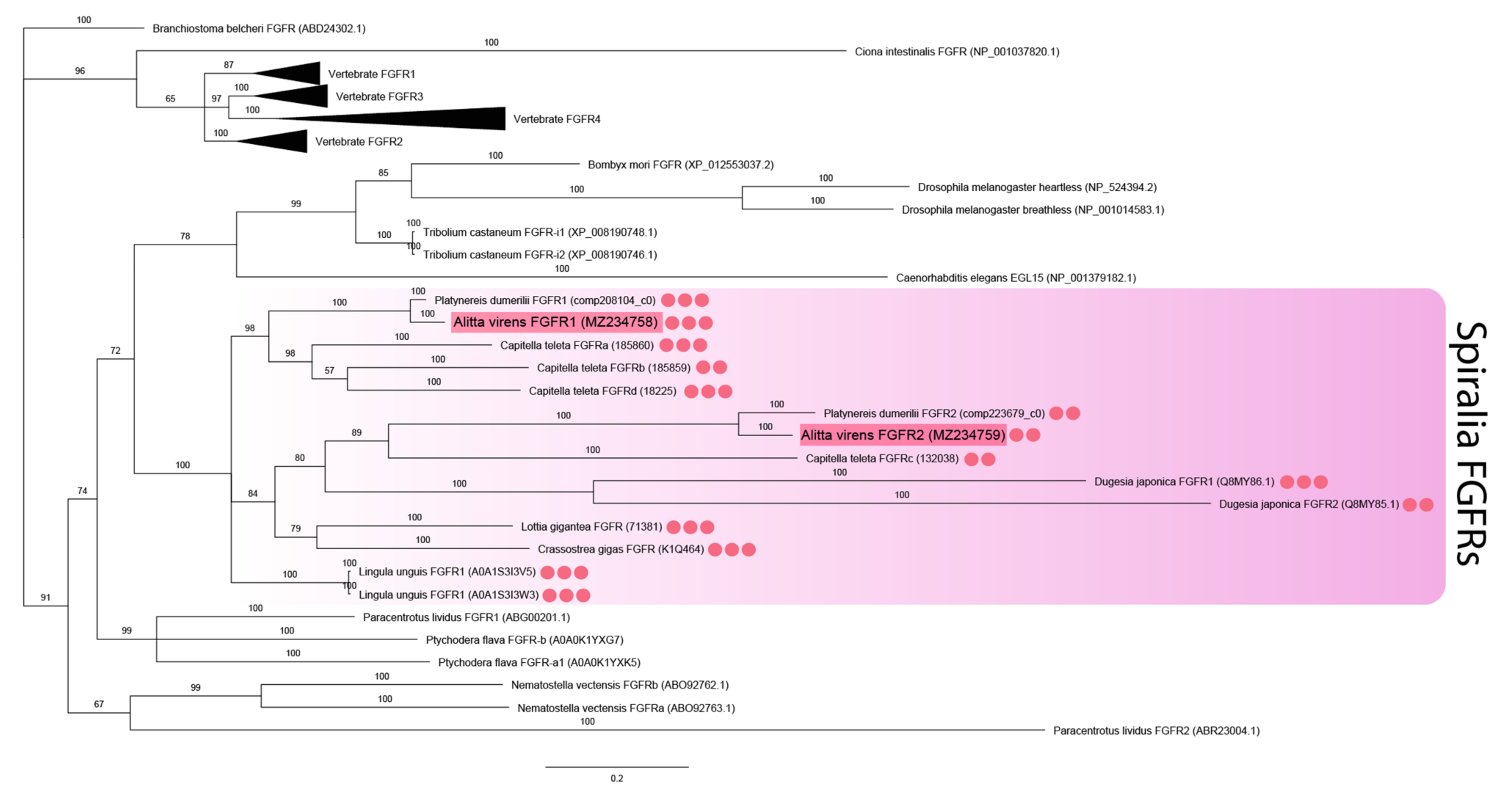
2.4. Sequence Retrieval and Phylogenetic Analysis
2.5. Cloning of the cDNA
2.6. Whole-Mount In Situ Hybridization
2.7. Data Visualization
2.8. Western Blotting
3. Results
3.1. Sequence Analysis
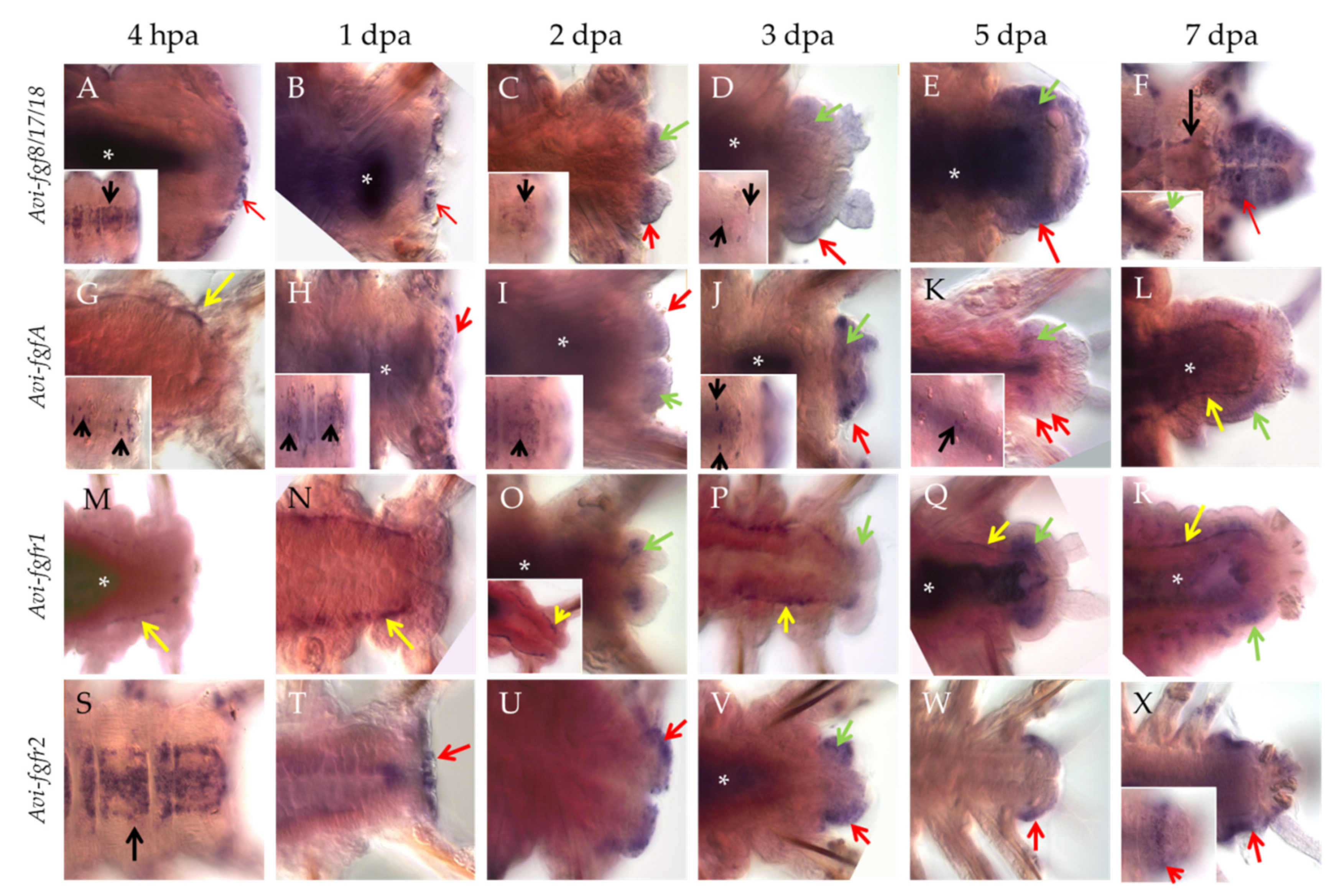
3.2. Expression Patterns of the FGF Signaling Genes
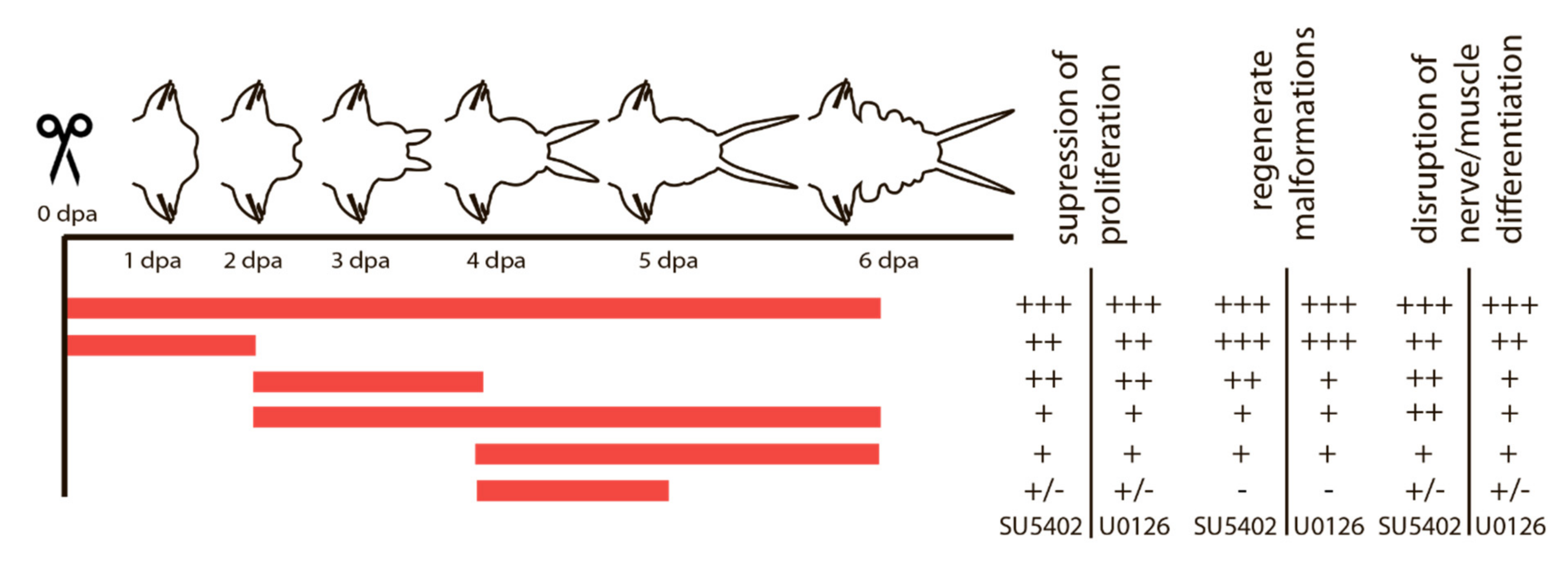
3.3. Inhibition of FGF signaling by SU5402 and U0126
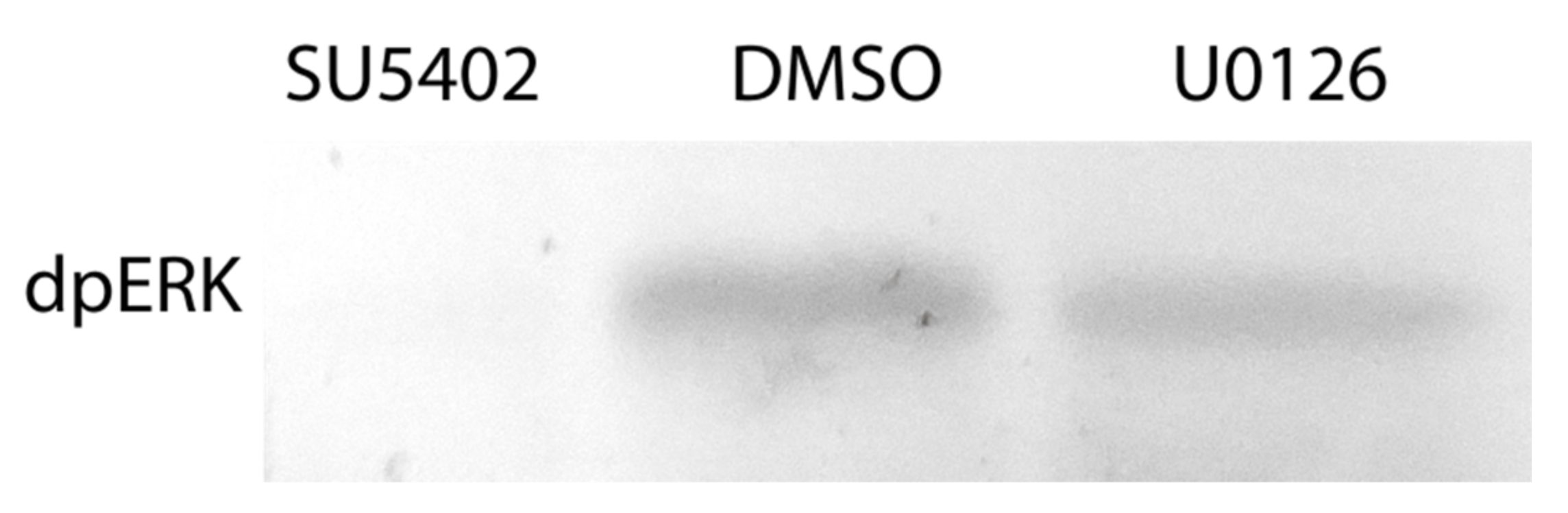
3.3.1. Suppression of FGF Signaling Immediately after Amputation
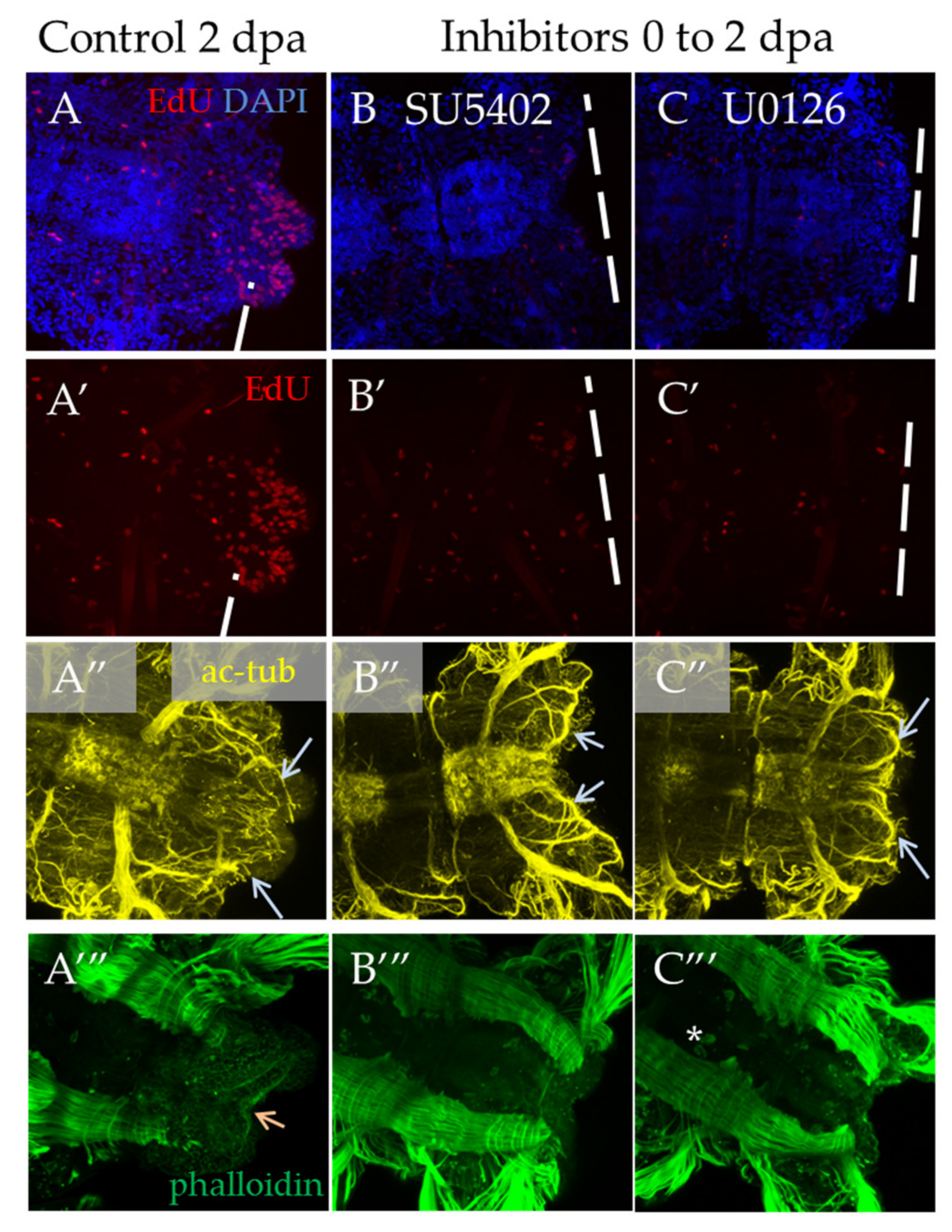
3.3.2. Suppression of FGF Signaling from the Moment of Amputation up to 2 dpa
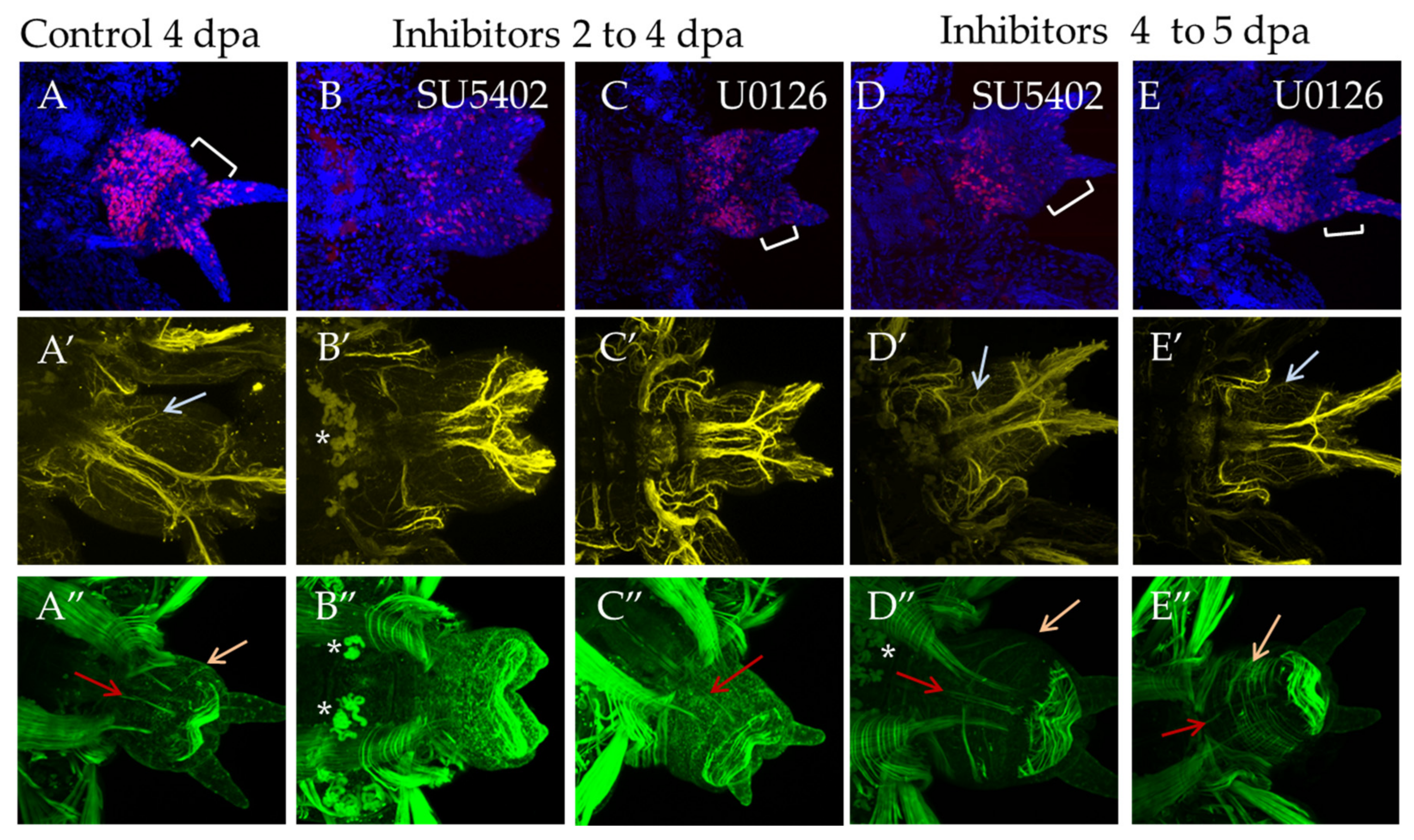
3.3.3. Suppression of FGF Signaling from 2 to 4 dpa and from 4 to 5 dpa
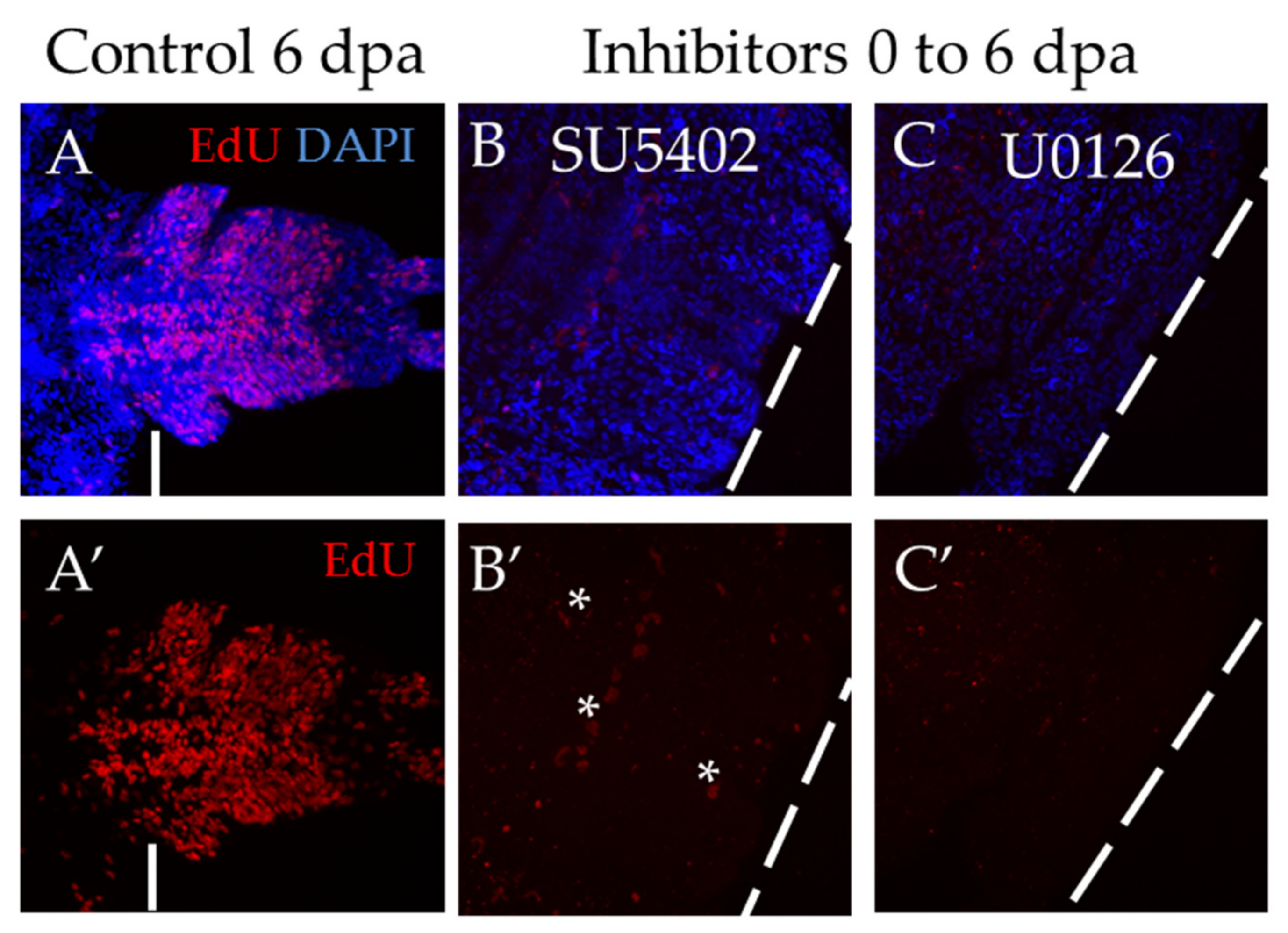
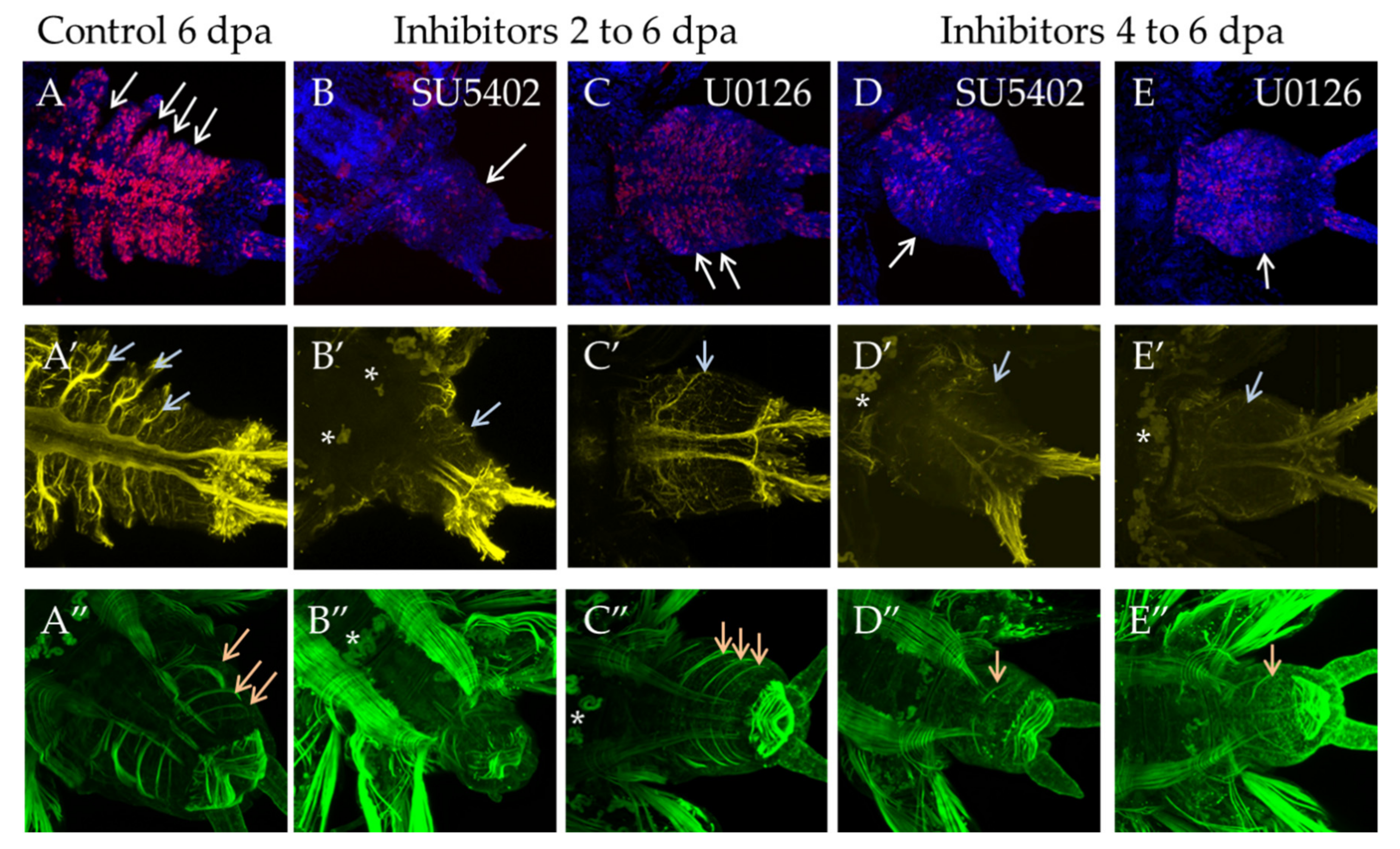
3.3.4. Suppression of FGF Signaling from 2 or 4 to 6 dpa
4. Discussion
4.1. Involvement of FGF Signaling in the Induction of Cellular Sources of Growth and Regeneration
4.2. Putative Roles of FGFs in Axial Patterning and Further Regenerative Bud Maturation
4.3. Molecular and Functional Evolution of the FGF signaling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostyuchenko, R.P.; Kozin, V.V.; Filippova, N.A.; Sorokina, E.V. FoxA Expression pattern in two polychaete species, Alitta virens and Platynereis dumerilii: Examination of the conserved key regulator of the gut development from cleavage through larval life, postlarval growth, and regeneration. Dev. Dyn. 2019, 248, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Kostyuchenko, R.P.; Kozin, V.V. Morphallaxis versus epimorphosis? Cellular and molecular aspects of regeneration and asexual reproduction in annelids. Biol. Bull. 2020, 47, 237–246. [Google Scholar] [CrossRef]
- Kozin, V.V.; Filippova, N.A.; Kostyuchenko, R.P. Regeneration of the nervous and muscular system after caudal amputation in the Polychaete Alitta virens (Annelida: Nereididae). Russ. J. Dev. Biol. 2017, 48, 198–210. [Google Scholar] [CrossRef]
- Kozin, V.V.; Kostyuchenko, R.P. Vasa, PL10, and Piwi gene expression during caudal regeneration of the polychaete annelid Alitta virens. Dev. Genes Evol. 2015, 225, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Planques, A.; Malem, J.; Parapar, J.; Vervoort, M.; Gazave, E. Morphological, cellular and molecular characterization of posterior regeneration in the marine annelid Platynereis dumerilii. Dev. Biol. 2019, 445, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Nikanorova, D.D.; Kupriashova, E.E.; Kostyuchenko, R.P. Regeneration in annelids: Cell sources, tissue remodeling, and differential gene expression. Russ. J. Dev. Biol. 2020, 51, 148–161. [Google Scholar] [CrossRef]
- Niwa, N.; Akimoto-Kato, A.; Sakuma, M.; Kuraku, S.; Hayashi, S. Homeogenetic inductive mechanism of segmentation in polychaete tail regeneration. Dev. Biol. 2013, 381, 460–470. [Google Scholar] [CrossRef]
- Shibata, E.; Yokota, Y.; Horita, N.; Kudo, A.; Abe, G.; Kawakami, K.; Kawakami, A. FGF signalling controls diverse aspects of fin regeneration. Development 2016, 143, 2920–2929. [Google Scholar] [CrossRef]
- Lange, E.; Bertrand, S.; Holz, O.; Rebscher, N.; Hassel, M. Dynamic expression of a hydra FGF at boundaries and termini. Dev. Genes Evol. 2014, 224, 235–244. [Google Scholar] [CrossRef]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast growth factors: Key players in regeneration and tissue repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef]
- Lin, G.; Slack, J.M.W. Requirement for Wnt and FGF signaling in xenopus tadpole tail regeneration. Dev. Biol. 2008, 316, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Czarkwiani, A.; Dylus, D.V.; Carballo, L.; Oliveri, P. FGF signalling plays similar roles in development and regeneration of the skeleton in the brittle star Amphiura filiformis. Development 2021. [Google Scholar] [CrossRef]
- Bertrand, S.; Iwema, T.; Escriva, H. FGF Signaling emerged concomitantly with the origin of eumetazoans. Mol. Biol. Evol. 2014, 31, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, reviews3005.1–reviews3005.12. [Google Scholar] [CrossRef]
- Oulion, S.; Bertrand, S.; Escriva, H. Evolution of the FGF gene family. Int. J. Evol. Biol. 2012, 2012, 298147. [Google Scholar] [CrossRef]
- Popovici, C.; Roubin, R.; Coulier, F.; Birnbaum, D. An evolutionary history of the FGF superfamily. BioEssays 2005, 27, 849–857. [Google Scholar] [CrossRef]
- Böttcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef]
- Tulin, S.; Stathopoulos, A. Extending the family table: Insights from beyond vertebrates into the regulation of embryonic development by FGFs. Birth Defects Res. C Embryo Today Rev. 2010, 90, 214–227. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Functional evolutionary history of the mouse FGF gene family. Dev. Dyn. 2008, 237, 18–27. [Google Scholar] [CrossRef]
- Dorey, K.; Amaya, E. FGF signalling: Diverse roles during early vertebrate embryogenesis. Development 2010, 137, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Jandzik, D.; Hawkins, M.B.; Cattell, M.V.; Cerny, R.; Square, T.A.; Medeiros, D.M. Roles for FGF in lamprey pharyngeal pouch formation and skeletogenesis highlight ancestral functions in the vertebrate head. Development 2014, 141, 629–638. [Google Scholar] [CrossRef]
- Bökel, C.; Brand, M. Generation and interpretation of FGF morphogen gradients in vertebrates. Curr. Opin. Genet. Dev. 2013, 23, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Diez del Corral, R.; Morales, A.V. The multiple roles of FGF signaling in the developing spinal cord. Front. Cell Dev. Biol. 2017, 5, 58. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factors in skeletal development. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 133, pp. 195–234. ISBN 978-0-12-810487-3. [Google Scholar]
- Fan, T.-P.; Su, Y.-H. FGF Signaling repertoire of the indirect developing hemichordate Ptychodera flava. Mar. Genom. 2015, 24, 167–175. [Google Scholar] [CrossRef]
- Bertrand, S.; Camasses, A.; Somorjai, I.; Belgacem, M.R.; Chabrol, O.; Escande, M.-L.; Pontarotti, P.; Escriva, H. Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 9160–9165. [Google Scholar] [CrossRef] [PubMed]
- Nacu, E.; Gromberg, E.; Oliveira, C.R.; Drechsel, D.; Tanaka, E.M. FGF8 and SHH substitute for anterior-posterior tissue interactions to induce limb regeneration. Nature 2016, 533, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.M. The molecular and cellular choreography of appendage regeneration. Cell 2016, 165, 1598–1608. [Google Scholar] [CrossRef]
- Grigoryan, E.N.; Markitantova, Y.V.; Avdonin, P.P.; Radugina, E.A. Study of regeneration in amphibians in age of molecular-genetic approaches and methods. Russ. J. Genet. 2013, 49, 46–62. [Google Scholar] [CrossRef]
- Makanae, A.; Satoh, A. Early regulation of axolotl limb regeneration. Anat. Rec. 2012, 295, 1566–1574. [Google Scholar] [CrossRef]
- Makanae, A.; Hirata, A.; Honjo, Y.; Mitogawa, K.; Satoh, A. Nerve independent limb induction in axolotls. Dev. Biol. 2013, 381, 213–226. [Google Scholar] [CrossRef]
- Makanae, A.; Mitogawa, K.; Satoh, A. Cooperative inputs of BMP and FGF signaling induce tail regeneration in urodele amphibians. Dev. Biol. 2016, 410, 45–55. [Google Scholar] [CrossRef]
- Blanckaert, V.; Hondermarck, H.; Baert, J.L.; Boilly-Marer, Y. Identification of a heparin-binding growth factor and of its affinity binding sites in the marine annelid nereis diversicolor. Comp. Biochem. Physiol. B Biochem. 1992, 103, 991–997. [Google Scholar] [CrossRef]
- DuBuc, T.Q.; Traylor-Knowles, N.; Martindale, M.Q. Initiating a regenerative response; Cellular and molecular features of wound healing in the cnidarian Nematostella vectensis. BMC Biol. 2014, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.A.; Bazarsky, M.; Levy, K.; Chalifa-Caspi, V.; Gat, U. A Transcriptional time-course analysis of oral vs. aboral whole-body regeneration in the sea anemone Nematostella vectensis. BMC Genom. 2016, 17, 718. [Google Scholar] [CrossRef]
- Ogawa, K.; Kobayashi, C.; Hayashi, T.; Orii, H.; Watanabe, K.; Agata, K. Planarian fibroblast growth factor receptor homologs expressed in stem cells and cephalic ganglions. Dev. Growth Differ. 2002, 44, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Auwal, M.A.; Kashima, M.; Nishimura, O.; Hosoda, K.; Motoishi, M.; Kamimura, A.; Okumura, A.; Agata, K.; Umesono, Y. Identification and characterization of a fibroblast growth factor gene in the planarian Dugesia japonica. Dev. Growth Differ. 2020, 62, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Vellutini, B.C.; Hejnol, A. Expression of segment polarity genes in brachiopods supports a non-segmental ancestral role of engrailed for bilaterians. Sci. Rep. 2016, 6, 32387. [Google Scholar] [CrossRef]
- Andrikou, C.; Hejnol, A. FGF Signaling acts on different levels of mesoderm development within spiralia. Development 2021. [Google Scholar] [CrossRef]
- Martín-Durán, J.M.; Vellutini, B.C.; Marlétaz, F.; Cetrangolo, V.; Cvetesic, N.; Thiel, D.; Henriet, S.; Grau-Bové, X.; Carrillo-Baltodano, A.M.; Gu, W.; et al. Conservative route to genome compaction in a miniature annelid. Nat. Ecol. Evol. 2020, 5, 231–242. [Google Scholar] [CrossRef]
- Dondua, A.K. Influence of actinomycin D and sibiromycin upon the embryonic and larval development in Nereis virens (Sars.). Ontogenez 1975, 6, 475–484. (In Russian) [Google Scholar]
- Arimoto, A.; Hikosaka-Katayama, T.; Hikosaka, A.; Tagawa, K.; Inoue, T.; Ueki, T.; Yoshida, M.; Kanda, M.; Shoguchi, E.; Hisata, K.; et al. A draft nuclear-genome assembly of the acoel flatworm Praesagittifera naikaiensis. GigaScience 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Simakov, O.; Marletaz, F.; Cho, S.-J.; Edsinger-Gonzales, E.; Havlak, P.; Hellsten, U.; Kuo, D.-H.; Larsson, T.; Lv, J.; Arendt, D.; et al. Insights into bilaterian evolution from three spiralian genomes. Nature 2013, 493, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, P.; Eppinger, B.; van Goor, H.; Horobin, R.W. A Versatile, Low toxicity glycol methacrylate embedding medium for use in biological research, and for recovered biomaterials prostheses. Cells Mater. 1991, 1, 1. [Google Scholar]
- Rebscher, N.; Deichmann, C.; Sudhop, S.; Fritzenwanker, J.H.; Green, S.; Hassel, M. Conserved intron positions in FGFR genes reflect the modular structure of FGFR and reveal stepwise addition of domains to an already complex ancestral FGFR. Dev. Genes Evol. 2009, 219, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Kozin, V.V.; Filimonova, D.A.; Kupriashova, E.E.; Kostyuchenko, R.P. Mesoderm patterning and morphogenesis in the polychaete Alitta virens (Spiralia, Annelida): Expression of mesodermal markers twist, mox, evx and functional role for MAP kinase signaling. Mech. Dev. 2016, 140, 1–11. [Google Scholar] [CrossRef]
- Boilly, B. Origine des cellules régénératrices chez Nereis diversicolor O. F. Müller (Annélide Polychète). Wilhelm Roux Arch. Für Entwickl. Org. 1969, 162, 286–305. [Google Scholar] [CrossRef]
- Hill, S.D. Origin of the regeneration blastema in polychaete annelids. Integr. Comp. Biol. 1970, 10, 101–112. [Google Scholar] [CrossRef]
- Bely, A.E. Early events in annelid regeneration: A cellular perspective. Integr. Comp. Biol. 2014, 54, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Kostyuchenko, R.P.; Kozin, V.V.; Kupriashova, E.E. Regeneration and asexual reproduction in annelids: Cells, genes, and evolution. Biol. Bull. 2016, 43, 185–194. [Google Scholar] [CrossRef]
- Avel, M. L’influence du systeme nerveux sur la regeneration chez les urodeles et les oligochetes. Bull. Soc. Zool. Fr. 1961, 86, 464–483. [Google Scholar]
- Müller, M.C.M.; Berenzen, A.; Westheide, W. Experiments on anterior regeneration in Eurythoe complanata (“Polychaeta”, Amphinomidae): Reconfiguration of the nervous system and its function for Regeneration. Zoomorphology 2003, 122, 95–103. [Google Scholar] [CrossRef]
- Von Haffner, K. Die überzähligen Bildungen des Körperstammes von Lumbriculus variegatus Müll. und ihre kausale Analyse. Wilhelm Roux Arch. Entwickl. Org. 1931, 123, 649–681. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Principles of Regenerative Biology; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780123694393. [Google Scholar]
- Pirotte, N.; Leynen, N.; Artois, T.; Smeets, K. Do you have the nerves to regenerate? The importance of neural signalling in the regeneration process. Dev. Biol. 2016, 409, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Heber-Katz, E.; Zhang, Y.; Bedelbaeva, K.; Song, F.; Chen, X.; Stocum, D.L. Cell cycle regulation and regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 253–276. [Google Scholar] [CrossRef]
- McMahon, A.; Reeves, G.T.; Supatto, W.; Stathopoulos, A. Mesoderm migration in drosophila is a multi-step process requiring FGF signaling and integrin activity. Development 2010, 137, 2167–2175. [Google Scholar] [CrossRef]
- Saera-Vila, A.; Kish, P.E.; Kahana, A. FGF regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell. Signal. 2016, 28, 1196–1204. [Google Scholar] [CrossRef]
- Novikova, E.L.; Bakalenko, N.I.; Nesterenko, A.Y.; Kulakova, M.A. Expression of hox genes during regeneration of nereid polychaete Alitta (Nereis) nirens (Annelida, Lophotrochozoa). EvoDevo 2013, 4, 1–16. [Google Scholar] [CrossRef]
- Herlant-Meewis, H.; Nokin, A. Cicatrisation et premiers stades de regeneration pygidiale chez nereis diversicolor. Ann. Soc. Zool. Belg. 1962, 93, 137–154. [Google Scholar]
- Chipman, A. Cellular Processes in Segmentation; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-42360-4. [Google Scholar]
- Balavoine, G. Segment formation in annelids: Patterns, processes and evolution. Int. J. Dev. Biol. 2014, 58, 469–483. [Google Scholar] [CrossRef]
- Matus, D.Q.; Thomsen, G.H.; Martindale, M.Q. FGF signaling in gastrulation and neural development in Nematostella vectensis, an Anthozoan cnidarian. Dev. Genes. Evol. 2007, 217, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Babonis, L.S.; Martindale, M.Q. Phylogenetic evidence for the modular evolution of metazoan signalling pathways. Philos. Trans. R. Soc. B 2017, 372, 20150477. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, F.; Fritzenwanker, J.H.; Scholz, C.B.; Technau, U. FGF Signalling controls formation of the apical sensory organ in the cnidarian Nematostella vectensis. Development 2008, 135, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Norris, R.P.; Terasaki, M.; Lowe, C.J. FGF signaling induces mesoderm in the hemichordate Saccoglossus kowalevskii. Development 2013, 140, 1024–1033. [Google Scholar] [CrossRef]
- Sharma, R.; Beer, K.; Iwanov, K.; Schmöhl, F.; Beckmann, P.I.; Schröder, R. The single FGF receptor gene in the beetle Tribolium castaneum codes for two isoforms that integrate FGF8-and branchless-dependent signals. Dev. Biol. 2015, 402, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Röttinger, E.; Saudemont, A.; Duboc, V.; Besnardeau, L.; McClay, D.; Lepage, T. FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis and regulate gastrulation during sea urchin development. Development 2008, 135, 353–365. [Google Scholar] [CrossRef]
- Kim, G.J.; Nishida, H. Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev. Growth Differ. 2001, 43, 521–533. [Google Scholar] [CrossRef]
- Fan, T.-P.; Ting, H.-C.; Yu, J.-K.; Su, Y.-H. Reiterative Use of FGF signaling in mesoderm development during embryogenesis and metamorphosis in the hemichordate Ptychodera flava. BMC Evol. Biol. 2018, 18, 120. [Google Scholar] [CrossRef]
- Beermann, A.; Schröder, R. Sites of FGF signalling and perception during embryogenesis of the beetle Tribolium castaneum. Dev. Genes Evol. 2008, 218, 153–167. [Google Scholar] [CrossRef]
- Kozin, V.V.; Kostyuchenko, R.P. Evolutionary conservation and variability of the mesoderm development in spiralia: A peculiar pattern of nereid polychaetes. Biol. Bull. 2016, 43, 216–225. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalaeva, A.Y.; Kostyuchenko, R.P.; Kozin, V.V. Structural and Functional Characterization of the FGF Signaling Pathway in Regeneration of the Polychaete Worm Alitta virens (Annelida, Errantia). Genes 2021, 12, 788. https://doi.org/10.3390/genes12060788
Shalaeva AY, Kostyuchenko RP, Kozin VV. Structural and Functional Characterization of the FGF Signaling Pathway in Regeneration of the Polychaete Worm Alitta virens (Annelida, Errantia). Genes. 2021; 12(6):788. https://doi.org/10.3390/genes12060788
Chicago/Turabian StyleShalaeva, Alexandra Y., Roman P. Kostyuchenko, and Vitaly V. Kozin. 2021. "Structural and Functional Characterization of the FGF Signaling Pathway in Regeneration of the Polychaete Worm Alitta virens (Annelida, Errantia)" Genes 12, no. 6: 788. https://doi.org/10.3390/genes12060788
APA StyleShalaeva, A. Y., Kostyuchenko, R. P., & Kozin, V. V. (2021). Structural and Functional Characterization of the FGF Signaling Pathway in Regeneration of the Polychaete Worm Alitta virens (Annelida, Errantia). Genes, 12(6), 788. https://doi.org/10.3390/genes12060788





