MicroRNA Analysis of Human Stroke Brain Tissue Resected during Decompressive Craniectomy/Stroke-Ectomy Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Sample Collection
2.2. Human Brain Tissue Processing
2.3. Histological Staining
2.4. Immunohistochemistry and Fluorescence Microscopy
2.5. Next Generation Sequencing (NGS) Analysis
2.6. Statistical Analysis
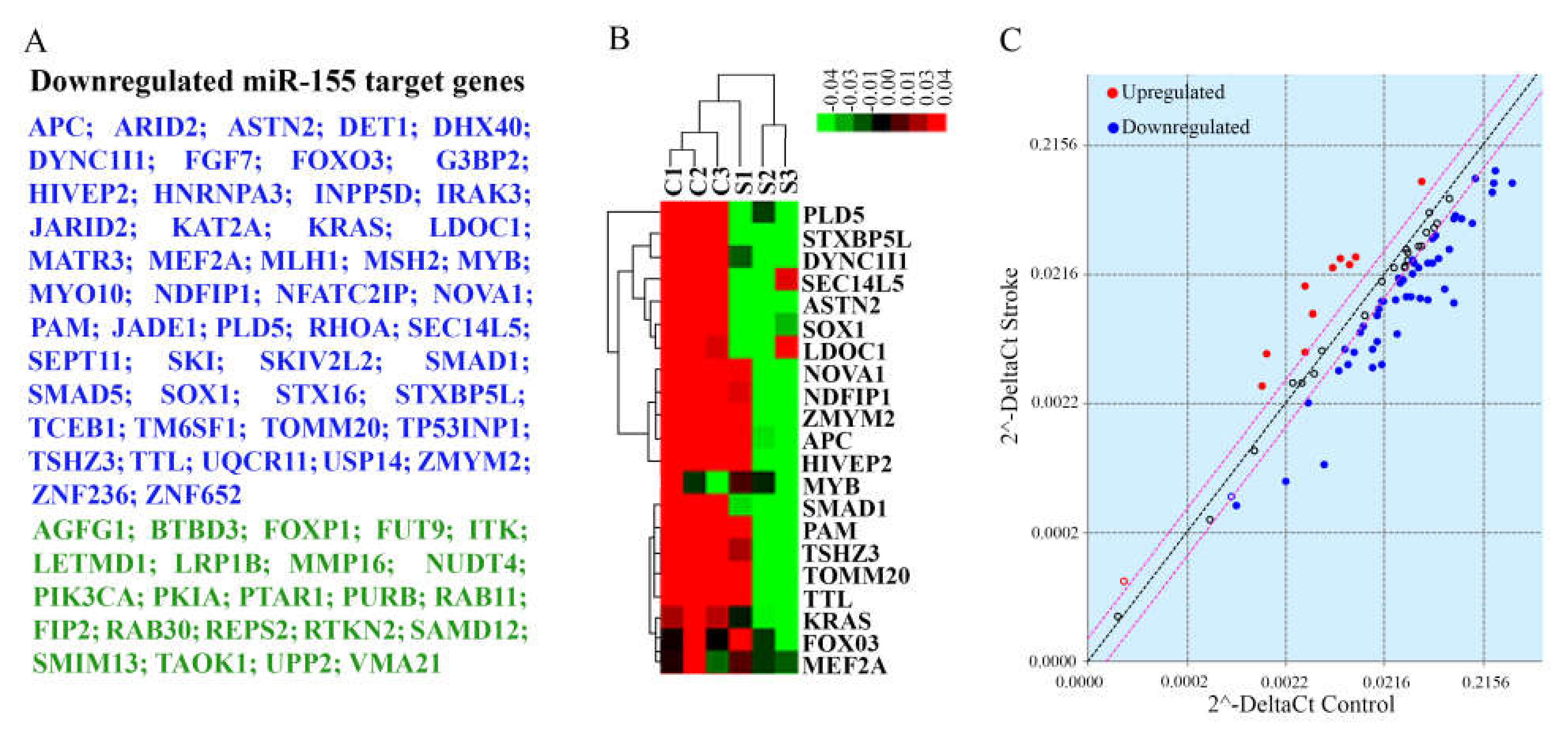
2.7. Evaluation of miR-155 Target Genes
2.8. Bioinformatics Analysis of miRNA Sequencing, Whole Transcriptome RNA-Sequencing, and PCR Array Data
3. Results
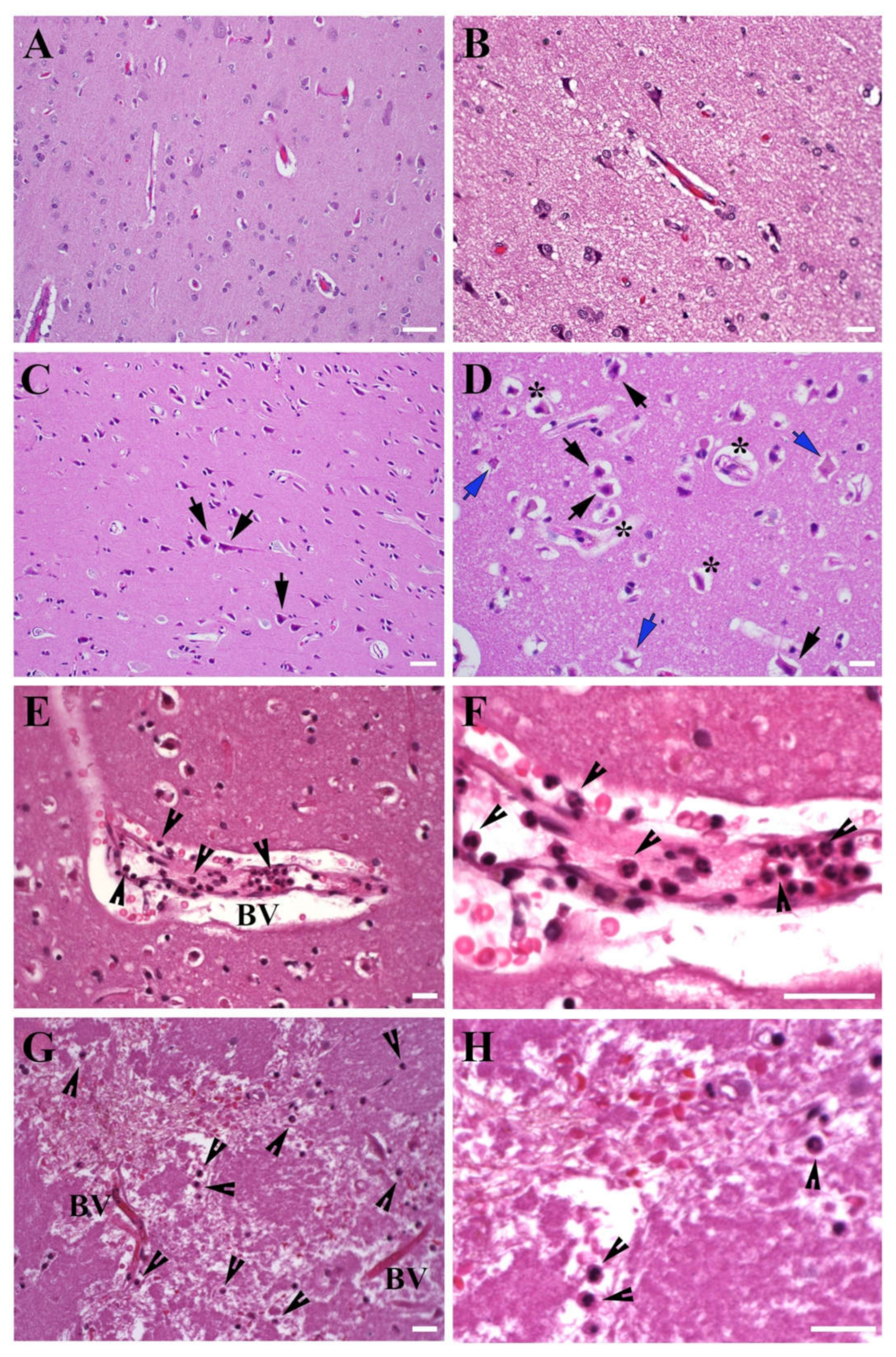
3.1. Morphology of the Brain Tissue Collected from Stroke Patients
3.1.1. Brain Tissue Damage
3.1.2. Leukocyte Infiltration
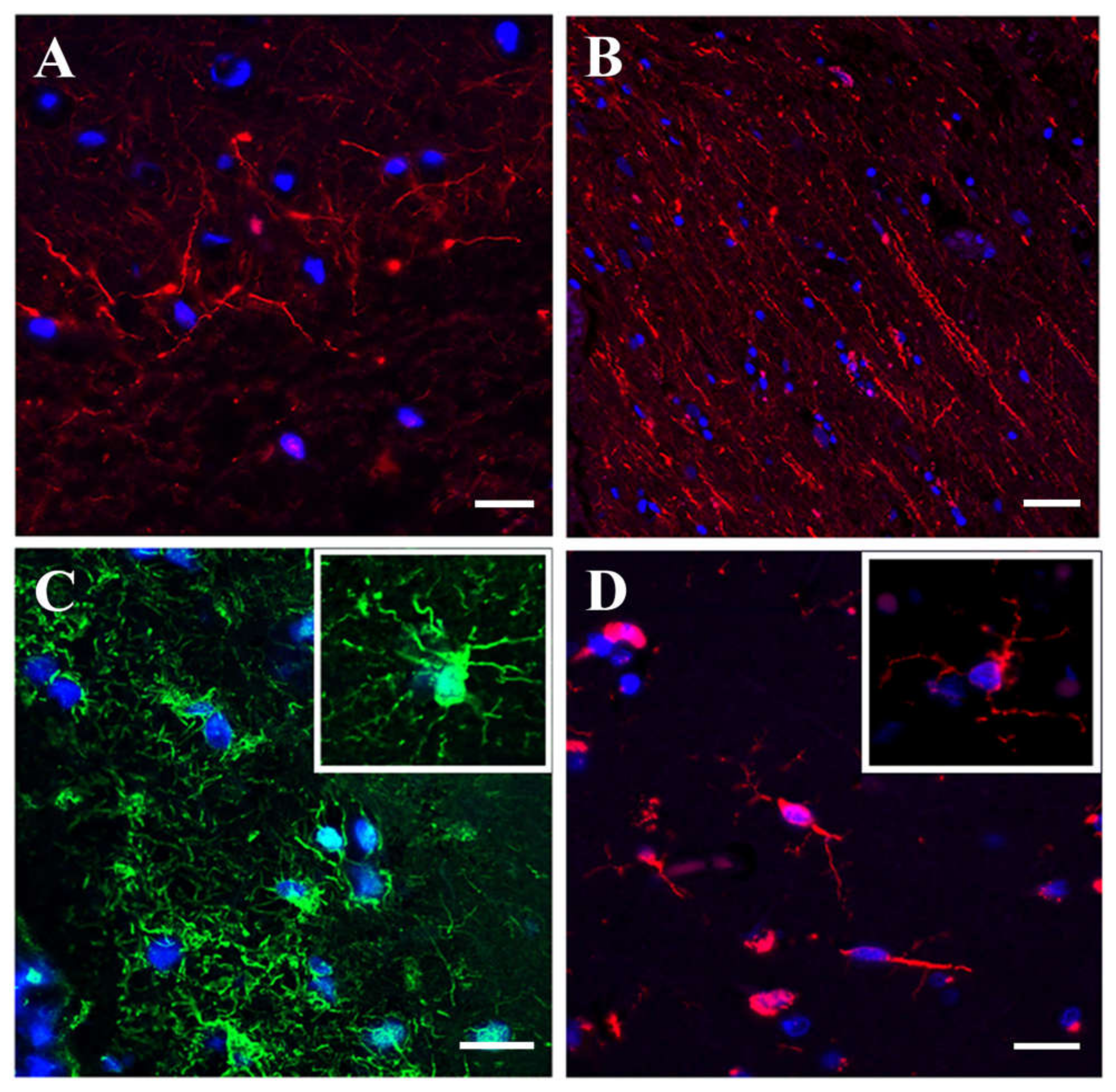
3.1.3. Immunofluorescence Microscopy
3.2. miRNAs Differentially Expressed in Stroke Brain Tissue
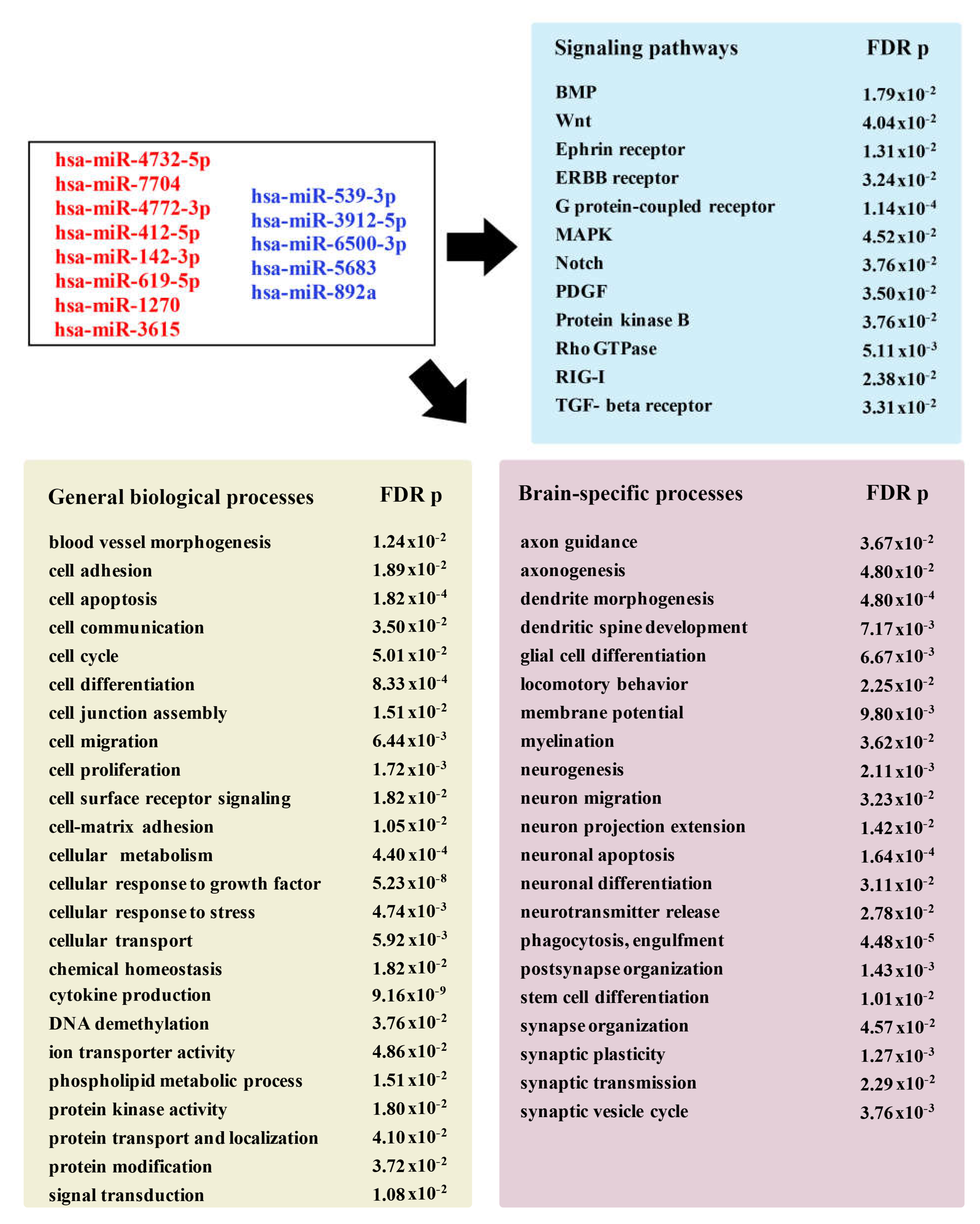
Newly Detected Group of miRNAs Dysregulated in Stroke Brain Tissue
3.3. miR-155 Expression in Stroke Brain Tissue
4. Discussion
4.1. Dysregulation of miRNAs with Known Association with Stroke
4.2. Newly Detected miRNAs Dysregulated in Human Stroke Tissue
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Norrving, B.; George, M.G.; Foltz, J.L.; Roth, G.A.; Mensah, G.A. Prevention of stroke: A strategic global imperative. Nat. Rev. Neurol. 2016, 12, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouns, R.; De Deyn, P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.N.; Ma, H.K.; Arakawa, S.; Howells, D.W.; Markus, R.; Rowe, C.C.; Donnan, G.A. Inflammation following stroke. J. Clin. Neurosci. 2006, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Barreto, G.E.; Xu, L.; Ouyang, Y.B.; Xie, X.; Giffard, R.G. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke 2011, 42, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Dreier, J.P.; Lemale, C.L.; Kola, V.; Friedman, A.; Schoknecht, K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology 2018, 134, 189–207. [Google Scholar] [CrossRef]
- Jickling, G.C.; Xu, H.; Stamova, B.; Ander, B.P.; Zhan, X.; Tian, Y.; Liu, D.; Turner, R.J.; Mesias, M.; Verro, P.; et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann. Neurol. 2010, 68, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Barr, T.L.; Conley, Y.; Ding, J.; Dillman, A.; Warach, S.; Singleton, A.; Matarin, M. Genomic biomarkers and cellular pathways of ischemic stroke by RNA gene expression profiling. Neurology 2010, 75, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Theofilatos, K.; Korfiati, A.; Mavroudi, S.; Cowperthwaite, M.C.; Shpak, M. Discovery of stroke-related blood biomarkers from gene expression network models. BMC Med. Genom. 2019, 12, 118. [Google Scholar] [CrossRef]
- Koutsis, G.; Siasos, G.; Spengos, K. The emerging role of microRNA in stroke. Curr. Top. Med. Chem. 2013, 13, 1573–1588. [Google Scholar] [CrossRef]
- Vijayan, M.; Reddy, P.H. Peripheral biomarkers of stroke: Focus on circulatory microRNAs. Biochim. Biophys. Acta 2016, 1862, 1984–1993. [Google Scholar] [CrossRef]
- Li, G.; Morris-Blanco, K.C.; Lopez, M.S.; Yang, T.; Zhao, H.; Vemuganti, R.; Luo, Y. Impact of microRNAs on ischemic stroke: From pre- to post-disease. Prog. Neurobiol. 2017, 163, 59–78. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Czlonkowska, A.; Postula, M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke—A Comprehensive Review and Bioinformatic Analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef] [Green Version]
- Roitbak, T. MicroRNAs and Regeneration in Animal Models of CNS Disorders. Neurochem. Res. 2020, 45, 188–203. [Google Scholar] [CrossRef]
- Dewdney, B.; Trollope, A.; Moxon, J.; Manapurathe, D.T.; Biros, E.; Golledge, J. Circulating MicroRNAs as Biomarkers for Acute Ischemic Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 522–530. [Google Scholar] [CrossRef]
- Mitsios, N.; Saka, M.; Krupinski, J.; Pennucci, R.; Sanfeliu, C.; Wang, Q.; Rubio, F.; Gaffney, J.; Kumar, P.; Kumar, S.; et al. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC Neurosci. 2007, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Sepramaniam, S.; Tan, J.R.; Tan, K.S.; DeSilva, D.A.; Tavintharan, S.; Woon, F.P.; Wang, C.W.; Yong, F.L.; Karolina, D.S.; Kaur, P.; et al. Circulating MicroRNAs as biomarkers of acute stroke. Int. J. Mol. Sci. 2014, 15, 1418–1432. [Google Scholar] [CrossRef] [Green Version]
- Jickling, G.C.; Ander, B.P.; Zhan, X.; Noblett, D.; Stamova, B.; Liu, D. microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS ONE 2014, 9, e99283. [Google Scholar] [CrossRef]
- Asano, S.; Chantler, P.D.; Barr, T.L. Gene expression profiling in stroke: Relevance of blood–brain interaction. Curr. Opin. Pharmacol. 2016, 26, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Bardutzky, J.; Schwab, S. Antiedema therapy in ischemic stroke. Stroke 2007, 38, 3084–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, W.D. Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions. Cerebrovasc. Dis. 2016, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S.; Juttler, E.; Shapovalov, Y.; Yegin, A.; Landen, J.; Jauch, E.C. Cerebral Edema Associated With Large Hemispheric Infarction. Stroke 2019, 50, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Guanci, M.M. Management of the Patient with Malignant Hemispheric Stroke. Crit. Care Nurs. Clin. N. Am. 2020, 32, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Goedemans, T.; Verbaan, D.; Coert, B.A.; Kerklaan, B.; van den Berg, R.; Coutinho, J.M.; van Middelaar, T.; Nederkoorn, P.J.; Vandertop, W.P.; van den Munckhof, P. Outcome After Decompressive Craniectomy for Middle Cerebral Artery Infarction: Timing of the Intervention. Neurosurgery 2020, 86, E318–E325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Jia, F.M.; Yin, H.X.; Guo, Z.L. Decompressive hemicraniectomy versus medical treatment of malignant middle cerebral artery infarction: A systematic review and meta-analysis. Biosci. Rep. 2020, 40, BSR20191448. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, W.; Gao, Y.H.; Liang, F.-T.; Gao, Y.L.; Yu, H.G.; Huang, Q.L.; Long, X.Q.; Zhou, Y.F. Surgical Decompression versus Conservative Treatment in Patients with Malignant Infarction of the Middle Cerebral Artery: Direct Comparison of Death-Related Complications. World Neurosurg. 2020, 135, e366–e374. [Google Scholar] [CrossRef]
- Kalia, K.K.; Yonas, H. An aggressive approach to massive middle cerebral artery infarction. Arch. Neurol. 1993, 50, 1293–1297. [Google Scholar] [CrossRef]
- Robertson, S.C.; Lennarson, P.; Hasan, D.M.; Traynelis, V.C. Clinical course and surgical management of massive cerebral infarction. Neurosurgery 2004, 55, 55–61; discussion 61–62. [Google Scholar] [CrossRef]
- Gross, A.M.; Kreisberg, J.F.; Ideker, T. Analysis of Matched Tumor and Normal Profiles Reveals Common Transcriptional and Epigenetic Signals Shared across Cancer Types. PLoS ONE 2015, 10, e0142618. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.Z.D.; Glicksberg, B.S.; Li, Y.; Chen, B. Selecting precise reference normal tissue samples for cancer research using a deep learning approach. BMC Med. Genom. 2019, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Torres-Platas, S.G.; Comeau, S.; Rachalski, A.; Bo, G.D.; Cruceanu, C.; Turecki, G.; Giros, B.; Mechawar, N. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuro Inflamm. 2014, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Waldvogel, H.J.; Curtis, M.A.; Baer, K.; Rees, M.I.; Faull, R.L. Immunohistochemical staining of post-mortem adult human brain sections. Nat. Protoc. 2006, 1, 2719–2732. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Buck, B.H.; Liebeskind, D.S.; Saver, J.L.; Bang, O.Y.; Yun, S.W.; Starkman, S.; Ali, L.K.; Kim, D.; Villablanca, J.P.; Salamon, N.; et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 2008, 39, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Planas, A.M. Role of Immune Cells Migrating to the Ischemic Brain. Stroke 2018, 49, 2261–2267. [Google Scholar] [CrossRef]
- Zrzavy, T.; Machado-Santos, J.; Christine, S.; Baumgartner, C.; Weiner, H.L.; Butovsky, O.; Lassmann, H. Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol. 2018, 28, 791–805. [Google Scholar] [CrossRef]
- Jin, F.; Xing, J. Circulating pro-angiogenic and anti-angiogenic microRNA expressions in patients with acute ischemic stroke and their association with disease severity. Neurol. Sci. 2017, 38, 2015–2023. [Google Scholar] [CrossRef]
- Gui, Y.; Xu, Z.; Jin, T.; Zhang, L.; Chen, L.; Hong, B.; Xie, F.; Lv, W.; Hu, X. Using Extracellular Circulating microRNAs to Classify the Etiological Subtypes of Ischemic Stroke. Transl. Stroke Res. 2019, 10, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-C.; Liao, Y.-C.; Wang, Y.-S.; Lin, H.-F.; Lin, R.-T.; Juo, S.-H.H. Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease. J. Vasc. Res. 2013, 50, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Takuma, A.; Abe, A.; Saito, Y.; Nito, C.; Ueda, M.; Ishimaru, Y.; Harada, H.; Abe, K.; Kimura, K.; Asakura, T. Gene Expression Analysis of the Effect of Ischemic Infarction in Whole Blood. Int. J. Mol. Sci. 2017, 18, 2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, J.; Lin, H.; Aparico, H.J.; Huan, T.; Liu, C.; Rong, J.; Beiser, A.; Himali, J.J.; Freedman, J.E.; Larson, M.G.; et al. Whole blood microRNA expression associated with stroke: Results from the Framingham Heart Study. PLoS ONE 2019, 14, e0219261. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Hou, R.; Ma, A.; Yang, S.; Pan, X. Associations of miR-146a, miR-149, miR-196a2, and miR-499 Polymorphisms with Ischemic Stroke in the Northern Chinese Han Population. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7366–7374. [Google Scholar] [CrossRef]
- Yuan, Y.; Kang, R.; Yu, Y.; Liu, J.; Zhang, Y.; Shen, C.; Wang, J.; Wu, P.; Shen, C.; Wang, Z. Crosstalk between miRNAs and their regulated genes network in stroke. Sci. Rep. 2016, 6, 20429. [Google Scholar] [CrossRef] [Green Version]
- Bam, M.; Yang, X.; Sen, S.; Zumbrun, E.E.; Dennis, L.; Zhang, J.; Nagarkatti, P.S.; Nagarkatti, M. Characterization of Dysregulated miRNA in Peripheral Blood Mononuclear Cells from Ischemic Stroke Patients. Mol. Neurobiol. 2018, 55, 1419–1429. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, S.; Zhang, J.; Xu, X.; Guan, W.; Jing, L.; Liu, P.; Lu, J.; Teng, J.; Peng, T.; et al. Initial research on the relationship between let-7 family members in the serum and massive cerebral infarction. J. Neurol. Sci. 2016, 361, 150–157. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Chen, S.; Chen, X.; Li, S.; Chen, W. Bioinformatic Analysis of Potential microRNAs in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 1753–1759. [Google Scholar] [CrossRef]
- Tan, K.S.; Armugam, A.; Sepramaniam, S.; Lim, K.Y.; Setyowati, K.D.; Wang, C.W.; Jeyaseelan, K. Expression profile of MicroRNAs in young stroke patients. PLoS ONE 2009, 4, e7689. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Teng, F.; Gao, F.; Zhang, M.; Wu, J.; Zhang, C. Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke. Cell. Mol. Neurobiol. 2015, 35, 433–447. [Google Scholar] [CrossRef]
- Sorensen, S.S.; Nygaard, A.B.; Carlsen, A.L.; Heegaard, N.H.H.; Bak, M.; Christensen, T. Elevation of brain-enriched miRNAs in cerebrospinal fluid of patients with acute ischemic stroke. Biomark. Res. 2017, 5, 24. [Google Scholar] [CrossRef]
- Shi, Q.; Ge, D.; Yang, Q.; Wang, L.; Fu, J. MicroRNA profiling of cerebrospinal fluid from patients with intracerebral haemorrhage. Front. Lab. Med. 2018, 2, 141–145. [Google Scholar] [CrossRef]
- Sepramaniam, S.; Armugam, A.; Lim, K.Y.; Karolina, D.S.; Swaminathan, P.; Tan, J.R.; Jeyaseelan, K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J. Biol. Chem. 2010, 285, 29223–29230. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Du, K.; Lu, X. Elevated expressions of serum miR-15a, miR-16, and miR-17-5p are associated with acute ischemic stroke. Int. J. Clin. Exp. Med. 2015, 8, 21071–21079. [Google Scholar]
- Xu, W.; Gao, L.; Zheng, J.; Li, T.; Shao, A.; Reis, C.; Chen, S.; Zhang, J. The Roles of MicroRNAs in Stroke: Possible Therapeutic Targets. Cell Transplant. 2018, 27, 1778–1788. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Li, Z.; Yang, Z.; Huang, Q.; Liu, J.; Hong, B. Plasma MicroRNA-16 Is a Biomarker for Diagnosis, Stratification, and Prognosis of Hyperacute Cerebral Infarction. PLoS ONE 2016, 11, e0166688. [Google Scholar] [CrossRef]
- Mick, E.; Shah, R.; Tanriverdi, K.; Murthy, V.; Gerstein, M.; Rozowsky, J.; Kitchen, R.; Larson, M.G.; Levy, D.; Freedman, J.E. Stroke and Circulating Extracellular RNAs. Stroke 2017, 48, 828–834. [Google Scholar] [CrossRef]
- Edwardson, M.A.; Zhong, X.; Fiandaca, M.S.; Federoff, H.J.; Cheema, A.K.; Dromerick, A.W. Plasma microRNA markers of upper limb recovery following human stroke. Sci. Rep. 2018, 8, 12558. [Google Scholar] [CrossRef]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Böck, J.; Northoff, B.H.; Klein, M.; et al. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Grinspan, J.B. Inhibitors of Myelination and Remyelination, Bone Morphogenetic Proteins, are Upregulated in Human Neurological Disease. Neurochem. Res. 2020, 45, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef] [PubMed]
- Sawe, N.; Steinberg, G.; Zhao, H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J. Neurosci. Res. 2008, 86, 1659–1669. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Baik, S.-H.; Balaganapathy, P.; Sobey, C.G.; Mattson, M.P.; Jo, D.-G. Notch signaling and neuronal death in stroke. Prog. Neurobiol. 2018, 165-167, 103–116. [Google Scholar] [CrossRef]
- Narasimhalu, K.; Ma, L.; De Silva, D.A.; Wong, M.-C.; Chang, H.-M.; Chen, C. Elevated platelet-derived growth factor AB/BB is associated with a lower risk of recurrent vascular events in stroke patients. Int. J. Stroke Off. J. Int. Stroke Soc. 2015, 10, 85–89. [Google Scholar] [CrossRef]
- Rienks, M.; Barallobre-Barreiro, J.; Mayr, M. The Emerging Role of the ADAMTS Family in Vascular Diseases. Circ. Res. 2018, 123, 1279–1281. [Google Scholar] [CrossRef]
- Dordoe, C.; Chen, K.; Huang, W.; Chen, J.; Hu, J.; Wang, X.; Lin, L. Roles of Fibroblast Growth Factors and Their Therapeutic Potential in Treatment of Ischemic Stroke. Front. Pharmacol. 2021, 12, 671131. [Google Scholar] [CrossRef]
- Bolaños, J.P.; Almeida, A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim. Biophys. Acta 1999, 1411, 415–436. [Google Scholar] [CrossRef] [Green Version]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 497–505. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Caballero-Garrido, E.; Pena-Philippides, J.C.; Lordkipanidze, T.; Bragin, D.; Yang, Y.; Erhardt, E.B.; Roitbak, T. In Vivo Inhibition of miR-155 Promotes Recovery after Experimental Mouse Stroke. J. Neurosci. 2015, 35, 12446–12464. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.; Luo, Z.; Zhong, C.; Pan, X.; Xu, X. Influence of miR-155 on Cell Apoptosis in Rats with Ischemic Stroke: Role of the Ras Homolog Enriched in Brain (Rheb)/mTOR Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 5141–5153. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, V.; Lamb, D.G.; Williamson, J.B.; Stead, T.S.; Penumudi, R.; Bidari, S.; Ganti, L.; Heilman, K.M.; Hedna, V.S. Hemispheric differences in malignant middle cerebral artery stroke. J. Neurol. Sci. 2015, 353, 20–27. [Google Scholar] [CrossRef]
- Nakamura, H.; Strong, A.J.; Dohmen, C.; Sakowitz, O.W.; Vollmar, S.; Sué, M.; Kracht, L.; Hashemi, P.; Bhatia, R.; Yoshimine, T.; et al. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain 2010, 133, 1994–2006. [Google Scholar] [CrossRef] [Green Version]
- Hartings, J.A.; Shuttleworth, C.W.; Kirov, S.A.; Ayata, C.; Hinzman, J.M.; Foreman, B.; Andrew, R.D.; Boutelle, M.G.; Brennan, K.C.; Carlson, A.P.; et al. The continuum of spreading depolarizations in acute cortical lesion development: Examining Leão’s legacy. J. Cereb. Blood Flow Metab. 2017, 37, 1571–1594. [Google Scholar] [CrossRef] [Green Version]
- Mestre, H.; Du, T.; Sweeney, A.M.; Liu, G.; Samson, A.J.; Peng, W.; Mortensen, K.N.; Stæger, F.F.; Bork, P.A.R.; Bashford, L.; et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 2020, 367, eaax7171. [Google Scholar] [CrossRef]
- Zador, Z.; Stiver, S.; Wang, V.; Manley, G.T. Role of Aquaporin-4 in Cerebral Edema and Stroke. Handb Exp. Pharm. 2009, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Pirici, I.; Balsanu, T.A.; Bogdan, C.; Margaritescu, C.; Divan, T.; Vitalie, V.; Mogoanta, L.; Pirici, D.; Carare, R.O.; Muresanu, D.F. Inhibition of Aquaporin-4 Improves the Outcome of Ischaemic Stroke and Modulates Brain Paravascular Drainage Pathways. Int. J. Mol. Sci. 2017, 19, 46. [Google Scholar] [CrossRef] [Green Version]

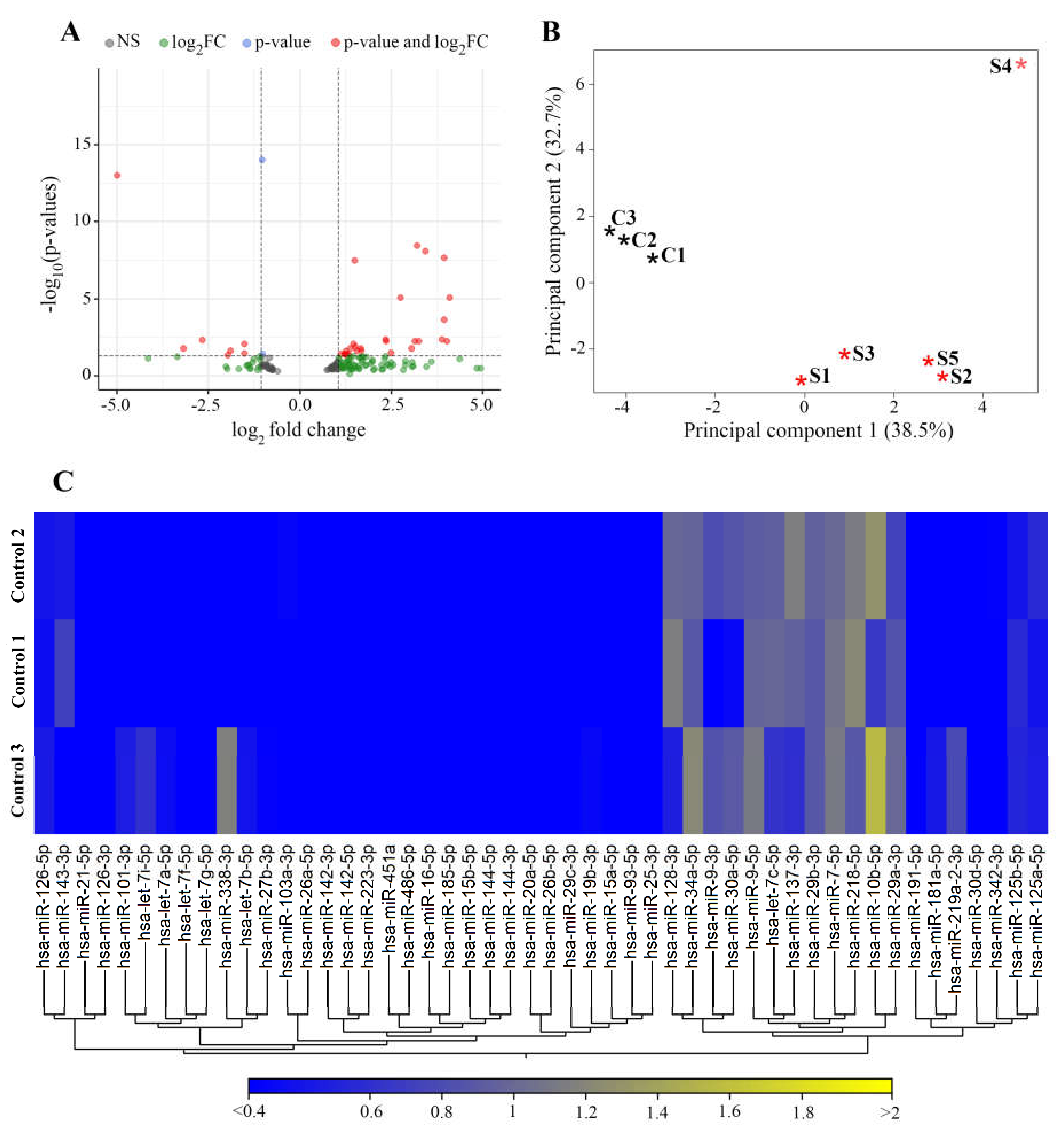
| Name | Fold-Change | p-Value | FDR p-Value | Reported in: |
|---|---|---|---|---|
| hsa-miR-1246 hsa-miR-4516 hsa-miR-182-5p hsa-miR-320d hsa-miR-1255b-5p hsa-miR-320c hsa-miR-183-5p hsa-miR-196b-5p hsa-miR-204-3p hsa-miR-17-5p hsa-miR-193b-5p hsa-miR-16-5p hsa-miR-320b hsa-miR-423-5p hsa-miR-320a-3p hsa-miR-505-5p hsa-miR-652-3p hsa-miR-135a-3p hsa-miR-196a-5p | 334.46 32.68 15.44 10.80 9.53 9.29 8.87 5.64 3.23 3.20 2.87 2.78 2.54 2.46 2.41 2.34 2.19 −9.00 −31.65 | 0 0 3.98 × 10−6 5.73 × 10−11 0.0001 2.30 × 10−11 0.0001 0.0011 0.0008 0.0005 0.0004 0.0002 0.0017 0.0015 0.0008 0.0014 0.0015 0.000536 3.33 × 10−16 | 0 0 0.0002 8.67 × 10−9 0.0059 3.79 × 10−9 0.0059 0.0347 0.0251 0.0180 0.0142 0.0090 0.0465 0.0416 0.0263 0.0416 0.0416 0.0180 1.01 × 10−13 | [51,52,53] [54] [18] [18,59] [60] [51] [18] [18] [57] [56] [61] [58] [18] [52] [55] [18] [18] [57] [18] |
| Name | Fold-Change | p-Value | FDR p-Value |
|---|---|---|---|
| hsa-miR-10395-3p hsa-miR-4732-5p hsa-miR-7704 hsa-miR-9901 hsa-miR-4772-3p hsa-miR-412-5p hsa-miR-142-3p hsa-miR-619-5p hsa-miR-1270 hsa-miR-3615 hsa-miR-539-3p hsa-miR-3912-5p hsa-miR-6500-3p hsa-miR-5683 hsa-miR-892a | 17.13 16.35 15.42 14.91 8.35 6.75 5.14 5.08 2.96 2.62 −2.08 −2.65 −2.83 −3.68 −3.88 | 1.00 × 10−7 0.0002 1.61 × 10−10 0.0001 0.0005 1.10 × 10−7 0.0001 0.0001 0.0008 0.0005 0.0012 0.0001 0.0002 0.0007 0.0019 | 9.08 × 10−6 0.0062 2.24 × 10−8 0.0048 0.0172 9.08 × 10−6 0.0060 0.0050 0.0254 0.0175 0.0363 0.0051 0.0090 0.0243 0.0514 |
| miRNA | Differentially Expressed Target Genes |
|---|---|
| hsa-miR-4732-5p | UHMK1, TOX *, TMEM183A *, TMED8, TGOLN2, SMAD2, SAMD12, RORB, RAP2A, RALYL*, PURG *, PTCH1 *, PSD3 *, PRPF40A, PKIA *, PHKA1 *, PDE4D, PCDH17, PARM1, PAPOLA * |
| hsa-miR-7704 | SPATA17, LDLRAP1, IWS1, DERL3, SEMA6A *, CACNA1E, ATG12, OPA1, PAK3, EPS8, CHMP3, POLR2L, GPR85, C4orf33, LETMD1, MEIS2 *, SPATA6, KLF12 *, ROBO1, PTCH1 * |
| hsa-miR-4772-3p | CTDSP2, TMEM183A *, PURA *, RHOU, CSRNP3, ARHGAP31 *, SH3BGRL2, CADM2 *, LRRC28 *, METTL15 *, PHKA1 *, TAOK1 *, GUCY1A2, MLLT3 *, CHIC1 *, RNF141 *, TOMM20 *, ZDHHC21 *, HS6ST3 *, PURG * |
| hsa-miR-142-3p | PDGFRA, TSPAN3, MTF2, FOXP1, REPS2 *, MTX3, MEIS2 *, PIK3CA, MAP2K6, CLSTN2, HMGCLL1 *, FGF12 *, SNTB2 *, NUCKS1, PURA *, CADM2 *, MAGI2, CHIC1 *, LRP1B, TOX * |
| hsa-miR-619-5p | EIF4EBP2, CADM2 *, LRRC28 *, METTL15 *, ATRNL1, NKAIN2, TAOK1 *, MLLT3 *, GALC, CHIC1 *, KDM3B, AMBRA1, IL1RAPL1, RNF141 *, TOMM20 *, RPS6KA6, ZDHHC21 *, KCNH5, HS6ST3 *, PURG * |
| hsa-miR-1270 | LRRTM4, THADA, PCDH9, KLF12 *, STX17, MMP16, HMGCLL1 *, CNOT7, PURB, RALYL *, PTAR1, PKIA *, NOVA1, OLFM3, NUBPL, FGF12 *, SNTB2 *, PAPOLA *, DPP6 *, CNR1 |
| hsa-miR-3615 | REPS2 *, SEMA6A *, ARHGAP15, MAMLD1, FOXB1, CCDC80, MEIS2 *, SLITRK5, PSD3 *, CBX5, DIRAS2, PKIA *, BTBD3, DPP6 *, ARHGAP31 *, TAOK1 * |
| hsa-miR-539-3p | AQP7 *, ADAMTS5, HAVCR1 *, GOLGA8G, ZNF266, TMCO5A, NOTCH2 *, PLCL1 *, HCN4, RYR2, PHKB *, ABCA13, LYN *, C18orf25, WIPF2, GATA3 *, FBLN2, TMPRSS4 *, TNFAIP3, ZC3H4 * |
| hsa-miR-3912-5p | FZD8 *, WNT1, PID1 *, ADAM8, GATA3 *, KLHDC7B, JAG2, DLST, RBMS3, FIG4, TMEM63A, IQSEC3 |
| hsa-miR-6500-3p | FZD8 *, HIC1, AQP7 *, MRGPRF, DMRT1, CTCFL, HSPA1A, UNC45B, SLCO5A1, SBSPON, FOXJ3, HAVCR1 *, PID1 *, NR5A1, COX20, NOXRED1, SCNN1G *, UBAP1, PLCL1 *, LYN * |
| hsa-miR-5683 | LSM4, LOXL4, PPM1N, ARSI, GATA3 *, NUTM2D *, TMOD4, NLRP2, ZNF217, NGB, HOXD3, CYTH3, DACT3, CTPS2, MOB2, CD74, CLDN10, GALNT1, FZD8 *, MAPK8IP2 |
| hsa-miR-892a | NOTCH2 *, SCNN1G *, ACTG2, PHKB *, LYN *, ZBTB42, CASC4, NUTM2D *, FTCDNL1, IRX2, SLC16A3, RCL1, TMPRSS4 *, DPEP1, VSIG10, ZC3H4 *, SAP30BP, POTEG, NOS1, IRAK3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlson, A.P.; McKay, W.; Edwards, J.S.; Swaminathan, R.; SantaCruz, K.S.; Mims, R.L.; Yonas, H.; Roitbak, T. MicroRNA Analysis of Human Stroke Brain Tissue Resected during Decompressive Craniectomy/Stroke-Ectomy Surgery. Genes 2021, 12, 1860. https://doi.org/10.3390/genes12121860
Carlson AP, McKay W, Edwards JS, Swaminathan R, SantaCruz KS, Mims RL, Yonas H, Roitbak T. MicroRNA Analysis of Human Stroke Brain Tissue Resected during Decompressive Craniectomy/Stroke-Ectomy Surgery. Genes. 2021; 12(12):1860. https://doi.org/10.3390/genes12121860
Chicago/Turabian StyleCarlson, Andrew P., William McKay, Jeremy S. Edwards, Radha Swaminathan, Karen S. SantaCruz, Ron L. Mims, Howard Yonas, and Tamara Roitbak. 2021. "MicroRNA Analysis of Human Stroke Brain Tissue Resected during Decompressive Craniectomy/Stroke-Ectomy Surgery" Genes 12, no. 12: 1860. https://doi.org/10.3390/genes12121860
APA StyleCarlson, A. P., McKay, W., Edwards, J. S., Swaminathan, R., SantaCruz, K. S., Mims, R. L., Yonas, H., & Roitbak, T. (2021). MicroRNA Analysis of Human Stroke Brain Tissue Resected during Decompressive Craniectomy/Stroke-Ectomy Surgery. Genes, 12(12), 1860. https://doi.org/10.3390/genes12121860






