Whole-Body Regeneration in the Lobate Ctenophore Mnemiopsis leidyi
Abstract
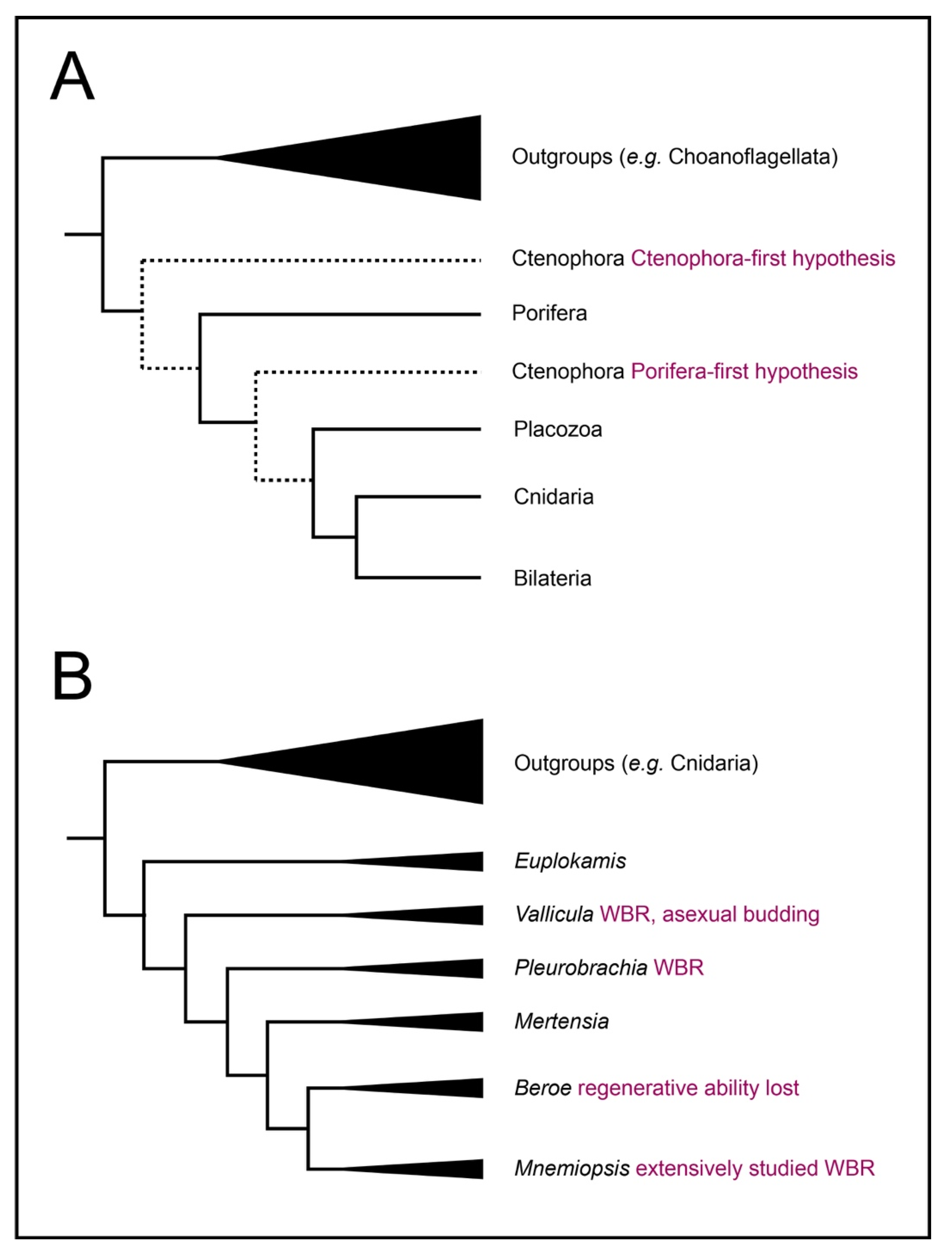
1. Introduction: Ctenophores Are a Key Model for Understanding Animal Regeneration
2. Regeneration in Ctenophores
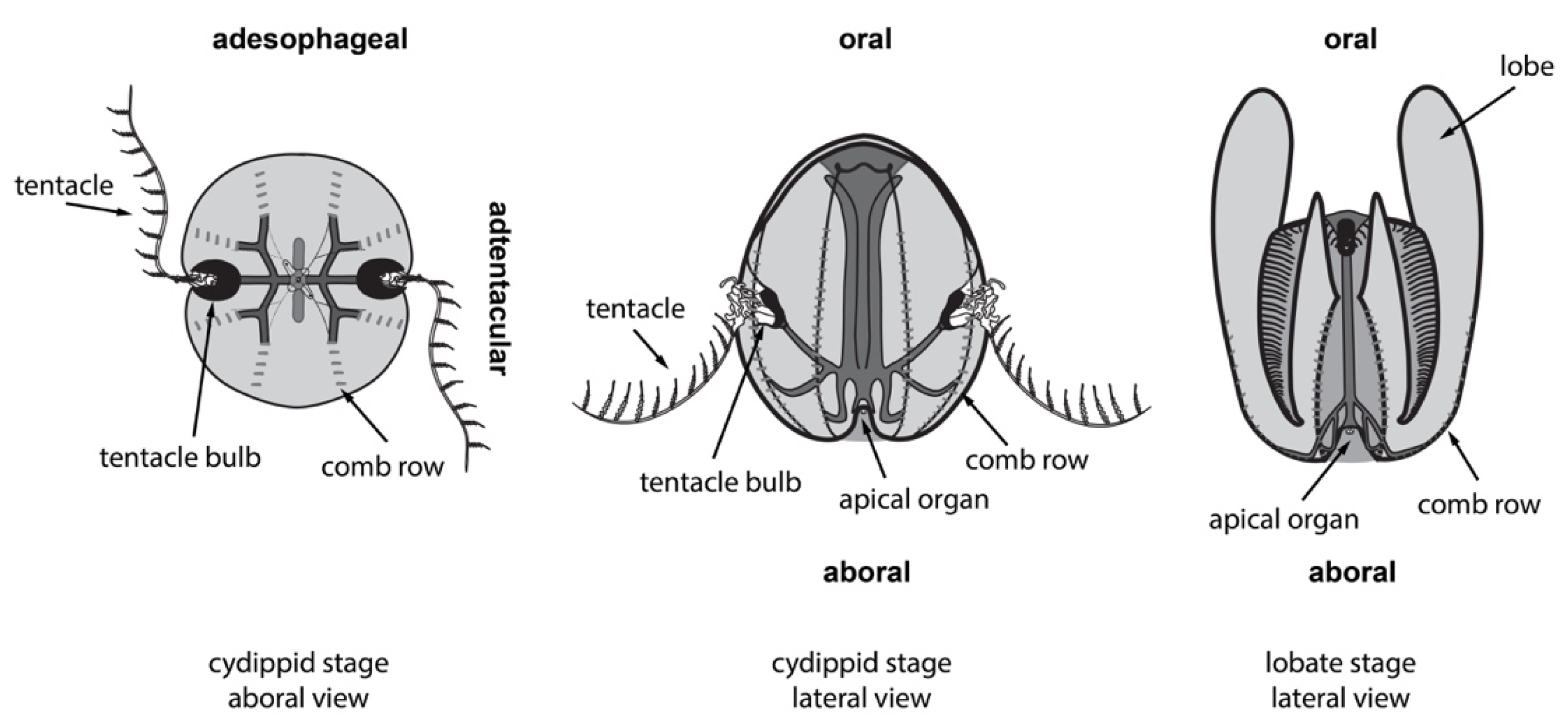
2.1. Ctenophores Have Diverse Cell Types and Structures, All of Which Can Regenerate
2.2. Classic Experiments Demonstrated Ctenophores’ Capacity for Extensive Regeneration
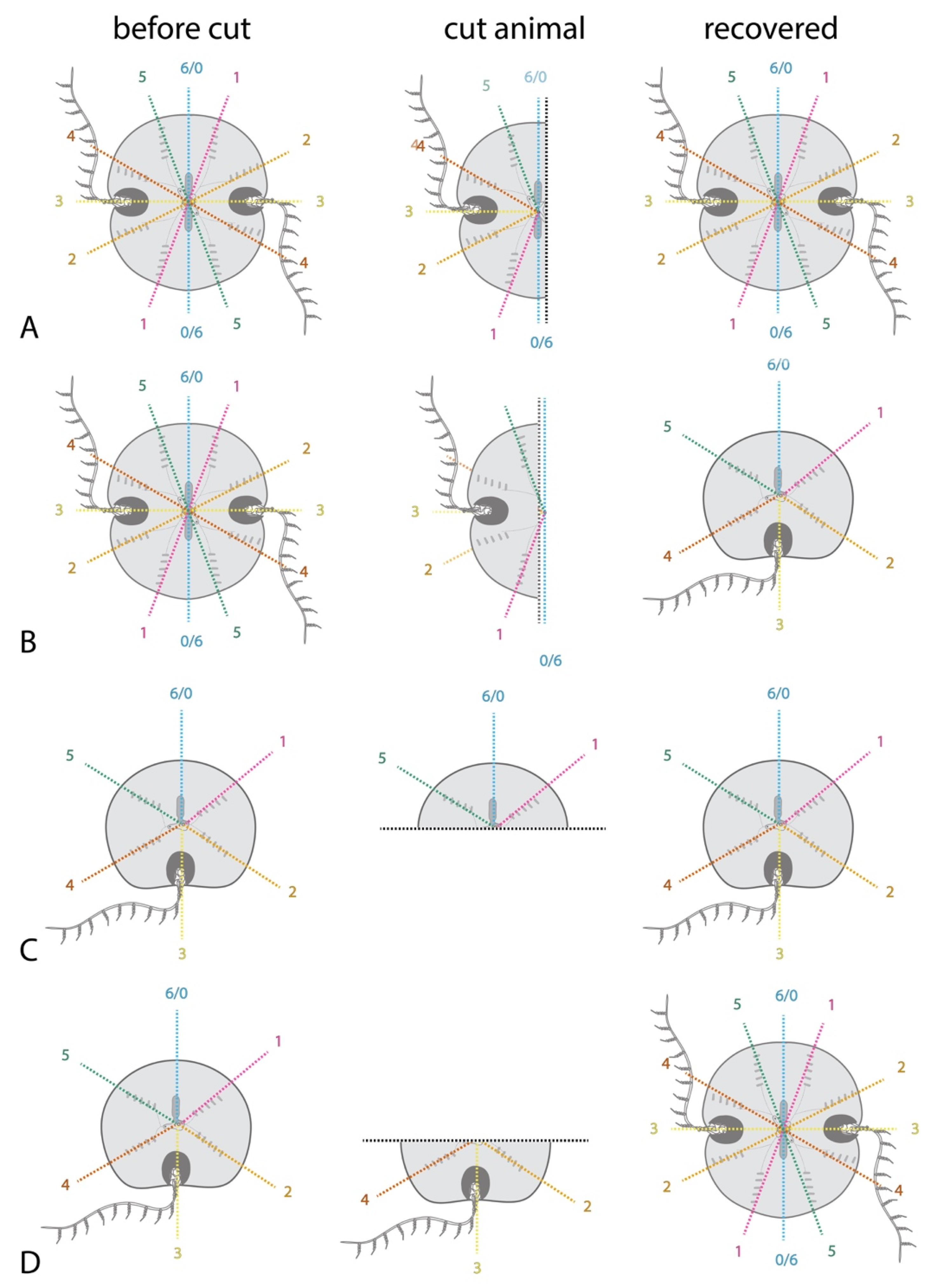
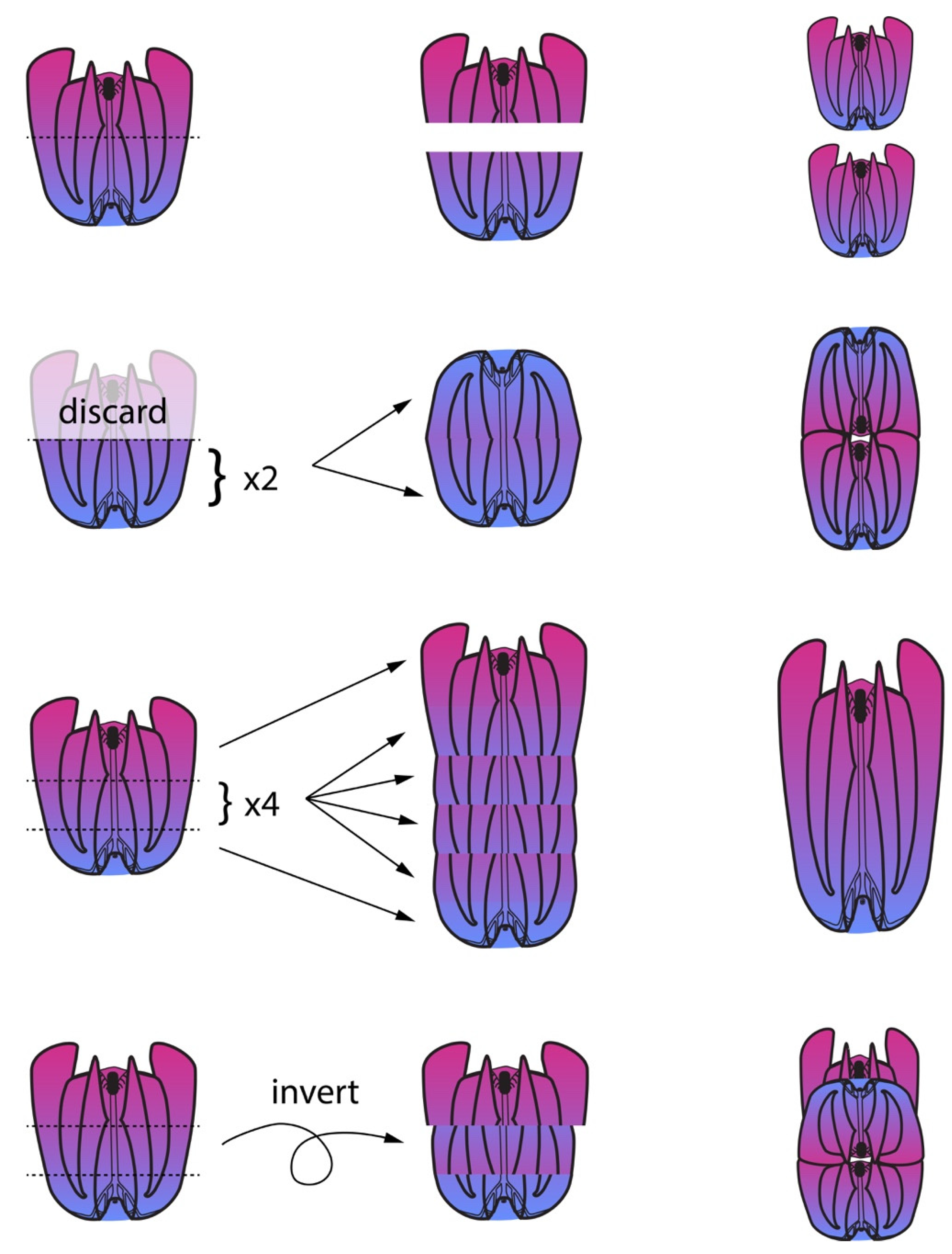
2.3. Regeneration Experiments Demonstrate a Global Axial Patterning System in Adult Ctenophores
2.4. Several Requirements for Ctenophore Regeneration Are Known: Developmental Stage, Nutrition, and Cell Division
2.5. Ctenophore Regeneration Does Not Appear to Use Dedicated Pluripotent Stem Cells
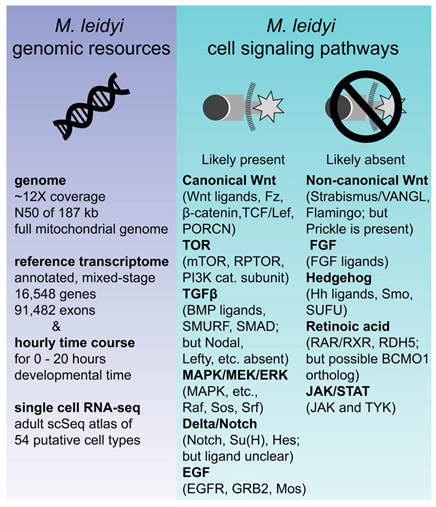
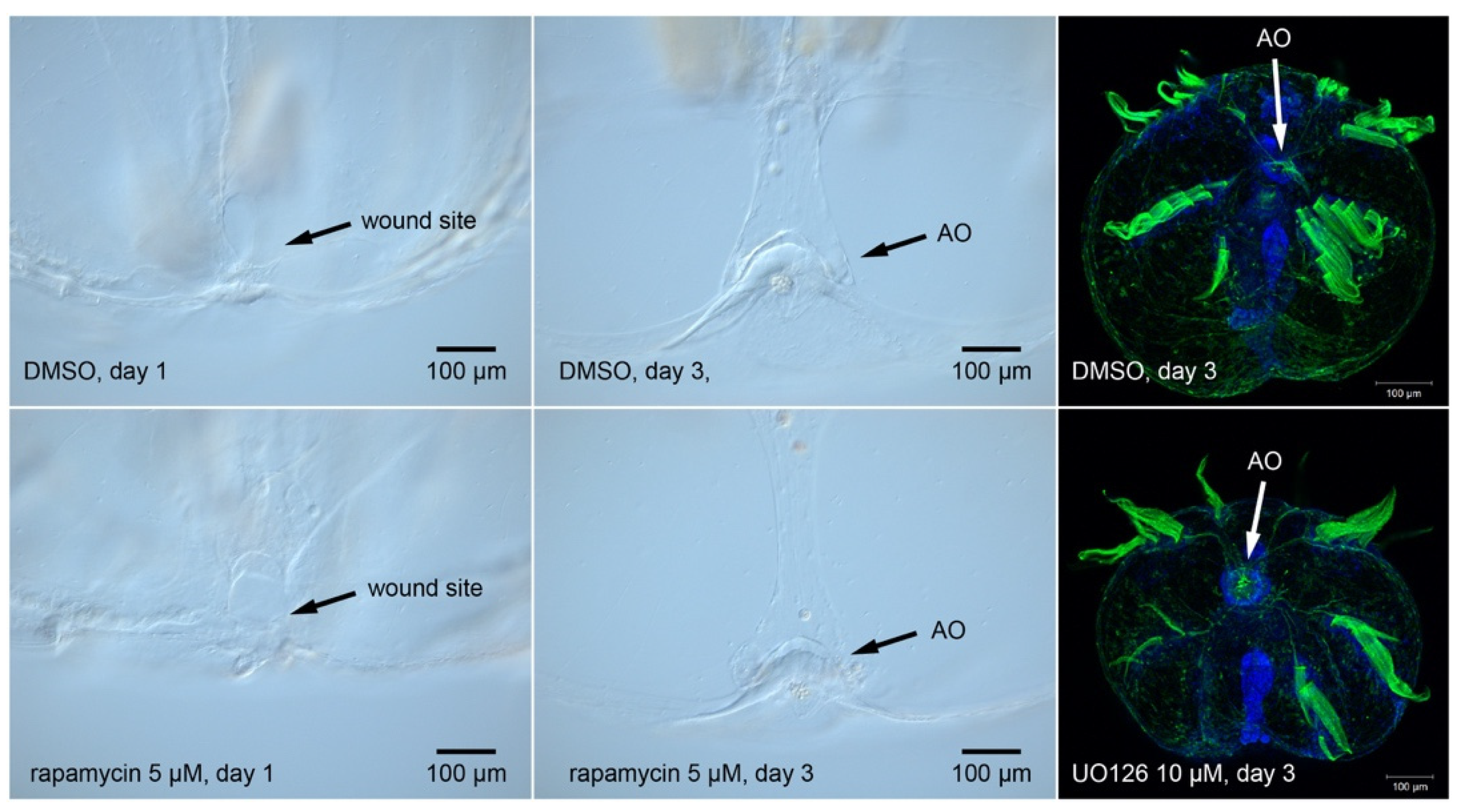
2.6. M. leidyi Genomic Information Eliminates Several Candidate Cell Signaling Pathways
3. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bely, A.E.; Nyberg, K.G. Evolution of Animal Regeneration: Re-Emergence of a Field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef]
- Martindale, M.Q. The Ontogeny and Maintenance of Adult Symmetry Properties in the Ctenophore. Mnemiopsis mccradyi. Dev. Biol. 1986, 118, 556–576. [Google Scholar] [CrossRef]
- Coonfield, B.R. Regeneration in Mnemiopsis Leidyi, Agassiz. Biol. Bull. 1936, 71, 421–428. [Google Scholar] [CrossRef]
- Freeman, G. Studies on Regeneration in the Creeping Ctenophore, Vallicula Multiformis. J. Morphol. 2004, 123, 71–84. [Google Scholar] [CrossRef]
- Jager, M.; Manuel, M. Ctenophores: An Evolutionary-Developmental Perspective. Curr. Opin. Genet. Dev. 2016, 39, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jékely, G.; Paps, J.; Nielsen, C. The Phylogenetic Position of Ctenophores and the Origin(s) of Nervous Systems. EvoDevo 2015, 6. [Google Scholar] [CrossRef]
- Whelan, N.V.; Kocot, K.M.; Moroz, T.P.; Mukherjee, K.; Williams, P.; Paulay, G.; Moroz, L.L.; Halanych, K.M. Ctenophore Relationships and Their Placement as the Sister Group to All Other Animals. Nat. Ecol. Evol. 2017, 1, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Kapli, P.; Telford, M.J. Topology-Dependent Asymmetry in Systematic Errors Affects Phylogenetic Placement of Ctenophora and Xenacoelomorpha. Sci. Adv. 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.W.; Hejnol, A.; Matus, D.Q.; Pang, K.; Browne, W.E.; Smith, S.A.; Seaver, E.; Rouse, G.W.; Obst, M.; Edgecombe, G.D.; et al. Broad Phylogenomic Sampling Improves Resolution of the Animal Tree of Life. Nature 2008, 452, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.F.; Pang, K.; Schnitzler, C.E.; Nguyen, A.D.; Moreland, R.T.; Simmons, D.K.; Koch, B.J.; Francis, W.R.; Havlak, P.; Program, N.C.S.; et al. The Genome of the Ctenophore Mnemiopsis leidyi and Its Implications for Cell Type Evolution. Science 2013, 342, 1242592. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.S.; Tsonis, P.A. Bridging the Regeneration Gap: Genetic Insights from Diverse Animal Models. Nat. Rev. Genet. 2006, 7, 873–884. [Google Scholar] [CrossRef]
- Tanaka, E.M.; Reddien, P.W. The Cellular Basis for Animal Regeneration. Dev. Cell 2011, 21, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.R.; Zapperi, S.; la Porta, C.A.M. Regeneration in Distantly Related Species: Common Strategies and Pathways. npj Syst. Biol. Appl. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Aboobaker, A.A. EvoRegen in Animals: Time to Uncover Deep Conservation or Convergence of Adult Stem Cell Evolution and Regenerative Processes. Dev. Biol. 2018, 433, 118–131. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Zhong, T.P. Regeneration across Metazoan Phylogeny: Lessons from Model Organisms. J. Genet. Genom. 2015, 42, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Cary, G.A.; Wolff, A.; Zueva, O.; Pattinato, J.; Hinman, V.F. Analysis of Sea Star Larval Regeneration Reveals Conserved Processes of Whole-Body Regeneration across the Metazoa. BMC Biol. 2019, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Seravin, L. The Systematic Revision of the Genus Mnemiopsis (Ctenophora, Lobata). Zool. J. 1994, 73, 9–18. [Google Scholar]
- Coonfield, B.R. Symmetry and Regulation in Mnemiopsis leidyi, Agassiz. Biol. Bull. 1937, 72, 299–310. [Google Scholar] [CrossRef]
- Presnell, J.S.; Vandepas, L.E.; Warren, K.J.; Swalla, B.J.; Amemiya, C.T.; Browne, W.E. The Presence of a Functionally Tripartite Through-Gut in Ctenophora Has Implications for Metazoan Character Trait Evolution. Curr. Biol. 2016, 26, 2814–2820. [Google Scholar] [CrossRef]
- Bumann, D.; Puls, G. The Ctenophore Mnemiopsis leidyi Has a Flow-Through System for Digestion with Three Consecutive Phases of Extracellular Digestion. Physiol. Zool. 1997, 70, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Main, R.J. Observations of the Feeding Mechanism of a Ctenophore. Mnemiopsis leidyi. Biol. Bull. 1928, 55, 69–78. [Google Scholar] [CrossRef]
- Coonfield, B.R. Apical Dominance and Polarity in Mnemiopsis leidyi, Agassiz. Biol. Bull. 1936, 70, 460–471. [Google Scholar] [CrossRef]
- Tamm, S.L. Functional Consequences of the Asymmetric Architecture of the Ctenophore Statocyst. Biol. Bull. 2015, 229, 173–184. [Google Scholar] [CrossRef]
- Schnitzler, C.E.; Pang, K.; Powers, M.L.; Reitzel, A.M.; Ryan, J.F.; Simmons, D.; Tada, T.; Park, M.; Gupta, J.; Brooks, S.Y.; et al. Genomic Organization, Evolution, and Expression of Photoprotein and Opsin Genes in Mnemiopsis Leidyi: A New View of Ctenophore Photocytes. BMC Biol. 2012, 10, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Tamm, S.L. Cilia and the Life of Ctenophores. Invertebr. Biol. 2014, 133, 1–46. [Google Scholar] [CrossRef]
- Chun, C. Die Dissogonie, eine neue Form der geschlechtlichen Zeugung. In Festschrift Zum Siebzigsten Geburtstage Rudorf Leuckarts; Engelmarm: Leipzig, Germany, 1892; pp. 77–108. [Google Scholar]
- Mortensen, T. On Regeneration in Ctenophores. Vidensk. Medd. Fra Dan. Nat. Foren. I Kjøbenhavn. 1913, 66, 45–51. [Google Scholar]
- Coonfield, B.R. The Regeneration of Plate Rows in Mnemiopsis leidyi, Agassiz. Proc. Natl. Acad. Sci. USA 1937, 23, 152–158. [Google Scholar] [CrossRef]
- Ramon-Mateu, J.; Ellison, S.T.; Angelini, T.E.; Martindale, M.Q. Regeneration in the Ctenophore Mnemiopsis leidyi Occurs in the Absence of a Blastema, Requires Cell Division, and Is Temporally Separable from Wound Healing. BMC Biol. 2019, 17, 1–25. [Google Scholar] [CrossRef]
- Driesch, H.; Morgan, T.H. Zur Analysis Der Ersten Entwickelungsstadien Des Ctenophoreneies. Arch. Für Entwickl. Der Org. 1895, 2, 216–224. [Google Scholar] [CrossRef]
- Martindale, M.Q. The Onset of Regenerative Properties in Ctenophores. Curr. Opin. Genet. Dev. 2016, 40, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bading, K.T.; Kaehlert, S.; Chi, X.; Jaspers, C.; Martindale, M.Q.; Javidpour, J. Food Availability Drives Plastic Self-Repair Response in a Basal Metazoan-Case Study on the Ctenophore Mnemiopsis Leidyi A. Agassiz. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Henry, J.Q.; Martindale, M.Q. Regulation and Regeneration in the Ctenophore Mnemiopsis Leidyi. Dev. Biol. 2000, 227, 720–733. [Google Scholar] [CrossRef]
- Martindale, M.Q.; Henry, J.Q. Development and Regeneration of Comb Plates in the Ctenophore Mnemiopsis leidyi. Biol. Bull. 1996, 191, 290–292. [Google Scholar] [CrossRef]
- Martindale, M.Q.; Henry, J.J. Experimental Analysis of Tentacle Formation in the Ctenophore Mnemiopsis leidyi. Biol. Bull. 1997, 193, 245–247. [Google Scholar] [CrossRef]
- Franc, J.-M. Organization and Function of Ctenophore Colloblasts: An Ultrastructural Study. Biol. Bull. 1978, 155, 527–541. [Google Scholar] [CrossRef]
- Martindale, M.Q.; Henry, J.Q. Intracellular Fate Mapping in a Basal Metazoan, the Ctenophore Mnemiopsis leidyi, Reveals the Origins of Mesoderm and the Existence of Indeterminate Cell Lineages. Dev. Biol. 1999, 214, 243–257. [Google Scholar] [CrossRef]
- Kragl, M.; Knapp, D.; Nacu, E.; Khattak, S.; Maden, M.; Epperlein, H.H.; Tanaka, E.M. Cells Keep a Memory of Their Tissue Origin during Axolotl Limb Regeneration. Nature 2009, 460, 60–65. [Google Scholar] [CrossRef]
- Gurley, K.A.; Rink, J.C.; Alvarado, A.S. β-Catenin Defines Head Versus Tail Identity During Planarian Regeneration and Homeostasis. Science 2008, 319, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Gurley, K.A.; Elliott, S.A.; Simakov, O.; Schmidt, H.A.; Holstein, T.W.; Alvarado, A.S. Expression of Secreted Wnt Pathway Components Reveals Unexpected Complexity of the Planarian Amputation Response. Dev. Biol. 2010, 347, 24–39. [Google Scholar] [CrossRef]
- Petersen, C.P.; Reddien, P.W. Wnt Signaling and the Polarity of the Primary Body Axis. Cell 2009, 139, 1056–1068. [Google Scholar] [CrossRef]
- Srivastava, M.; Mazza-Curll, K.L.; van Wolfswinkel, J.C.; Reddien, P.W. Whole-Body Acoel Regeneration Is Controlled by Wnt and Bmp-Admp Signaling. Curr. Biol. 2014, 24, 1107–1113. [Google Scholar] [CrossRef]
- Lengfeld, T.; Watanabe, H.; Simakov, O.; Lindgens, D.; Gee, L.; Law, L.; Schmidt, H.A.; Özbek, S.; Bode, H.; Holstein, T.W. Multiple Wnts Are Involved in Hydra Organizer Formation and Regeneration. Dev. Biol. 2009, 330, 186–199. [Google Scholar] [CrossRef]
- Ramirez, A.N.; Loubet-Senear, K.; Srivastava, M. A Regulatory Program for Initiation of Wnt Signaling during Posterior Regeneration. Cell Rep. 2020, 32, 108098. [Google Scholar] [CrossRef] [PubMed]
- Stoick-Cooper, C.L.; Weidinger, G.; Riehle, K.J.; Hubbert, C.; Major, M.B.; Fausto, N.; Moon, R.T. Distinct Wnt Signaling Pathways Have Opposing Roles in Appendage Regeneration. Development 2007, 134, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Adams, D.S.; Qiu, D.; Koustubhan, P.; Levin, M. Apoptosis Is Required during Early Stages of Tail Regeneration in Xenopus laevis. Dev. Biol. 2007, 301, 62–69. [Google Scholar] [CrossRef]
- Chera, S.; Ghila, L.; Dobretz, K.; Wenger, Y.; Bauer, C.; Buzgariu, W.; Martinou, J.C.; Galliot, B. Apoptotic Cells Provide an Unexpected Source of Wnt3 Signaling to Drive Hydra Head Regeneration. Dev. Cell 2009, 17, 279–289. [Google Scholar] [CrossRef]
- Pellettieri, J.; Fitzgerald, P.; Watanabe, S.; Mancuso, J.; Green, D.R.; Alvarado, A.S. Cell Death and Tissue Remodeling in Planarian Regeneration. Dev. Biol. 2010, 338, 76–85. [Google Scholar] [CrossRef]
- Todd, J.T. On the Process of Reproduction of the Members of the Aquatic Salamander. Q. J. Sci. Lit. Arts 1823, 16, 84–96. [Google Scholar]
- Kumar, A.; Godwin, J.W.; Gates, P.B.; Garza-Garcia, A.A.; Brockes, J.P. Molecular Basis for the Nerve Dependence of Limb Regeneration in an Adult Vertebrate. Science 2007, 318, 772–777. [Google Scholar] [CrossRef]
- Farkas, J.E.; Freitas, P.D.; Bryant, D.M.; Whited, J.L.; Monaghan, J.R. Neuregulin-1 Signaling Is Essential for Nerve-Dependent Axolotl Limb Regeneration. Development 2016, 143, 2724–2731. [Google Scholar] [CrossRef]
- Kumar, A.; Brockes, J.P. Nerve Dependence in Tissue, Organ, and Appendage Regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, C.; Averof, M. The Multifaceted Role of Nerves in Animal Regeneration. Curr. Opin. Genet. Dev. 2019, 57, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Toma, J.S.; Johnston, A.P.W.; Steadman, P.E.; Yuzwa, S.A.; Mahmud, N.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Mesenchymal Precursor Cells in Adult Nerves Contribute to Mammalian Tissue Repair and Regeneration. Cell Stem Cell 2019, 24, 240–256.e9. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.E.; Monaghan, J.R. A Brief History of the Study of Nerve Dependent Regeneration. Neurogenesis 2017, 4, e1302216. [Google Scholar] [CrossRef]
- Oviedo, N.; Morokuma, J.; Walentek, P.; Kema, I.; Gu, M.; Ahn, J.; Gojobori, T.; Levin, M. Long-Range Neural and Gap Junction Protein-Mediated Cues Control Polarity during Planarian Regeneration. Dev. Biol. 2010, 339, 188–199. [Google Scholar] [CrossRef]
- Newmark, P.A.; Sánchez Alvarado, A. Bromodeoxyuridine Specifically Labels the Regenerative Stem Cells of Planarians. Dev. Biol. 2000, 220, 142–153. [Google Scholar] [CrossRef]
- Passamaneck, Y.J.; Martindale, M.Q. Cell Proliferation Is Necessary for the Regeneration of Oral Structures in the Anthozoan Cnidarian Nematostella vectensis. BMC Dev. Biol. 2012, 12, 1–13. [Google Scholar] [CrossRef]
- Wenemoser, D.; Reddien, P.W. Planarian Regeneration Involves Distinct Stem Cell Responses to Wounds and Tissue Absence. Dev. Biol. 2010, 344, 979–991. [Google Scholar] [CrossRef]
- Beane, W.S.; Morokuma, J.; Lemire, J.M.; Levin, M. Bioelectric Signaling Regulates Head and Organ Size during Planarian Regeneration. Development 2012, 140, 313–322. [Google Scholar] [CrossRef]
- Levin, M.; Martyniuk, C.J. The Bioelectric Code: An Ancient Computational Medium for Dynamic Control of Growth and Form. Biosystems 2018, 164, 76–93. [Google Scholar] [CrossRef]
- Barghouth, P.G.; Thiruvalluvan, M.; Oviedo, N.J. Bioelectrical Regulation of Cell Cycle and the Planarian Model System. Biochim. Et Biophys. Acta Biomembr. 2015, 1848, 2629–2637. [Google Scholar] [CrossRef]
- Almuedo-Castillo, M.; Crespo, X.; Seebeck, F.; Bartscherer, K.; Salò, E.; Adell, T. JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef]
- DuBuc, T.Q.; Traylor-Knowles, N.; Martindale, M.Q. Initiating a Regenerative Response; Cellular and Molecular Features of Wound Healing in the Cnidarian Nematostella vectensis. BMC Biol. 2014, 12, 1–20. [Google Scholar] [CrossRef]
- Tasaki, J.; Shibata, N.; Nishimura, O.; Itomi, K.; Tabata, Y.; Son, F.; Suzuki, N.; Araki, R.; Abe, M.; Agata, K.; et al. ERK Signaling Controls Blastema Cell Differentiation during Planarian Regeneration. Development 2011, 138, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, J.; Shibata, N.; Sakurai, T.; Agata, K.; Umesono, Y. Role of C-Jun N-Terminal Kinase Activation in Blastema Formation during Planarian Regeneration. Dev. Growth Differ. 2011, 53, 389–400. [Google Scholar] [CrossRef]
- Tejada-Romero, B.; Carter, J.M.; Mihaylova, Y.; Neumann, B.; Aziz Aboobaker, A. JNK Signalling Is Necessary for a Wnt- and Stem Cell-Dependent Regeneration Programme. Development 2015, 142, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Luo, L.; Chen, J. Roles of MTOR Signaling in Tissue Regeneration. Cells 2019, 8, 1075. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.C.; Pearson, B.J.; Sánchez Alvarado, A. TORC1 Is Required to Balance Cell Proliferation and Cell Death in Planarians. Dev. Biol. 2012, 365, 458–469. [Google Scholar] [CrossRef]
- Oviedo, N.J.; Pearson, B.J.; Levin, M.; Alvarado, A.S. Planarian PTEN Homologs Regulate Stem Cells and Regeneration through TOR Signaling. DMM Dis. Models Mech. 2008, 1, 131–143. [Google Scholar] [CrossRef]
- Maiese, K. Driving Neural Regeneration through the Mammalian Target of Rapamycin. Neural Regen. Res. 2014, 9, 1413–1417. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, A.L.; Warnes, C.; Liu, J.; Zhang, C.; Kawasome, H.; Terada, N.; Boppart, M.D.; Schoenherr, C.J.; Chen, J. MTOR Regulates Skeletal Muscle Regeneration in Vivo through Kinase-Dependent and Kinase-Independent Mechanisms. Am. J. Physiol. Cell Physiol. 2009, 297, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Moroz, L.L.; Kocot, K.M.; Citarella, M.R.; Dosung, S.; Norekian, T.P.; Povolotskaya, I.S.; Grigorenko, A.P.; Dailey, C.; Berezikov, E.; Buckley, K.M.; et al. The Ctenophore Genome and the Evolutionary Origins of Neural Systems. Nature 2014, 510, 109–114. [Google Scholar] [CrossRef]
- Finenko, G.A.; Kideys, A.E.; Anninsky, B.E.; Shiganova, T.A.; Roohi, A.; Tabari, M.R.; Rostami, H.; Bagheri, S. Invasive Ctenophore Mnemiopsis Leidyi in the Caspian Sea: Feeding, Respiration, Reproduction and Predatory Impact on the Zooplankton Community. Mar. Ecol. Prog. Ser. 2006, 314, 171–185. [Google Scholar] [CrossRef]
- Babonis, L.S.; DeBiasse, M.B.; Francis, W.R.; Christianson, L.M.; Moss, A.G.; Haddock, S.H.D.; Martindale, M.Q.; Ryan, J.F. Integrating Embryonic Development and Evolutionary History to Characterize Tentacle-Specific Cell Types in a Ctenophore. Mol. Biol. Evol. 2018, 35, 2940–2956. [Google Scholar] [CrossRef]
- Spence, J.R.; Aycinena, J.-C.; del Rio-Tsonis, K.; Rio-Tsonis, K. del FGF Hedgehog Interdependence During Retina Regeneration. Dev. Dyn. 2007, 236, 1161–1174. [Google Scholar] [CrossRef]
- Turwankar, A.; Ghaskadbi, S. VEGF and FGF Signaling during Head Regeneration in Hydra. Gene 2019, 717, 144047. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Grill, S.; Sanchez, A.; Murphy-Ryan, M.; Poss, K.D. Fgf Signaling Instructs Position-Dependent Growth Rate during Zebrafish Fin Regeneration. Development 2005, 132, 5173–5183. [Google Scholar] [CrossRef]
- Lin, G.; Slack, J.M.W. Requirement for Wnt and FGF Signaling in Xenopus Tadpole Tail Regeneration. Dev. Biol. 2008, 316, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Madhavan, M.; Ewing, J.D.; Jones, D.K.; Lehman, B.M.; del Rio-Tsonis, K. The Hedgehog Pathway Is a Modulator of Retina Regeneration. Development 2004, 131, 4607–4621. [Google Scholar] [CrossRef][Green Version]
- Sebé-Pedrós, A.; Chomsky, E.; Pang, K.; Lara-Astiaso, D.; Gaiti, F.; Mukamel, Z.; Amit, I.; Hejnol, A.; Degnan, B.M.; Tanay, A. Early Metazoan Cell Type Diversity and the Evolution of Multicellular Gene Regulation. Nat. Ecol. Evol. 2018, 2, 1–17. [Google Scholar] [CrossRef]
- Moreland, R.T.; Nguyen, A.D.; Ryan, J.F.; Schnitzler, C.E.; Koch, B.J.; Siewert, K.; Wolfsberg, T.G.; Baxevanis, A.D. A Customized Web Portal for the Genome of the Ctenophore Mnemiopsis Leidyi. BMC Genom. 2014, 15, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreland, R.T.; Nguyen, A.D.; Ryan, J.F.; Baxevanis, A.D. The Mnemiopsis Genome Project Portal: Integrating New Gene Expression Resources and Improving Data Visualization. Database 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Pang, K.; Ryan, J.F.; Mullikin, J.C.; Baxevanis, A.D.; Martindale, M.Q. Genomic Insights into Wnt Signaling in an Early Diverging Metazoan, the Ctenophore Mnemiopsis leidyi. EvoDevo 2010, 1, 1–15. [Google Scholar] [CrossRef]
- Hernandez-Nicaise, M.-L. The Nervous System of Ctenophores III. Ultrastructure of Synapses. J. Neurocytol. 1973, 2, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; de Mulder, K.; Hartenstein, V.; Kuales, G.; Borgonie, G.; Marx, F.; Morris, J.; Ladurner, P. Flatworm Stem Cells and the Germ Line: Developmental and Evolutionary Implications of Macvasa Expression in Macrostomum lignano. Dev. Biol. 2008, 319, 146–159. [Google Scholar] [CrossRef]
- Swartz, S.Z.; Chan, X.Y.; Lambert, J.D. Localization of Vasa mRNA during Early Cleavage of the Snail Ilyanassa. Dev. Genes Evol. 2008, 218, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.K.; Seaver, E.C. Vasa and Nanos Are Coexpressed in Somatic and Germ Line Tissue from Early Embryonic Cleavage Stages through Adulthood in the Polychaete Capitella Sp. I. Dev. Genes Evol. 2008, 218, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Alié, A.; Leclère, L.; Jager, M.; Dayraud, C.; Chang, P.; le Guyader, H.; Quéinnec, E.; Manuel, M. Somatic Stem Cells Express Piwi and Vasa Genes in an Adult Ctenophore: Ancient Association of “Germline Genes” with Stemness. Dev. Biol. 2011, 350, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Reitzel, A.M.; Pang, K.; Martindale, M.Q. Developmental Expression of “Germline”- and “Sex Determination”-Related Genes in the Ctenophore Mnemiopsis leidyi. EvoDevo 2016, 7, 1–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edgar, A.; Mitchell, D.G.; Martindale, M.Q. Whole-Body Regeneration in the Lobate Ctenophore Mnemiopsis leidyi. Genes 2021, 12, 867. https://doi.org/10.3390/genes12060867
Edgar A, Mitchell DG, Martindale MQ. Whole-Body Regeneration in the Lobate Ctenophore Mnemiopsis leidyi. Genes. 2021; 12(6):867. https://doi.org/10.3390/genes12060867
Chicago/Turabian StyleEdgar, Allison, Dorothy G. Mitchell, and Mark Q. Martindale. 2021. "Whole-Body Regeneration in the Lobate Ctenophore Mnemiopsis leidyi" Genes 12, no. 6: 867. https://doi.org/10.3390/genes12060867
APA StyleEdgar, A., Mitchell, D. G., & Martindale, M. Q. (2021). Whole-Body Regeneration in the Lobate Ctenophore Mnemiopsis leidyi. Genes, 12(6), 867. https://doi.org/10.3390/genes12060867





