Aquaporins in Cereals—Important Players in Maintaining Cell Homeostasis under Abiotic Stress
Abstract
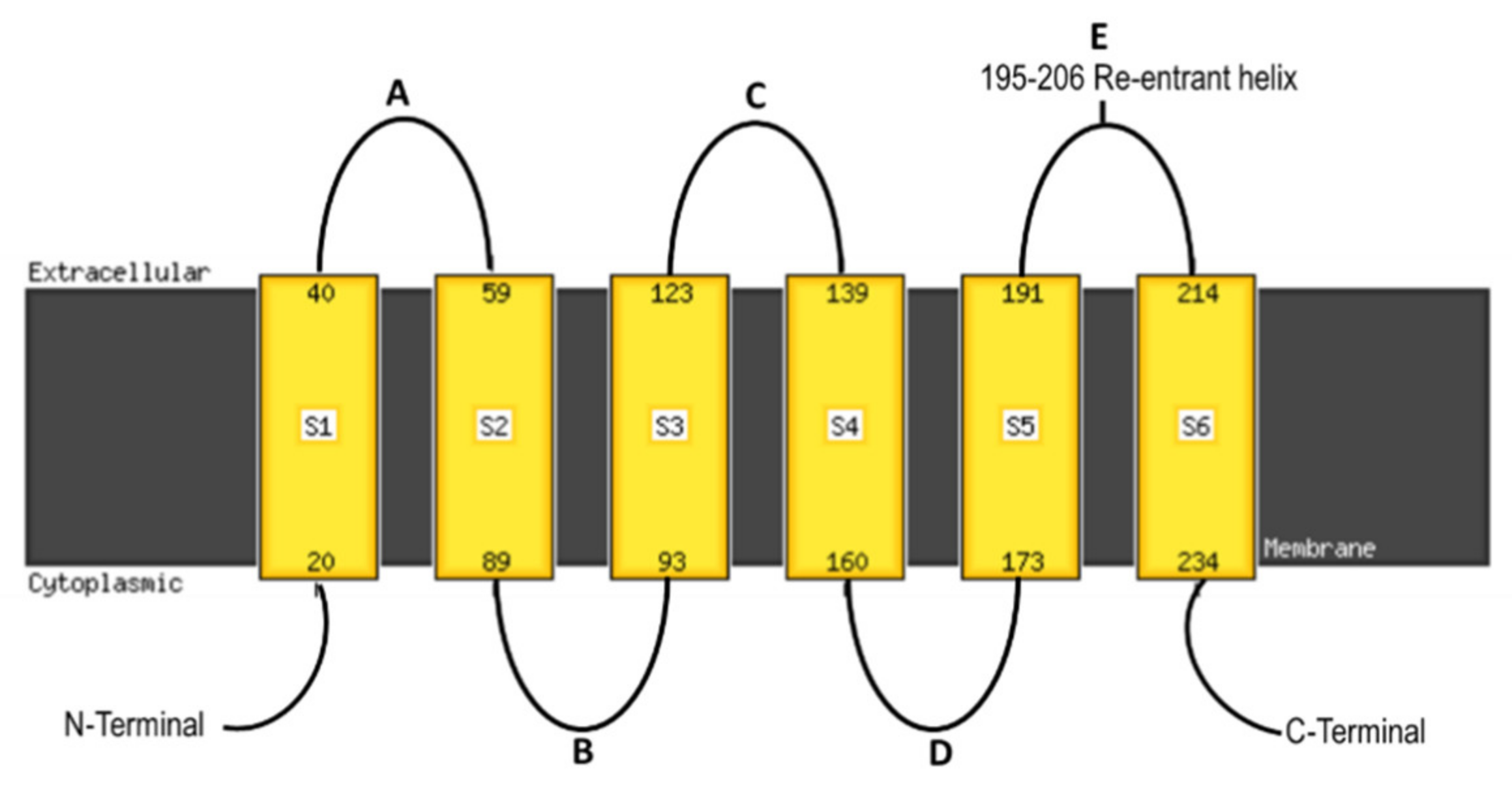
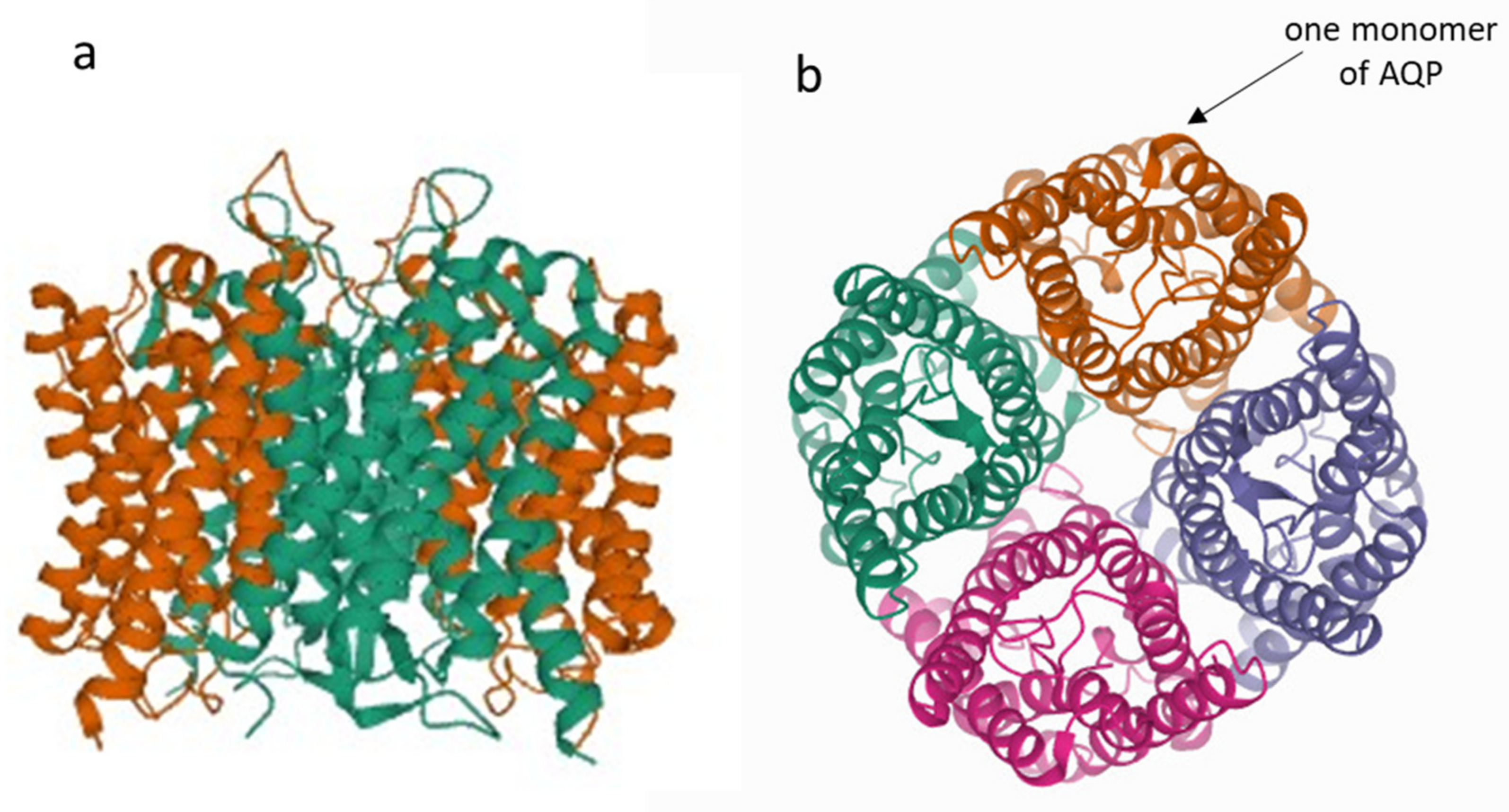
1. Introduction
2. Diversity of Aquaporins in Cereal Species
3. Study of the Expression and Role of Aquaporins in Maintaining the Cell Homeostasis of Cereals under Abiotic Stress
3.1. Drought and Heat Stress
3.2. Cold Stress
3.3. Salinity
3.4. Comparison between Genotypes, Which Are Characterised as Tolerant or Resistant to Specific Abiotic Stress
4. Overexpression and Mutant Lines in the Cereal Aquaporin-Encoding Genes
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chrispeels, M.J.; Agre, P. Aquaporins: Water Channel Proteins of Plant and Animal Cells. Trends Biochem. Sci. 1994, 19, 421–425. [Google Scholar] [CrossRef]
- Maurel, C.; Chrispeels, M.J. Aquaporins. A Molecular Entry into Plant Water Relations. Plant. Physiol. 2001, 125, 135–138. [Google Scholar] [CrossRef]
- Sutka, M.; Amodeo, G.; Ozu, M. Plant and Animal Aquaporins Crosstalk: What Can Be Revealed from Distinct Perspectives. Biophys. Rev. 2017, 9, 545–562. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; de Araújo, F.C.; Ferreira-Neto, J.R.C.; da Silva, M.D.; Pandolfi, V.; Aburjaile, F.F.; Sakamoto, T.; de Oliveira Silva, R.L.; Kido, E.A.; Barbosa Amorim, L.L.; et al. Plant Aquaporins: Diversity, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2019, 20, 368–395. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Benga, G.; Popescu, O.; Pop, V.I.; Holmes, R.P. p-(Chloromercuri)benzenesulfonate Binding by Membrane Proteins and the Inhibition of Water Transport in Human Erythrocytes. Biochemistry 1986, 25, 1535–1538. [Google Scholar] [CrossRef]
- Kuchel, P.W. The Story of the Discovery of Aquaporins: Convergent Evolution of Ideas—But Who Got There First? Cell Mol. Biol. 2006, 52, 2–5. [Google Scholar] [PubMed]
- Fortin, M.G.; Morrison, N.A.; Verma, D.P. Nodulin-26, a Peribacteroid Membrane Nodulin is Expressed Independently of the Development of the Peribacteroid Compartment. Nucleic Acids Res. 1987, 15, 813–824. [Google Scholar] [CrossRef]
- Maurel, C.; Reizer, J.; Schroeder, J.I.; Chrispeels, M.J. The Vacuolar Membrane Protein Gamma-TIP Creates Water Specific Channels in Xenopus Oocytes. EMBO J. 1993, 12, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of Aquaporins in Plants under Stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural Basis of Water-Specific Transport through the AQP1 Water Channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef]
- Wu, B.; Steinbronn, C.; Alsterfjord, M.; Zeuthen, T.; Beitz, E. Concerted Action of Two Cation Filters in the Aquaporin Water Channel. EMBO J. 2009, 28, 2188–2194. [Google Scholar] [CrossRef]
- Guan, X.-G.; Su, W.-H.; Yi, F.; Zhang, D.; Hao, F.; Zhang, H.-G.; Liu, Y.-J.; Feng, X.-C.; Ma, T.-H. NPA Motifs Play a Key Role in Plasma Membrane Targeting of Aquaporin-4. IUBMB Life 2010, 62, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Verma, R.K.; Gupta, A.B.; Sankararamakrishnan, R. Major Intrinsic Protein Superfamily: Channels with Unique Structural Features and Diverse Selectivity Filters. Methods Enzymol. 2015, 557, 485–520. [Google Scholar] [CrossRef]
- Verma, R.K.; Prabh, N.D.; Sankararamakrishnan, R. Intra-Helical Salt-Bridge and Helix Destabilizing Residues within the Same Helical Turn: Role of Functionally Important Loop E Half-Helix in Channel Regulation of Major Intrinsic Proteins. Biochim. Biophys. Acta 2015, 1848, 1436–1449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomes, D.; Agasse, A.; Thiébaud, P.; Delrot, S.; Gerós, H.; Chaumont, F. Aquaporins Are Multifunctional Water and Solute Transporters Highly Divergent in Living Organisms. Biochim. Biophys. Acta. 2009, 1788, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Available online: http://www.pdb.org (accessed on 21 September 2020).
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural Mechanism of Plant Aquaporin Gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of Aquaporin-Driven Hydrogen Peroxide Transport. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef]
- Berka, K.; Hanák, O.; Sehnal, D.; Banás, P.; Navrátilová, V.; Jaiswal, D.; Ionescu, C.M.; Svobodová Vareková, R.; Koca, J.; Otyepka, M. MOLEonline 2.0: Interactive Web-based Analysis of Biomacromolecular Channels. Nucleic Acids Res. 2012, 40, W222–W227. [Google Scholar] [CrossRef] [PubMed]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4. [Google Scholar] [CrossRef]
- Javot, H.; Lauvergeat, V.; Santoni, V.; Martin-Laurent, F.; Güçlü, J.; Vinh, J.; Heyes, J.; Franck, K.I.; Schäffner, A.R.; Bouchez, D.; et al. Role of a Single Aquaporin Isoform in Root Water Uptake. Plant. Cell 2003, 15, 509–522. [Google Scholar] [CrossRef]
- Vander Willigen, C.; Postaire, O.; Tournaire-Roux, C.; Boursiac, Y.; Maurel, C. Expression and Inhibition of Aquaporins in Germinating Arabidopsis Seeds. Plant. Cell Physiol. 2006, 47, 1241–1250. [Google Scholar] [CrossRef]
- Soto, G.; Alleva, K.; Mazzella, M.A.; Amodeo, G.; Muschietti, J.P. AtTIP1;3 and AtTIP5;1, the Only Highly Expressed Arabidopsis Pollen-Specific Aquaporins, Transport Water and Urea. FEBS Lett. 2008, 582, 4077–4082. [Google Scholar] [CrossRef]
- Soto, G.; Fox, R.; Ayub, N.; Alleva, K.; Guaimas, F.; Erijman, E.J.; Mazzella, A.; Amodeo, G.; Muschietti, J. TIP5;1 Is an Aquaporin Specifically Targeted to Pollen Mitochondria and Is Probably Involved in Nitrogen Remobilization in Arabidopsis thaliana. Plant. J. 2010, 64, 1038–1047. [Google Scholar] [CrossRef]
- Pérez Di Giorgio, J.A.; Barberini, M.L.; Amodeo, G.; Muschietti, J.P. Pollen Aquaporins: What Are They There For? Plant. Signal. Behav. 2016, 11, e1217375. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Maeshima, M. The ER-Localized Aquaporin SIP2;1 Is Involved in Pollen Germination and Pollen Tube Elongation in Arabidopsis thaliana. Plant. Mol. Biol. 2019, 100, 335–349. [Google Scholar] [CrossRef]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins Facilitate Hydrogen Peroxide Entry into Guard Cells to Mediate ABA- and Pathogen-Triggered Stomatal Closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chaumont, F. Are Aquaporins Expressed in Stomatal Complexes Promising Targets to Enhance Stomatal Dynamics? Front. Plant. Sci. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Calanca, P.P. Effects of Abiotic Stress in Crop Production. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2017; pp. 165–180. [Google Scholar]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate Regulates Leaf Senescence and Tolerance to Cold Stress: Crosstalk with Other Phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and Crop Yield. Plant. Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-Analysis of Drought and Heat Stress Combination Impact on Crop Yield and Yield Components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to Drought and Salt Stress in Plants: Unraveling the Signaling Networks. Front. Plant. Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Carvajal, M.; Martínez-Ballesta, M.C. Detergent Resistant Membrane Domains in Broccoli Plasma Membrane Associated to the Response to Salinity Stress. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, I.; Masajada, K. Aquaporins as a Link between Water Relations and Photosynthetic Pathway in Abiotic Stress Tolerance in Plants. Gene 2019, 687, 166–172. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Mishra, M.; Patil, G.; Mulkey, S.; Ramawat, N.; Pratap Singh, V.; Deshmukh, R.; Kumar Tripathi, D.; Nguyen, H.T.; Sharma, S. Avenues of the Membrane Transport System in Adaptation of Plants to Abiotic Stresses. Crit. Rev. Biotechnol. 2019, 39, 861–883. [Google Scholar] [CrossRef]
- Barzana, G.; Rios, J.J.; Lopez-Zaplana, A.; Nicolas-Espinosa, J.; Yepes-Molina, L.; Garcia-Ibañez, P.; Carvajal, M. Interrelations of Nutrient and Water Transporters in Plants Under Abiotic Stress. Physiol. Plant. 2020. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile Roles of Aquaporin in Physiological Processes and Stress Tolerance in Plants. Plant. Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Bárzana, G.; Carvajal, M. Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress. Plants 2020, 9(12), 1662. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Liu, F.; Sun, L.; Hao, F. Versatile Roles of Aquaporins in Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Mishra, A. Plant Aquaporins Alleviate Drought Tolerance in Plants by Modulating Cellular Biochemistry, Root-architecture, and Photosynthesis. Physiol. Plant. 2021. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Thakral, V.; Padalkar, G.; Rajora, N.; Dhiman, P.; Raturi, G.; Sharma, Y.; Tripathi, D.K.; Deshmukh, R.; Sharma, T.R.; et al. Significance of Solute Specificity, Expression, and Gating Mechanism of Tonoplast Intrinsic Protein during Development and Stress Response in Plants. Physiol. Plant. 2021. [Google Scholar] [CrossRef]
- Vats, S.; Sudhakaran, S.; Bhardwaj, A.; Mandlik, R.; Sharma, Y.; Kumar, S.; Tripathi, D.K.; Sonah, H.; Sharma, T.R.; Deshmukh, R. Targeting Aquaporins to Alleviate Hazardous Metal(loid)s Imposed Stress in Plants. J. Hazard. Mater. 2021, 408. [Google Scholar] [CrossRef]
- Major Intrinsic Proteins Database. Available online: http://genoweb1.irisa.fr/mipdb (accessed on 20 September 2020).
- Ishikawa, F.; Suga, S.; Uemura, T.; Sato, M.H.; Maeshima, M. Novel Type Aquaporin SIPs Are Mainly Localized to the ER Membrane and Show Cell-Specific Expression in Arabidopsis Thaliana. FEBS Lett. 2005, 579, 5814–5820. [Google Scholar] [CrossRef]
- Zybailov, B.; Rutschow, H.; Friso, G.; Rudella, A.; Emanuelsson, O.; Sun, Q.; van Wijk, K.J. Sorting Signals, N-Terminal Modifications and Abundance of the Chloroplast Proteome. PLoS ONE 2008, 3, e1994. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Brugière, S.; Salvi, D.; Seigneurin-Berny, D.; Court, M.; Moyet, L.; Ramus, C.; Miras, S.; Mellal, M.; Le Gall, S.; et al. AT_CHLORO, a Comprehensive Chloroplast Proteome Database with Subplastidial Localization and Curated Information on Envelope Proteins. Mol. Cell. Proteom. 2010, 9, 1063–1084. [Google Scholar] [CrossRef]
- Simm, S.; Papasotiriou, D.G.; Ibrahim, M.; Leisegang, M.S.; Müller, B.; Schorge, T.; Karas, M.; Mirus, O.; Sommer, M.S.; Schleiff, E. Defining the Core Proteome of the Chloroplast Envelope Membranes. Front. Plant. Sci. 2013, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Quigley, F.; Rosenberg, J.M.; Shachar-Hill, Y.; Bohnert, H.J. From Genome to Function: The Arabidopsis Aquaporins. Genome Biol. 2002, 3, 1. [Google Scholar] [CrossRef]
- Li, W.; Zhang, D.; Zhu, G.; Mi, X.; Guo, W. Combining Genome-wide and Transcriptome-wide Analyses Reveal the Evolutionary Conservation and Functional Diversity of Aquaporins in Cotton. BMC Genom. 2019, 20, 538. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Yang, L.; Liu, N.; Yang, Y.; Zhou, X.K.; Xia, Y.C.; He, Y.; He, Y.Q.; Gong, H.J.; Ma, D.F.; et al. Genome-wide Identification, Structure Characterization, and Expression Pattern Profiling of Aquaporin Gene Family in Cucumber. BMC Plant. Biol. 2019, 19, 345. [Google Scholar] [CrossRef]
- Faize, M.; Fumanal, B.; Luque, F.; Ramírez-Tejero, J.A.; Zou, Z.; Qiao, X.; Faize, L.; Gousset-Dupont, A.; Roeckel-Drevet, P.; Label, P.; et al. Genome Wild Analysis and Molecular Understanding of the Aquaporin Diversity in Olive Trees (Olea europaea L.). Int J. Mol. Sci. 2020, 21(11), 4183. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Yu, J.W.; Park, S.W. Genome-wide Analysis and Expression Profiling of the Solanum tuberosum Aquaporins. Plant. Physiol. Biochem. 2013, 73, 392–404. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Watson-Lazowski, A.; Evans, J.R.; Groszmann, M. Genome-wide Identification and Characterisation of Aquaporins in Nicotiana tabacum and Their Relationships with other Solanaceae Species. BMC Plant. Biol. 2020, 20, 266. [Google Scholar] [CrossRef]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-wide Identification and Expression Analysis of Aquaporins in Tomato. PLoS ONE 2013, 8, e79052. [Google Scholar] [CrossRef]
- Fox, A.R.; Maistriaux, L.C.; Chaumont, F. Toward Understanding of the High Number of Plant Aquaporin Isoforms and Multiple Regulation Mechanisms. Plant. Sci. 2017, 264, 179–187. [Google Scholar] [CrossRef]
- FAO Stat. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 11 January 2021).
- Madrid-Espinoza, J.; Brunel-Saldias, N.; Guerra, F.P.; Gutiérrez, A.; Del Pozo, A. Genome-Wide Identification and Transcriptional Regulation of Aquaporin Genes in Bread Wheat (Triticum aestivum L.) under Water Stress. Genes 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Farooq, M.; Hussain, A.; Qamar, M.T.; Abbas, M.W.; Mustafa, G.; Karim, A.; Ahmed, I.; Hussain, T. Genome-Wide Bioinformatics Analysis of Aquaporin Gene Family in Maize (Zea mays L.). J. Phylogenetics. Evol. Biol. 2018, 6. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 Rice Aquaporin Genes and Analysis of Their Expression and Function. Plant. Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Hove, R.M.; Ziemann, M.; Bhave, M. Identification and Expression Analysis of the Barley (Hordeum vulgare L.) Aquaporin Gene Family. PLoS ONE 2015, 10, e0128025. [Google Scholar] [CrossRef]
- Azad, A.K.; Ahmed, J.; Alum, M.A.; Hasan, M.M.; Ishikawa, T.; Sawa, Y.; Katsuhara, M. Genome-Wide Characterization of Major Intrinsic Proteins in Four Grass Plants and Their Non-Aqua Transport Selectivity Profiles with Comparative Perspective. PLoS ONE 2016, 11, e0157735. [Google Scholar] [CrossRef]
- Singh, R.K.; Shweta, S.; Muthamilarasan, M.; Rani, R.; Prasad, M. Study on Aquaporins of Setaria italica Suggests the Involvement of SiPIP3;1 and SiSIP1;1 in Abiotic Stress Response. Funct. Integr. Genom. 2019, 19, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Affortit, P.; Tranchant-Dubreuil, C.; de la Fuente-Cantó, C.; Mariac, C.; Gantet, P.; Vadez, V.; Vigouroux, Y.; Laplaze, L. Aquaporins Are Main Contributors to Root Hydraulic Conductivity in Pearl Millet [Pennisetum glaucum (L.) R. Br.]. PLoS ONE 2020, 15, e0233481. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Fischer, M. Functional Aquaporin Diversity in Plants. Biochim. Biophys. Acta 2006, 1758, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant Aquaporins: Membrane Channels with Multiple Integrated Functions. Annu. Rev. Plant. Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef]
- Heckwolf, M.; Pater, D.; Hanson, D.T.; Kaldenhoff, R. The Arabidopsis Thaliana Aquaporin AtPIP1;2 Is a Physiologically Relevant CO2 Transport Facilitator. Plant. J. 2011, 67, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lu, Z.; Ding, L.; Guo, J.; Wang, M.; Ling, N.; Guo, S.; Shen, Q. Role of Aquaporins in Determining Carbon and Nitrogen Status in Higher Plants. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.M.; Daszkowska-Golec, A.; Gajecka, M.; Kościelniak, P.; Bierza, W.; Szarejko, I. Methyl Jasmonate Affects Photosynthesis Efficiency, Expression of HvTIP Genes and Nitrogen Homeostasis in Barley. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-Facilitated Transmembrane Diffusion of Hydrogen Peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schüssler, M.D.; Jahn, T.P. Metalloids: Essential, Beneficial or Toxic? Major Intrinsic Proteins Sort It Out. Trends Biochem. Sci. 2008, 33, 20–26. [Google Scholar] [CrossRef]
- Li, G.-W.; Peng, Y.-H.; Yu, X.; Zhang, M.-H.; Cai, W.-M.; Sun, W.-N.; Su, W.-A. Transport Functions and Expression Analysis of Vacuolar Membrane Aquaporins in Response to Various Stresses in Rice. J. Plant. Physiol. 2008, 165, 1879–1888. [Google Scholar] [CrossRef]
- Bacelar, E.L.V.A.; Moutinho-Pereira, J.M.; Gonçalves, B.M.C.; Brito, C.V.Q.; Gomes-Laranjo, J.; Ferreira, H.M.F.; Correia, C.M. Water Use Strategies of Plants under Drought Conditions. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–170. [Google Scholar] [CrossRef]
- Signor, S.A.; Nuzhdin, S.V. The Evolution of Gene Expression in Cis and Trans. Trends Genet. 2018, 34, 532–544. [Google Scholar] [CrossRef]
- Xu, F.; Wang, K.; Yuan, W.; Xu, W.; Shuang, L.; Kronzucker, H.J.; Chen, G.; Miao, R.; Zhang, M.; Ding, M.; et al. Overexpression of Rice Aquaporin OsPIP1;2 Improves Yield by Enhancing Mesophyll CO2 Conductance and Phloem Sucrose Transport. J. Exp. Bot. 2019, 7, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.; Murai-Hatano, M.; Ishikawa-Sakurai, J.; Hayashi, H.; Kawamura, Y.; Uemura, M. Cold Stress-Induced Acclimation in Rice Is Mediated by Root-Specific Aquaporins. Plant. Cell Physiol. 2012, 53, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, M.M.; Wiecha, K.; Gajek, K.; Szarejko, I. Drought Stress and Re-Watering Affect the Abundance of TIP Aquaporin Transcripts in Barley. PLoS ONE 2019, 14, e0226423. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.E.; Chen, J.; Liu, M.; Chen, Z.L.; Qu, L.J.; Gu, H. Expression and Functional Analysis of the Rice Plasma-Membrane Intrinsic Protein Gene Family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Mauleon, R.; Vadez, V.; Henry, A. Root Aquaporins Contribute to Whole Plant Water Fluxes under Drought Stress in Rice (Oryza sativa L.). Plant. Cell Environ. 2016, 39, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Akiyama, Y.; Koshio, K.; Shibasaka, M.; Kasamo, K. Functional Analysis of Water Channels in Barley Roots. Plant. Cell Physiol. 2002, 43, 885–893. [Google Scholar] [CrossRef]
- Horie, T.; Kaneko, T.; Sugimoto, G.; Sasano, S.; Panda, S.K.; Shibasaka, M.; Katsuhara, M. Mechanisms of Water Transport Mediated by PIP Aquaporins and Their Regulation via Phosphorylation Events under Salinity Stress in Barley Roots. Plant. Cell Physiol. 2011, 52, 663–675. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.-D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Ikram, K.; Ullah, A.; Alghanem, S.M.S. Conferring Drought-Tolerant Wheat Genotypes through Morpho-Physiological and Chlorophyll Indices at Seedling Stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Plassard, C. Aquaporins: For More than Water at the Plant-Fungus Interface? New Phytol. 2011, 190, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Hellal, F.A.; El-Shabrawi, H.M.; Abd El-Hady, M.; Khatab, I.A.; El-Sayed, S.a.A.; Abdelly, C. Influence of PEG Induced Drought Stress on Molecular and Biochemical Constituents and Seedling Growth of Egyptian Barley Cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Besse, M.; Knipfer, T.; Miller, A.J.; Verdeil, J.-L.; Jahn, T.P.; Fricke, W. Developmental Pattern of Aquaporin Expression in Barley (Hordeum vulgare L.) Leaves. J. Exp. Bot. 2011, 62, 4127–4142. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, N.C.; Turner, D.W.; Tyerman, S.D. Comparison between Gradient-Dependent Hydraulic Conductivities of Roots Using the Root Pressure Probe: The Role of Pressure Propagations and Implications for the Relative Roles of Parallel Radial Pathways. Plant. Cell Environ. 2007, 30, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Bramley, H.; Turner, N.C.; Turner, D.W.; Tyerman, S.D. Roles of Morphology, Anatomy, and Aquaporins in Determining Contrasting Hydraulic Behavior of Roots. Plant. Physiol. 2009, 150, 348–364. [Google Scholar] [CrossRef]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. A PIP1 Aquaporin Contributes to Hydrostatic Pressure-Induced Water Transport in Both the Root and Rosette of Arabidopsis. Plant. Physiol. 2010, 152, 1418–1430. [Google Scholar] [CrossRef]
- Prado, K.; Maurel, C. Regulation of Leaf Hydraulics: From Molecular to Whole Plant Levels. Front. Plant. Sci. 2013, 4, 255. [Google Scholar] [CrossRef] [PubMed]
- Gitto, A.; Fricke, W. Zinc Treatment of Hydroponically Grown Barley Plants Causes a Reduction in Root and Cell Hydraulic Conductivity and Isoform-Dependent Decrease in Aquaporin Gene Expression. Physiol. Plant. 2018, 164, 176–190. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The Role of Aquaporins in Root Water Uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Sakurai-Ishikawa, J.; Murai-Hatano, M.; Hayashi, H.; Ahamed, A.; Fukushi, K.; Matsumoto, T.; Kitagawa, Y. Transpiration from Shoots Triggers Diurnal Changes in Root Aquaporin Expression. Plant. Cell Environ. 2011, 34, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Nada, R.M.; Abogadallah, G.M. Aquaporins Are Major Determinants of Water Use Efficiency of Rice Plants in the Field. Plant. Sci. 2014, 227, 165–180. [Google Scholar] [CrossRef]
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef]
- Smirnoff, N. Plant Resistance to Environmental Stress. Curr. Opin. Biotechnol. 1998, 9, 214–219. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature Stress and Redox Homeostasis in Agricultural Crops. Front. Environ. Sci. 2015. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Markhart, A.H.; Fiscus, E.L.; Naylor, A.W.; Kramer, P.J. Effect of Temperature on Water and Ion Transport in Soybean and Broccoli Systems. Plant. Physiol. 1979, 64, 83–87. [Google Scholar] [CrossRef]
- Ranieri, E. Hydraulics of Sub-Superficial Flow Constructed Wetlands in Semi Arid Climate Conditions. Water Sci. Technol. 2003, 47, 49–55. [Google Scholar] [CrossRef]
- Aroca, R.; Amodeo, G.; Fernández-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The Role of Aquaporins and Membrane Damage in Chilling and Hydrogen Peroxide Induced Changes in the Hydraulic Conductance of Maize Roots. Plant. Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Phenotypic Plasticity in Photosynthetic Temperature Acclimation among Crop Species with Different Cold Tolerances. Plant. Physiol. 2010, 152, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Murai-Hatano, M.; Kuwagata, T.; Sakurai, J.; Nonami, H.; Ahamed, A.; Nagasuga, K.; Matsunami, T.; Fukushi, K.; Maeshima, M.; Okada, M. Effect of Low Root Temperature on Hydraulic Conductivity of Rice Plants and the Possible Role of Aquaporins. Plant. Cell Physiol. 2008, 49, 1294–1305. [Google Scholar] [CrossRef]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. Plasma Membrane ATPase and the Aquaporin HvPIP1 in Barley Brassinosteroid Mutants Acclimated to High and Low Temperature. J. Plant. Physiol. 2020, 244, 153090. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from Crop Plants Struggling with Salinity. Plant. Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Over-Expression of a Barley Aquaporin Increased the Shoot/Root Ratio and Raised Salt Sensitivity in Transgenic Rice Plants. Plant. Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Price, A.H.; Cairns, J.E.; Horton, P.; Jones, H.G.; Griffiths, H. Linking Drought-Resistance Mechanisms to Drought Avoidance in Upland Rice Using a QTL Approach: Progress and New Opportunities to Integrate Stomatal and Mesophyll Responses. J. Exp. Bot. 2002, 53, 989–1004. [Google Scholar] [CrossRef]
- Lian, H.-L.; Yu, X.; Ye, Q.; Ding, X.; Kitagawa, Y.; Kwak, S.-S.; Su, W.-A.; Tang, Z.-C.; Ding, X.-S. The Role of Aquaporin RWC3 in Drought Avoidance in Rice. Plant. Cell Physiol. 2004, 45, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Debieu, M.; Kanfany, G.; Laplaze, L. Pearl Millet Genome: Lessons from a Tough Crop. Trends Plant. Sci. 2017, 22, 911–913. [Google Scholar] [CrossRef]
- Reddy, P.S.; Tharanya, M.; Sivasakthi, K.; Srikanth, M.; Hash, C.T.; Kholova, J.; Sharma, K.K.; Vadez, V. Molecular Cloning and Expression Analysis of Aquaporin Genes in Pearl Millet [Pennisetum glaucum (L.) R. Br.] Genotypes Contrasting in Their Transpiration Response to High Vapour Pressure Deficits. Plant. Sci. 2017, 265, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Kholova, J.; Zaman-Allah, M.; Belko, N. Water: The Most Important “Molecular” Component of Water Stress Tolerance Research. Funct. Plant. Biol. 2013, 40, 1310–1322. [Google Scholar] [CrossRef]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.; Sun, X.; Zhu, Y.; et al. Aquaporin OsPIP1;1 Promotes Rice Salt Resistance and Seed Germination. Plant. Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef]
- Hu, W.; Yuan, Q.; Wang, Y.; Cai, R.; Deng, X.; Wang, J.; Zhou, S.; Chen, M.; Chen, L.; Huang, C.; et al. Overexpression of a Wheat Aquaporin Gene, TaAQP8, Enhances Salt Stress Tolerance in Transgenic Tobacco. Plant. Cell Physiol. 2012, 53, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, W.; Deng, X.; Ma, Z.; Chen, L.; Huang, C.; Wang, C.; Wang, J.; He, Y.; Yang, G.; et al. Overexpression of the Wheat Aquaporin Gene, TaAQP7, Enhances Drought Tolerance in Transgenic Tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Brini, F.; Masmoudi, K. Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat Cv. Maali. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Ding, L.; Milhiet, T.; Couvreur, V.; Nelissen, H.; Meziane, A.; Parent, B.; Aesaert, S.; Van Lijsebettens, M.; Inzé, D.; Tardieu, F.; et al. Modification of the Expression of the Aquaporin ZmPIP2;5 Affects Water Relations and Plant Growth. Plant. Physiol. 2020, 182, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Alavilli, H.; Awasthi, J.P.; Rout, G.R.; Sahoo, L.; Lee, B.-H.; Panda, S.K. Overexpression of a Barley Aquaporin Gene, HvPIP2;5 Confers Salt and Osmotic Stress Tolerance in Yeast and Plants. Front. Plant. Sci. 2016, 7, 1566. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-K.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.J.; Ma, J.F.; Zhao, F.-J. Decreasing Arsenic Accumulation in Rice by Overexpressing OsNIP1;1 and OsNIP3;3 through Disrupting Arsenite Radial Transport in Roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.T.; Lamarque, J. The RCP Greenhouse Gas Concentrations and Their Extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213–241. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant. Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
| Species | Area Harvested (ha) [60] | Genome Size/Ploidy x/ No. of Chromosomes | AQP | PIP | TIP | NIP | SIP | References |
|---|---|---|---|---|---|---|---|---|
| Bread wheat (Triticum aestivum L.) | 215,901,958 | ~17,000 Mb 2n = 6x = 42 AABBDD hexaploid | 113 (65-A, 42-B, 36-D) | 51 | 29 | 29 | 4 | [61] |
| Maize (Zea mays L.) | 197,204,250 | 2400 Mb 2n = 2x = 20 | 41 | 12 | 18 | 8 | 3 | [62] |
| Rice Japonica (Oryza sativa) | 162,055,938 | 500 Mb 2n = 2x = 24 | 33 | 11 | 10 | 10 | 2 | [63] |
| Barley (Hordeum vulgare L.) | 51,149,869 | ~5300 Mb 2n = 2x = 14 | 39 | 18 | 11 | 8 | 2 | [64] |
| Sorghum (Sorghum bicolor (L.) Moench) | 40,074,667 | ~730 Mb 2n = 2x = 20 | 37 | 13 | 11 | 11 | 2 | [65] |
| Foxtail millet (Setaria italica) | 31,653,878 ^ | ~490 Mb 2n = 2x = 18 | 39 | 12 | 11 | 13 | 3 | [66] |
| Pearl millet (Pennisetum glaucum (L.) R Br.) | 31,653,878 ^ | ~1790 Mb 2n = 2x = 14 | 33 | 10 | 9 | 11 | 3 | [67] |
| Abiotic Stress | Cereal Species | Stage of Growth | Treatment | Method | Analysed AQP Genes | Effect on the Expression Level/Tissue | References | |
|---|---|---|---|---|---|---|---|---|
| Increased | Decreased | |||||||
| Cold | Rice (Oryza sativa L. spp. japonica) | 16-week-old seedlings | 4 °C treatment, 96 h | RT-PCR ** | 8 PIPs 2 TIPs | Roots: OsPIP1;1, OsPIP1;2, OsPIP2;1, OsPIP2;2, OsPIP2;3, OsPIP2;4, OsPIP2;5, OsPIP2;6, OsTIP1;1 OsTIP2;2 | [63] | |
| Rice (Oryza sativa L. spp. japonica) | 16–20-day-old seedlings | Root chilling: culture solution set in a water bath maintained at °C; whole-plant chilling: plants were placed in a 10 °C set growth chamber, 5 days ^ | qRT-PCR * | 9 PIPs 3 TIPs | Roots chilled: OsPIP1;3 OsPIP2;4 OsPIP2;5 | Roots chilled: OsPIP2;6 OsTIP1;1 OsTIP2;2 Whole plant chillled: all analysed | [79] | |
| Drought/dehydratation | Barley (Hordeum vulgare L.) | 24-day-old seedlings | ten days of drought in soil under a volumetric water content of 1.5% | qRT-PCR * | 8 TIPs | Leaves: HvTIP3;1 HvTIP4;1 | Leaves: HvTIP1;1 HvTIP1;2 HvTIP2;1 HvTIP2;2 HvTIP2;3 | [80] |
| Foxtail millet (Setaria italica L.), tolerant cultivar | 21-day-old seedlings | Cultivated with 20% PEG 6000, 24 h ^ | qRT-PCR * | 2 PIPs 1 TIP 1 NIP 1 SIP | Roots: SiPIP3;1 SiSIP1;1 SiTIP2;2 | Roots: SiPIP1;2 SiNIP1;2 | [66] | |
| Rice (Oryza sativa L. spp. japonica) | two-week-old seedlings | Cultivated with 10% PEG 6000, 24 h ^ | qRT-PCR * | 10 PIPs | Roots: OsPIP1-1 OsPIP1-2 OsPIP1-3 OsPIP2-4 OsPIP2-5 OsPIP2-7 | Leaves: OsPIP2-1 OsPIP2-4 OsPIP2-5 OsPIP2-6 | [81] | |
| Rice (Oryza sativa L. spp. japonica) | four-week-old seedlings | Cultivated hydroponically with 15% PEG 6000, 10 h ^ | qRT-PCR * | 7 TIPs | Root: OsTIP1;1 OsTIP1;2 OsTIP4;1 OsTIP4;2 | Shoot: OsTIP1;1 OsTIP2;2 OsTIP4;1 OsTIP4;2 | [75] | |
| Rice (Oryza sativa L.), 6 rice varieties | 29-day-old seedlings | Drought in soil | qRT-PCR * in Azucena variety (japonica group) ^ | 10 PIPs | Roots: All analysed | [82] | ||
| Wheat (Triticum aestivum L.) | one-week-old seedlings | cultivated with 20% PEG-6000, 6 h ^ | Microarray data obtained from WheatExp database | 113 AQPs | 17 PIPs 12 TIPs Examples *: TaTIP1-2 TaTIP1-4 TaTIP2-3 TaTIP2-4 TaTIP3-3 TaTIP3-4 TaTIP3-6 TaTIP4-1 TaTIP4-4 TaTIP4-5 | 16 PIPs 3 SIPs Examples *: TaNIP1-3 TaNIP1-4 TaNIP1-6 TaNIP2-1C1 TaTIP2-2 TaTIP4-1C1 TaPIP2-1 TaPIP2-3 TaPIP2-4C1 TaPIP2-6 TaPIP2-10 TaPIP2-12 TaPIP2-14 TaPIP2-19 | [61] | |
| Wheat (Triticum aestivum L.), drought-tolerant genotype compared to the control conditions | Anthesis stage (Z61) —grain filling | Drought stress—without irrigation | qRT-PCR * | 8 PIPs 4 TIPs 2 NIPs | Leaves: TaPIP1-1 TaPIP1-5 TaPIP2-24 Roots: TaPIP1-1 TaPIP1-5 TaPIP2-2C1 TaTIP1-2 TaTIP2-4 TaTIP3-4 TaNIP4-3 TaLEA | Leaves: TaNIP4-2 Roots: TaPIP1-6 TaPIP2-7 TaPIP2-24 TaNIP4-1 | [61] | |
| Heat | Foxtail millet (Setaria italica L.), tolerant cultivar | 21-day-old seedlings | 45 °C treatment, 24 h | qRT-PCR * | 2 PIPs 1 TIP 1 NIP 1 SIP | Roots: SiPIP1;2 SiPIP3;1 SiSIP1;1 SiTIP2;2 SiNIP1;2 | [66] | |
| Salinity | Barley (Hordeum vulgare L.) | four-day-old seedlings | cultivated with 200 mM NaCl, 48 h | RT-PCR ** | 3 PIPs | Shoots: HvPIP2;1 | Roots: HvPIP2;1 | [83] |
| Barley (Hordeum vulgare L.) | four-day-old seedlings | cultivated with 200 mM NaCl, 24 h | qRT-PCR * | 10 PIPs | Roots: HvPIP1;2, HvPIP1;3, HvPIP1;4, HvPIP2;1, HvPIP2;2 HvPIP2;3 | [84] | ||
| Foxtail millet (Setaria italica L.), tolerant cultivar | 21-day-old seedlings | cultivated with 200 mM NaCl, 24 h | qRT-PCR * | 2 PIPs 1 TIP 1 NIP 1 SIP | Roots: SiPIP1;2 SiPIP3;1 SiSIP1;1 SiTIP2;2 | Roots: SiNIP1;2 | [66] | |
| Rice (Oryza sativa) | two-week-old seedlings | cultivated with 250 mM NaCl, 24 h ^ | qRT-PCR * | 10 PIPs | Leaves: OsPIP1-2 Roots: OsPIP1-1 OsPIP1-2 OsPIP2-4 | Leaves: OsPIP1-1 OsPIP2-1 OsPIP2-3 OsPIP2-4 OsPIP2-6 Roots: OsPIP1-3 OsPIP2-2 OsPIP2-3 OsPIP2-5 | [81] | |
| Rice (Oryza sativa L. spp. japonica) | four-week-old seedlings | cultivated hydroponically with 150 mM NaCl, 10 h ^ | qRT-PCR * | 7 TIPs | Roots: OsTIP1;1 OsTIP1;2 OsTIP4;1 OsTIP4;2 | Shoots: OsTIP1;2 OsTIP2;2 OsTIP4;2 OsTIP4;3 Roots: OsTIP2;2 OsTIP4;3 | [75] | |
| Species | AQP Gene | Method/Expression in Species | Promoter | Improved Tolerance to Abiotic Stress/or Other Traits | Phenotype | References |
|---|---|---|---|---|---|---|
| Hordeum vulgare L. | HvPIP2;1 | OX Oryza sativa | CaMV35S | No | In control conditions, there was an increased radial hydraulic conductivity of roots (Lpr) of up to 140% and the mass ratio of the shoot to root of up to 150%. Under salt stress of 100 mM NaCl, reduction in growth was greater than in non-transgenic plants | [109] |
| HvPIP2;5 | OX Arabidopsis thaliana | CaMV35S | Yes | Better stress tolerance during germination and root growth under high salt and high osmotic stresses. Able to survive and recover after a three-week drought | [120] | |
| Oryza sativa L. | RWC3 (PIP1 group) | OX Oryza sativa, lowland | SWPA2 | Yes | Better water status under a water deficit. Increased root osmotic hydraulic conductivity (Lp), leaf water potential and relative cumulative transpiration at the end of ten-h treatment with 20% PEG 6000 | [111] |
| OsPIP1;1 | OX Oryza sativa | CaMV35S | Yes | Increased seed yield, salt resistance, root hydraulic conductivity and seed germination rate | [115] | |
| OsPIP1;1 OsPIP2;2 | OX Arabidopsis thaliana | CaMV35S | Yes | Improved tolerance to salt (100 mM of NaCl) and drought (200 mM of mannitol), but not to salt treatment at a higher concentration (150 mM of NaCl) | [81] | |
| OSPIP1;2 | OX Oryza sativa | CaMV35S | Yes | Improved growth and grain yield by facilitating leaf CO2 diffusion, which increases both the net CO2 assimilation rate and sucrose transport. | [78] | |
| OsNIP1;1, OsNIP3;3 | OX KO Oryza sativa | OsLsi1 | Yes | Knockout of either gene had little effect on arsenite uptake or translocation. Overexpression did not affect arsenite uptake but decreased the root-to-shoot translocation of arsenite and shoot arsenic concentration. When grown in arsenic-contaminated paddy soils, there was a significantly lower arsenic concentration in the rice grains | [121] | |
| Triticum aestivum/ turgidum L. | TaAQP7 (PIP2 group) | OX Nicotiana tabacum | CaMV35S | Yes | Increased drought tolerance. Lower levels of malondialdehyde (MDA) and H2O2 and less ion leakage (IL), but a higher relative water content (RWC) and superoxide dismutase (SOD) and catalase (CAT) activities | [117] |
| TaAQP8 (PIP1 group) | OX Nicotiana tabacum | CaMV35S | Yes | Increased root elongation compared to the controls under salt stress. Retaining a high K+/Na+ ratio and Ca2+ content, but also lowering the H2O2 accumulation and membrane damage by improving the antioxidant system | [116] | |
| TdPIP2;1 | OX Triticum durum | PrCaMV35S | Yes | Improved germination rates and biomass production and retained low Na+ and high K+ concentrations in the shoots under high salt and osmotic stress conditions. A long-term study under greenhouse conditions on salt or drought stress produced good quality grains | [118] | |
| Zea mays L. | ZmPIP2;5 | OX KO Zea mays | CaMV35S | Yes | Whole-root conductivity decreased in the KO lines; no difference was observed in the OX plants. At the leaf level, the hydraulic conductance was higher in the PIP2;5 OE lines whereas there was no difference in the pip2;5 KO lines. Leaf elongation rate was faster in the PIP2;5 OE plants after mild drought stress | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowska, M.M. Aquaporins in Cereals—Important Players in Maintaining Cell Homeostasis under Abiotic Stress. Genes 2021, 12, 477. https://doi.org/10.3390/genes12040477
Kurowska MM. Aquaporins in Cereals—Important Players in Maintaining Cell Homeostasis under Abiotic Stress. Genes. 2021; 12(4):477. https://doi.org/10.3390/genes12040477
Chicago/Turabian StyleKurowska, Marzena Małgorzata. 2021. "Aquaporins in Cereals—Important Players in Maintaining Cell Homeostasis under Abiotic Stress" Genes 12, no. 4: 477. https://doi.org/10.3390/genes12040477
APA StyleKurowska, M. M. (2021). Aquaporins in Cereals—Important Players in Maintaining Cell Homeostasis under Abiotic Stress. Genes, 12(4), 477. https://doi.org/10.3390/genes12040477






