Transcriptome Analysis of Neuroendocrine Regulation of Ovine Hypothalamus-Pituitary-Ovary Axis during Ovine Anestrus and the Breeding Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals, Tissue Collection and Hormone Determination
2.2. RNA Extraction, Library Preparation and RNA Sequencing
2.3. Quality Control and Bioinformatics Analysis of Sequenced RNAs
2.4. Gene Ontology and KEGG Pathway Analysis of Differentially Expressed Genes
2.5. Integral Protein-Protein Networks Analysis
2.6. Statistical Analyses
3. Results
3.1. Summary of RNA Sequencing Data
3.2. Identification of DE mRNAs by RNA Sequencing
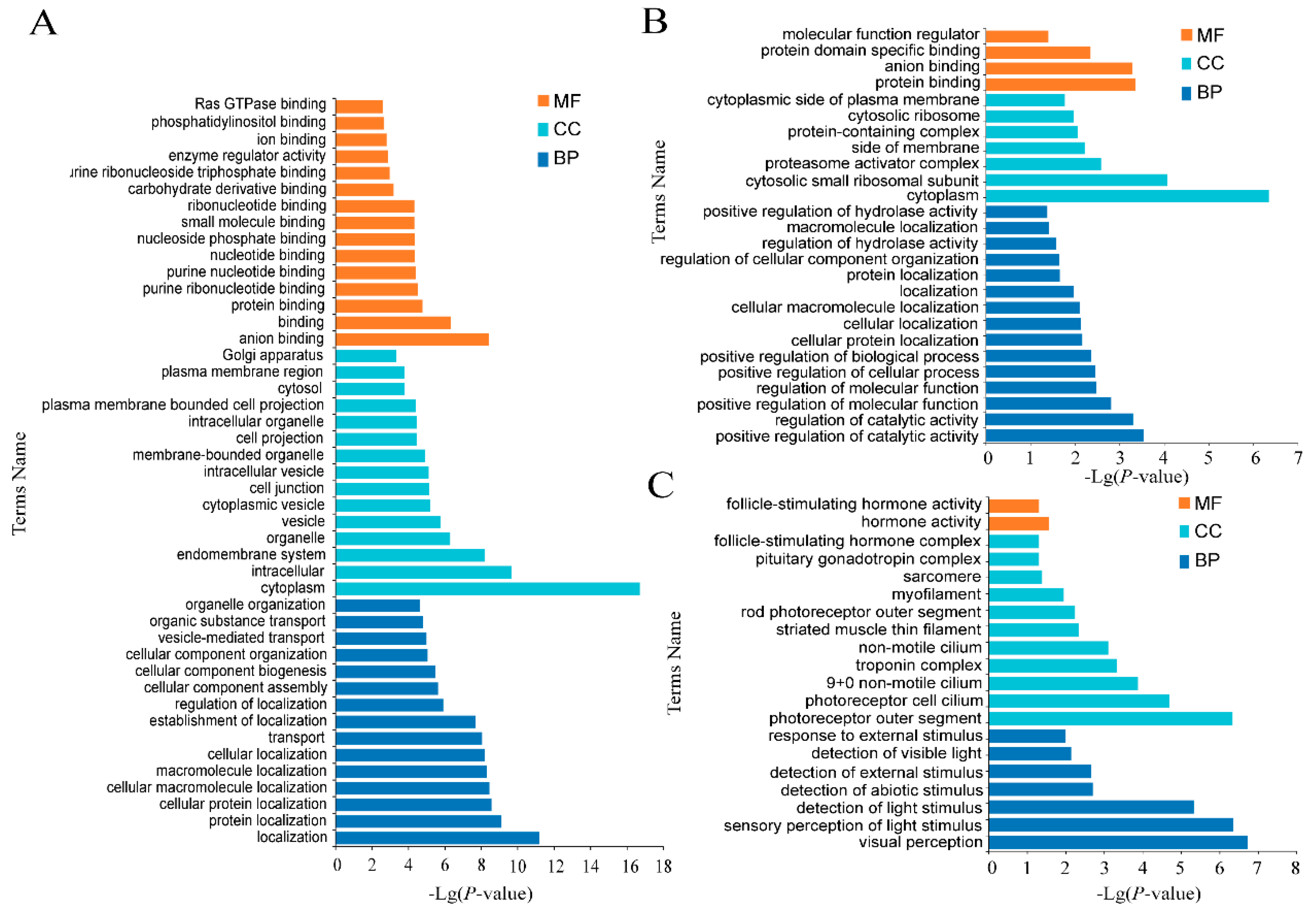
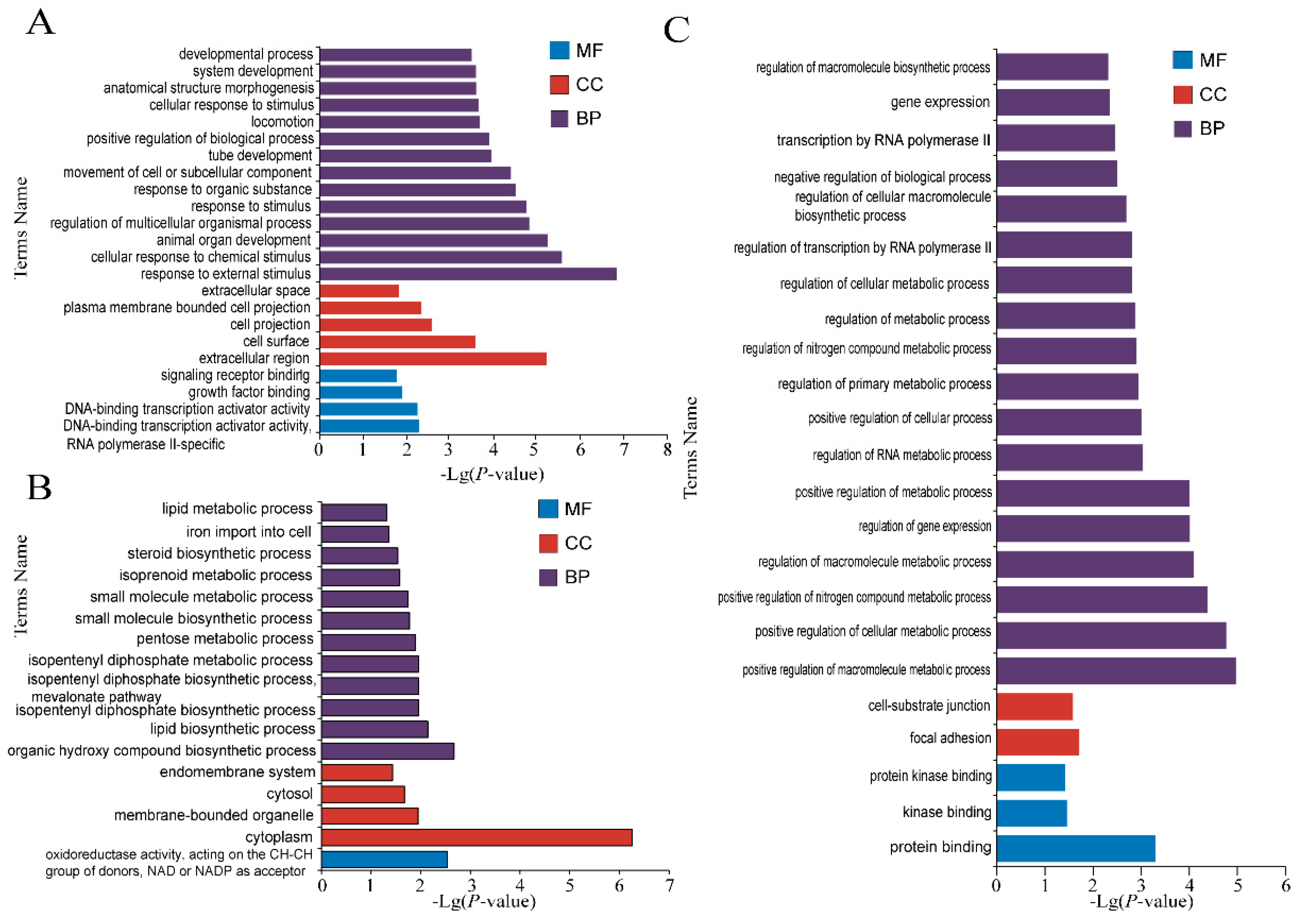
3.3. Enrichment Analysis of DE mRNAs
3.3.1. Enrichment Analysis of DE mRNAs of Three Tissues at Different Period
3.3.2. GO Enrichment Analysis of Specific mRNAs in HSE in Small Tail Han Sheep
3.4. Protein-Protein Interaction (PPI) Network Construction and Analysis
4. Discussion
4.1. Pathway Enrichment Analysis
4.2. Candidate Genes for Seasonality in Sheep
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RNA-seq | RNA sequencing |
| SD | short-day |
| LD | long-day |
| HPG | hypothalamus-pituitary-gonad |
| GnRH | gonadotropin releasing hormone |
| LH | luteinizing hormone |
| FSH | follicle stimulating hormone |
| STH | Small Tail Han sheep |
| TSA | Tan ewes in spring at anestrous stages |
| TAL | Tan ewes in autumn at luteal phase |
| TAP | Tan ewes in autumn at proestrus stage |
| TAE | Tan ewes in autumn at estrus stage |
| HSL | STH ewes in spring at luteal phase |
| HSP | STH ewes in spring at proestrus |
| HSE | STH ewes in spring at estrus stage |
| HAE | STH ewes in autumn at estrus stage |
| GO | Gene Ontology |
| DE mRNAs | Differentially Expressed mRNAs |
| Co-DE mRNAs | Co-Differentially Expressed mRNAs |
| PPI | protein-protein interaction |
| BP | biological process |
| CC | Cellular component |
| MF | Molecular function |
| CDC20 | cell division cycle 20 |
| TOP2A | DNA topoisomerase II α |
| EEF2 | eukaryotic translation elongation factor 2 |
| DLGAP5 | DLG associated protein 5 |
| CCNB2 | cyclin B2 |
| RPL23 | ribosomal protein L23 |
| HPO | hypothalamus-pituitary-ovarian |
| HPGA | hypothalamic-pituitary-gonadal axis |
| ODC1 | ornithine decarboxylase 1 |
| PRLH | prolactin releasing hormone |
| CRYBB2 | crystallin β B2 |
| RPS6 | ribosomal protein S6 |
| SMAD5 | SMAD family member 5 |
| FBXL7 | F-box and leucine rich repeat protein 7 |
| TPH1 | tryptophan hydroxylase 1 |
| MITF | melanocyte inducing transcription factor |
| DHCR24 | 24-dehydrocholesterol reductase |
| MPA | medial preoptic area |
| BMP | bone Morphogenetic protein |
| MLT | melatonin |
References
- Wayne, N.L.; Malpaux, B.; Karsch, F.J. Social cues can play a role in timing onset of the breeding season of the ewe. J. Reprod. Fertil. 1989, 87, 707–713. [Google Scholar] [CrossRef]
- Ebling, F.J. Photoperiodic regulation of puberty in seasonal species. Mol. Cell. Endocrinol. 2010, 324, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Guillaume, D.; Migaud, M.; Thiéry, J.C.; Pellicer-Rubio, M.T.; Malpaux, B. Seasonality of reproduction in mammals: Intimate regulatory mechanisms and practical implications. Reprod. Domest. Anim. Zuchthyg. 2008, 43 (Suppl. S2), 40–47. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Luo, Q.; Zhao, H.; Qin, X. Ovarian proteomic study reveals the possible molecular mechanism for hyperprolificacy of Small Tail Han sheep. Sci. Rep. 2016, 6, 27606. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, A.; Shimmura, T.; Nishiwaki-Ohkawa, T.; Yoshimura, T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front. Endocrinol. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Q.; Li, X.; Guo, X.; Wang, X.; Hu, W.; Di, R.; Chu, M. Molecular cloning and epigenetic change detection of Kiss1 during seasonal reproduction in Chinese indigenous sheep. Reprod. Fertil. Dev. 2018, 30, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B. The discovery, proof and reproof of neurosecretion (Speidel, 1917; Scharrer and Scharrer, 1934). Can. J. Neurol. Sci. 1983, 10, 208–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, G.W. Neural control of the pituitary gland. Physiol. Rev. 1948, 28, 139–179. [Google Scholar] [CrossRef]
- Maeda, K.; Ohkura, S.; Uenoyama, Y.; Wakabayashi, Y.; Oka, Y.; Tsukamura, H.; Okamura, H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010, 1364, 103–115. [Google Scholar] [CrossRef]
- Bittman, E.L.; Kaynard, A.H.; Olster, D.H.; Robinson, J.E.; Yellon, S.M.; Karsch, F.J. Pineal melatonin mediates photoperiodic control of pulsatile luteinizing hormone secretion in the ewe. Neuroendocrinology 1985, 40, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 1993, 133, 1624–1632. [Google Scholar] [CrossRef]
- Di, R.; He, J.; Song, S.; Tian, D.; Liu, Q.; Liang, X.; Ma, Q.; Sun, M.; Wang, J.; Zhao, W.; et al. Characterization and comparative profiling of ovarian microRNAs during ovine anestrus and the breeding season. BMC Genom. 2014, 15, 899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Song, F.H.; Zhu, J.W.; Zhang, S.S.; Yang, Y.D.; Chen, T.T.; Tang, B.X.; Dong, L.L.; Ding, N.; Zhang, Q.; et al. GSA: Genome sequence archive. Genom. Proteom. Bioinform. 2017, 15, 14–18. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, W.M.; Xiao, J.F.; Bao, Y.M.; He, S.M.; Zhang, G.Q.; Li, Y.X.; Zhao, G.P.; Chen, R.S.; Gao, Y.; et al. Database resources of the National Genomics Data Center in 2020. Nucleic Acids Res. 2020, 48, D24–D33. [Google Scholar]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Lewis, P.D. A review of lighting for broiler breeders. Br. Poult. Sci. 2006, 47, 393–404. [Google Scholar] [CrossRef]
- Thimonier, J. Control of seasonal reproduction in sheep and goats by light and hormones. J. Reprod. Fertil. Suppl. 1981, 30, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Burbridge, S.; Stewart, I.; Placzek, M. Development of the Neuroendocrine Hypothalamus. Compr. Physiol. 2016, 6, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Wei, J.; Ni, W.; Xu, Y.; Yao, R.; Zhang, M.; Li, H.; Liu, L.; Dang, H.; et al. Comprehensive Expression Profiling Analysis of Pituitary Indicates that circRNA Participates in the Regulation of Sheep Estrus. Genes 2019, 10, 90. [Google Scholar] [CrossRef]
- Richards, J.S. Hormonal control of gene expression in the ovary. Endocr. Rev. 1994, 15, 725–751. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Fitzpatrick, S.L.; Clemens, J.W.; Morris, J.K.; Alliston, T.; Sirois, J. Ovarian cell differentiation: A cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog. Horm. Res. 1995, 50, 223–254. [Google Scholar] [CrossRef]
- Anderson, L.L.; Jeftinija, S.; Scanes, C.G. Growth hormone secretion: Molecular and cellular mechanisms and in vivo approaches. Exp. Biol. Med. 2004, 229, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rekha, R.S.; Ahsan, K.B.; Doi, M.; Grandér, M.; Roy, A.K.; Ekström, E.C.; Wagatsuma, Y.; Vahter, M.; Raqib, R. Arsenic exposure affects plasma insulin-like growth factor 1 (IGF-1) in children in rural Bangladesh. PLoS ONE 2013, 8, e81530. [Google Scholar] [CrossRef]
- Oakley, O.R.; Frazer, M.L.; Ko, C. Pituitary-ovary-spleen axis in ovulation. Trends Endocrinol. Metab. 2011, 22, 345–352. [Google Scholar] [CrossRef]
- Bliss, S.P.; Navratil, A.M.; Xie, J.; Roberson, M.S. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocrinol. 2010, 31, 322–340. [Google Scholar] [CrossRef]
- De la Iglesia, H.O.; Schwartz, W.J. Minireview: Timely ovulation: Circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 2006, 147, 1148–1153. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, F.; Zhang, H.; Xu, C.; Wu, L.; Xia, C. Follicular fluid metabolite changes in dairy cows with inactive ovary identified using untargeted metabolomics. BioMed Res. Int. 2020, 2020, 9837543. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H. Melatonin-dependent timing of seasonal reproduction by the pars tuberalis: Pivotal roles for long daylengths and thyroid hormones. J. Neuroendocrinol. 2012, 24, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Hazlerigg, D.G.; Ebling, F.J. Thyroid Hormone and Seasonal Rhythmicity. Front. Endocrinol. 2014, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Lomet, D.; Robert, V.; Decourt, C.; Beltramo, M.; Pellicer-Rubio, M.T. Seasonal breeding in mammals: From basic science to applications and back. Theriogenology 2016, 86, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.R.D.; Jain, S.; Banerjee, A. Expression of ODC1, SPD, SPM and AZIN1 in the hypothalamus, ovary and uterus during rat estrous cycle. Gen. Comp. Endocrinol. 2017, 246, 9–22. [Google Scholar] [CrossRef]
- Maruyama, M.; Matsumoto, H.; Fujiwara, K.; Kitada, C.; Hinuma, S.; Onda, H.; Fujino, M.; Inoue, K. Immunocytochemical localization of prolactin-releasing peptide in the rat brain. Endocrinology 1999, 140, 2326–2333. [Google Scholar] [CrossRef][Green Version]
- Gao, Q.; Sun, L.L.; Xiang, F.F.; Gao, L.; Jia, Y.; Zhang, J.R.; Tao, H.B.; Zhang, J.J.; Li, W.J. Crybb2 deficiency impairs fertility in female mice. Biochem. Biophys. Res. Commun. 2014, 453, 37–42. [Google Scholar] [CrossRef]
- Kim, J.; Song, G.; Wu, G.; Gao, H.; Johnson, G.A.; Bazer, F.W. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol. Reprod. 2013, 88, 113. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Q.W.; Feng, J.G.; Xu, M.L.; Huang, R.H.; Shi, F.X. Expression of bone morphogenetic protein 2, 4, and related components of the BMP signaling pathway in the mouse uterus during the estrous cycle. J. Zhejiang Univ. Sci. B 2014, 15, 601–610. [Google Scholar] [CrossRef]
- Buhr, E.D.; Yue, W.W.; Ren, X.; Jiang, Z.; Liao, H.W.; Mei, X.; Vemaraju, S.; Nguyen, M.T.; Reed, R.R.; Lang, R.A.; et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. USA 2015, 112, 13093–13098. [Google Scholar] [CrossRef]
- Forde, N.; Duffy, G.B.; McGettigan, P.A.; Browne, J.A.; Mehta, J.P.; Kelly, A.K.; Mansouri-Attia, N.; Sandra, O.; Loftus, B.J.; Crowe, M.A.; et al. Evidence for an early endometrial response to pregnancy in cattle: Both dependent upon and independent of interferon tau. Physiol. Genom. 2012, 44, 799–810. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Liu, G.; Wassie, T.; Girmay, S. An ancient mutation in the TPH1 gene is consistent with the changes in mammalian reproductive rhythm. Int. J. Mol. Sci. 2019, 20, 6065. [Google Scholar] [CrossRef]
- Watanabe, H.; Tatsumi, K.; Yokoi, H.; Higuchi, T.; Iwai, M.; Fukuoka, M.; Fujiwara, H.; Fujita, K.; Nakayama, H.; Mori, T.; et al. Ovulation defect and its restoration by bone marrow transplantation in osteopetrotic mutant mice of Mitf(mi)/Mitf(mi) genotype. Biol. Reprod. 1997, 57, 1394–1400. [Google Scholar] [CrossRef]
- Agca, C.; Yakan, A.; Agca, Y. Estrus synchronization and ovarian hyper-stimulation treatments have negligible effects on cumulus oocyte complex gene expression whereas induction of ovulation causes major expression changes. Mol. Reprod. Dev. 2013, 80, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Lefèvre, P.L.; Palin, M.F.; Chen, G.; Turecki, G.; Murphy, B.D. Polyamines are implicated in the emergence of the embryo from obligate diapause. Endocrinology 2011, 152, 1627–1639. [Google Scholar] [CrossRef]
- McDougall, S.; Compton, C.W.; Anniss, F.M. Effect of exogenous progesterone and oestradiol on plasma progesterone concentrations and follicle wave dynamics in anovulatory anoestrous post-partum dairy cattle. Anim. Reprod. Sci. 2004, 84, 303–314. [Google Scholar] [CrossRef]
- Fortune, J.E.; Rivera, G.M.; Yang, M.Y. Follicular development: The role of the follicular microenvironment in selection of the dominant follicle. Anim. Reprod. Sci. 2004, 82–83, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Echternkamp, S.E. Insulin-like growth factor binding proteins in granulosa and thecal cells from bovine ovarian follicles at different stages of development. J. Anim. Sci. 2003, 81, 2826–2839. [Google Scholar] [CrossRef]
- Chen, D.D.; Zhu, S.Q. Whole-exome sequencing identification of a recurrent CRYBB2 variant in a four-generation Chinese family with congenital nuclear cataracts. Exp. Ther. Med. 2021, 22, 1375. [Google Scholar] [CrossRef]
- Wen, X.; He, J.; Zhang, X.; Zhao, L.; Du, S. Localization of Smad4 in the ovary of the European hedgehog (Erinaceus europaeus L.). Acta Histochem. 2011, 113, 382–386. [Google Scholar] [CrossRef]
- Massagué, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef]
- Pangas, S.A. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol. Cell. Endocrinol. 2012, 356, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Guerlotté, J.; Grève, P.; Gréchez-Cassiau, A.; Iuvone, M.P.; Zatz, M.; Chong, N.W.; Klein, D.C.; Voisin, P. Melatonin synthesis pathway: Circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reprod. Nutr. Dev. 1999, 39, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Weems, P.W.; Goodman, R.L.; Lehman, M.N. Neural mechanisms controlling seasonal reproduction: Principles derived from the sheep model and its comparison with hamsters. Front. Neuroendocrinol. 2015, 37, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Jiang, X.P.; Chi, S.X.; Bo, D.D.; Liu, G.Q. Contribution of the mutation T865G in TPH1 gene to the genetic potentiality of housed Mongolian sheep to year-round breeding. Reprod. Domest. Anim. 2021, 56, 1406–1412. [Google Scholar] [CrossRef] [PubMed]










| Luteal Phase | Proestrus | Estrus | ||||||
|---|---|---|---|---|---|---|---|---|
| TAL | HSL | TAP | HSP | TAE | HAE | HSE | ||
| Hypothalamus | Anestrus (TSA) | 1068 | 181 | 607 | 87 | 331 | 102 | 193 |
| Co-DE mRNAs | 21 | 15 | 0 | |||||
| Pituitary | Anestrus (TSA) | 374 | 205 | 640 | 100 | 556 | 170 | 285 |
| Co-DE mRNAs | 51 | 28 | 10 | |||||
| Ovary | Anestrus (TSA) | 676 | 884 | 385 | 606 | 526 | 772 | 307 |
| Co-DE mRNAs | 315 | 93 | 52 | |||||
| Tissue | Source | Term Name | p-Value |
|---|---|---|---|
| Hypothalamus estrus | KEGG | Cardiac muscle contraction | 0.0003 |
| Ribosome | 0.0094 | ||
| cAMP signaling pathway | 0.0144 | ||
| Phototransduction | 0.0266 | ||
| Pituitary luteal phase | Axon guidance | 0.0063 | |
| Ribosome | 0.0279 | ||
| Ovary Proestrus | Valine, leucine and isoleucine biosynthesis | 0.0043 | |
| Ovary estrus | Regulation of actin cytoskeleton | 0.0070 | |
| Focal adhesion | 0.0469 | ||
| Ovary Luteal phase | Biosynthesis of unsaturated fatty acids | 0.0455 |
| Proteins | Degree | Closeness | Betweenness | Class | Proteins | Degree | Closeness | Betweenness | Class |
|---|---|---|---|---|---|---|---|---|---|
| RPS18 | 45 | 0.80000000 | 0.26577395 | Hypothalamus Pituitary Ovary | RPLP2 | 21 | 0.60869565 | 0.00530952 | Hypothalamus Pituitary Ovary |
| RPS15 | 30 | 0.63636364 | 0.01884148 | Hypothalamus Pituitary Ovary | RPL23A | 21 | 0.59574468 | 0.00551146 | Hypothalamus Pituitary Ovary |
| RPS23 | 29 | 0.70000000 | 0.07679841 | Hypothalamus Ovary | RPL23 | 21 | 0.53846154 | 0.00659906 | Hypothalamus Pituitary Ovary |
| RPS26 | 26 | 0.62222222 | 0.01139418 | Hypothalamus Pituitary Ovary | RPS27A | 19 | 0.53846154 | 0.00659906 | Hypothalamus Pituitary Ovary |
| RPS27L | 25 | 0.60869565 | 0.00802717 | Hypothalamus Pituitary Ovary | RPS8 | 15 | 0.58333333 | 0.00052910 | Hypothalamus |
| RPS25 | 25 | 0.68292683 | 0.05605774 | Pituitary Ovary | CDC20 | 12 | 0.66666667 | 0.13099227 | Hypothalamus |
| RPS21 | 24 | 0.66666667 | 0.03142804 | Hypothalamus Ovary | TOP2A | 11 | 0.73076923 | 0.20673907 | Hypothalamus |
| OVUBQ-L40 | 23 | 0.65116279 | 0.08473492 | Pituitary Ovary | EEF2 | 11 | 0.67857143 | 0.09162490 | Hypothalamus |
| RPS3A | 21 | 0.58333333 | 0.00893936 | Hypothalamus Pituitary Ovary | DLGAP5 | 10 | 0.60869565 | 0.07270322 | Hypothalamus |
| RPS10 | 21 | 0.80000000 | 0.26577395 | Pituitary Ovary | CCNB2 | 10 | 0.65517241 | 0.14796018 | Hypothalamus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Di, R.; Yang, Y.; Liu, Q.; Chu, M. Transcriptome Analysis of Neuroendocrine Regulation of Ovine Hypothalamus-Pituitary-Ovary Axis during Ovine Anestrus and the Breeding Season. Genes 2021, 12, 1861. https://doi.org/10.3390/genes12121861
Zhong Y, Di R, Yang Y, Liu Q, Chu M. Transcriptome Analysis of Neuroendocrine Regulation of Ovine Hypothalamus-Pituitary-Ovary Axis during Ovine Anestrus and the Breeding Season. Genes. 2021; 12(12):1861. https://doi.org/10.3390/genes12121861
Chicago/Turabian StyleZhong, Yingjie, Ran Di, Yang Yang, Qiuyue Liu, and Mingxing Chu. 2021. "Transcriptome Analysis of Neuroendocrine Regulation of Ovine Hypothalamus-Pituitary-Ovary Axis during Ovine Anestrus and the Breeding Season" Genes 12, no. 12: 1861. https://doi.org/10.3390/genes12121861
APA StyleZhong, Y., Di, R., Yang, Y., Liu, Q., & Chu, M. (2021). Transcriptome Analysis of Neuroendocrine Regulation of Ovine Hypothalamus-Pituitary-Ovary Axis during Ovine Anestrus and the Breeding Season. Genes, 12(12), 1861. https://doi.org/10.3390/genes12121861








