Reproductive Stage Drought Tolerance in Wheat: Importance of Stomatal Conductance and Plant Growth Regulators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Controlled Environment Wheat Growing Conditions and Drought Treatments
2.2. Measurements of Stomatal Conductance and Stomatal Density
2.3. RNA Extraction, Gene Expression and RNA Sequencing Procedure
2.4. Phytohormone Quantification
3. Results
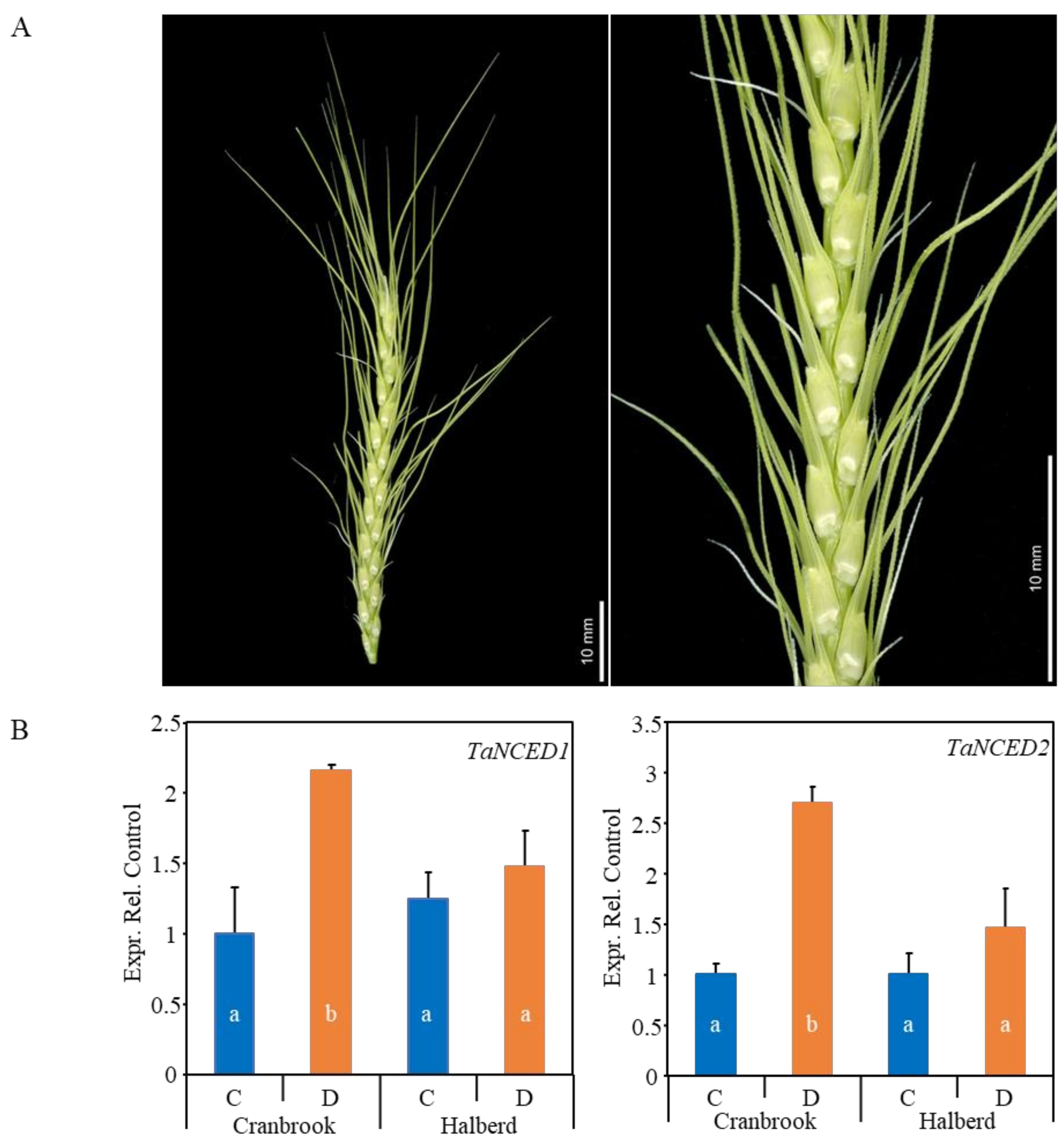
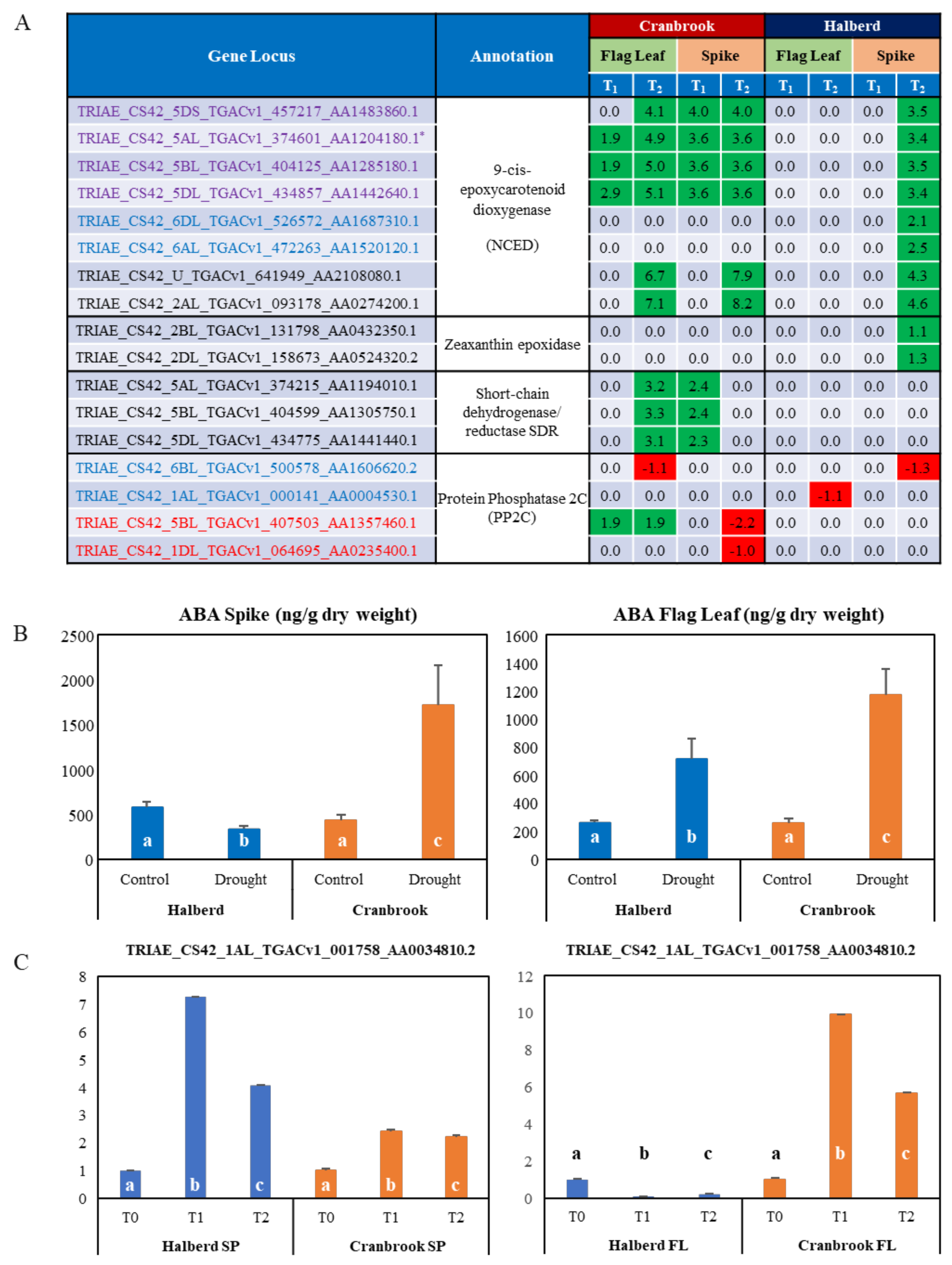
3.1. Drought Stress Does Not Induce ABA Biosynthesis Genes in Drought-tolerant Halberd Spikes
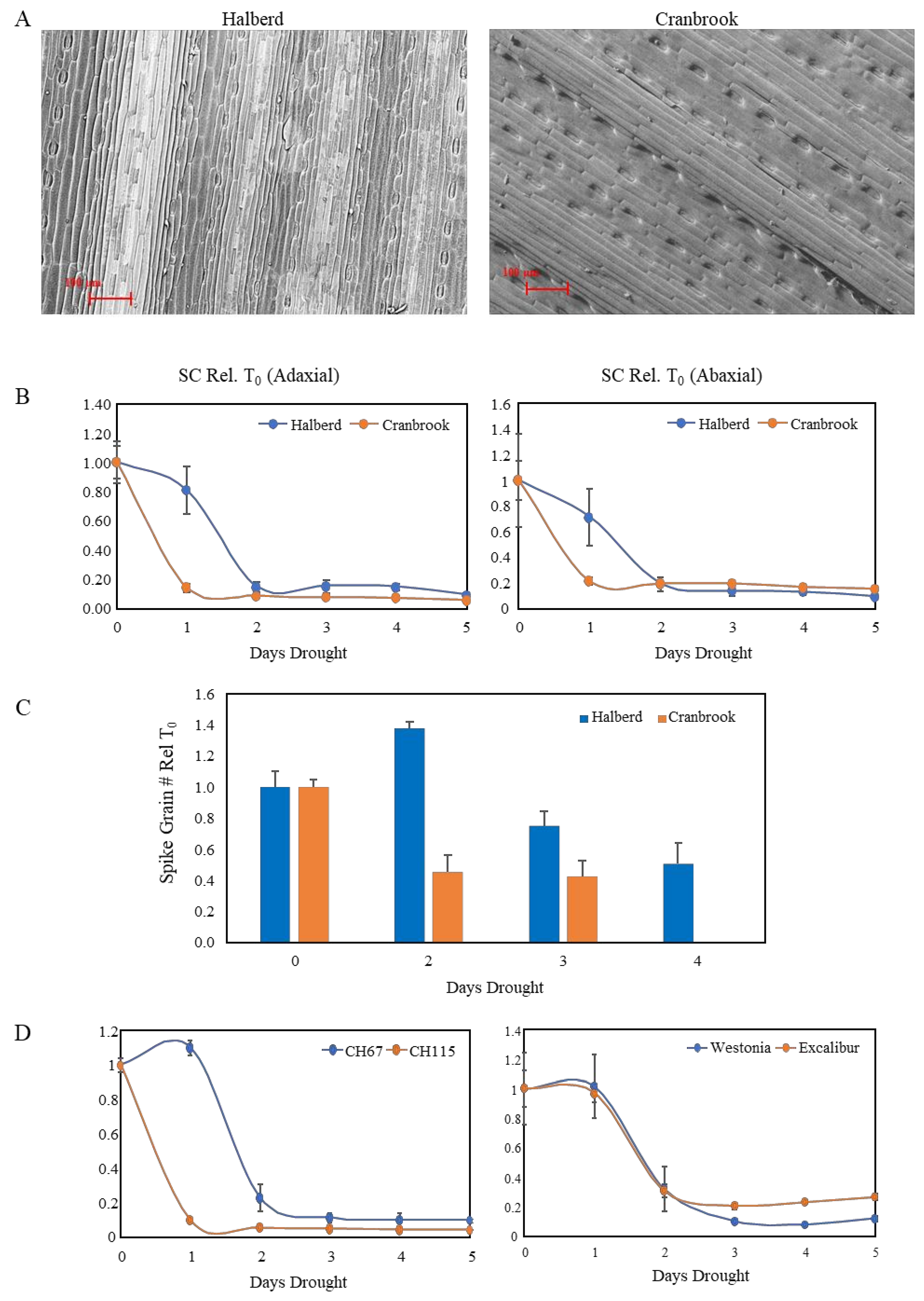
3.2. Drought-Tolerant Wheat Maintains Stomatal Conductance Longer under Drought Conditions
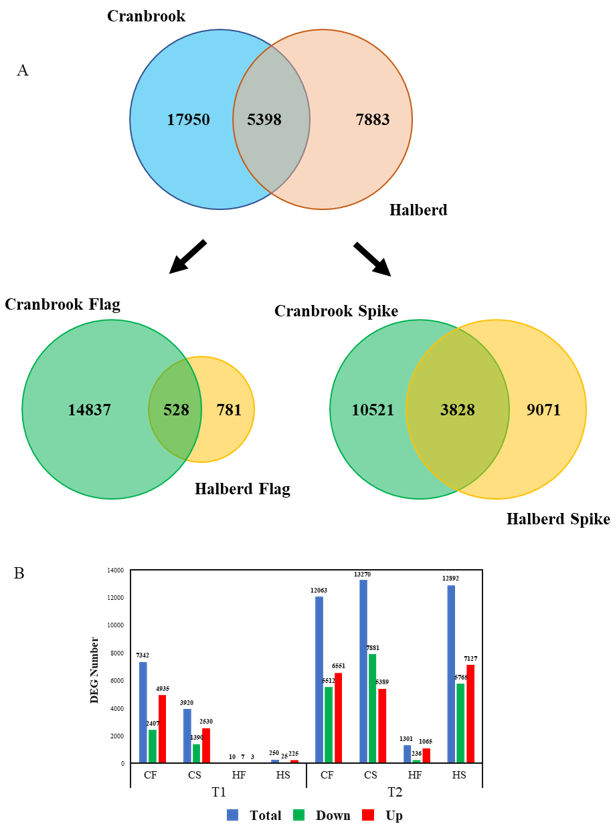
3.3. Quantitative Differences in the Cranbrook and Halberd Drought Response
3.4. Biological Processes Affected by Drought in Cranbrook and Halberd Spikes and Flag Leaves
3.5. Differences in Spike and Flag Leaf Drought Stress Response between Cranbrook and Halberd
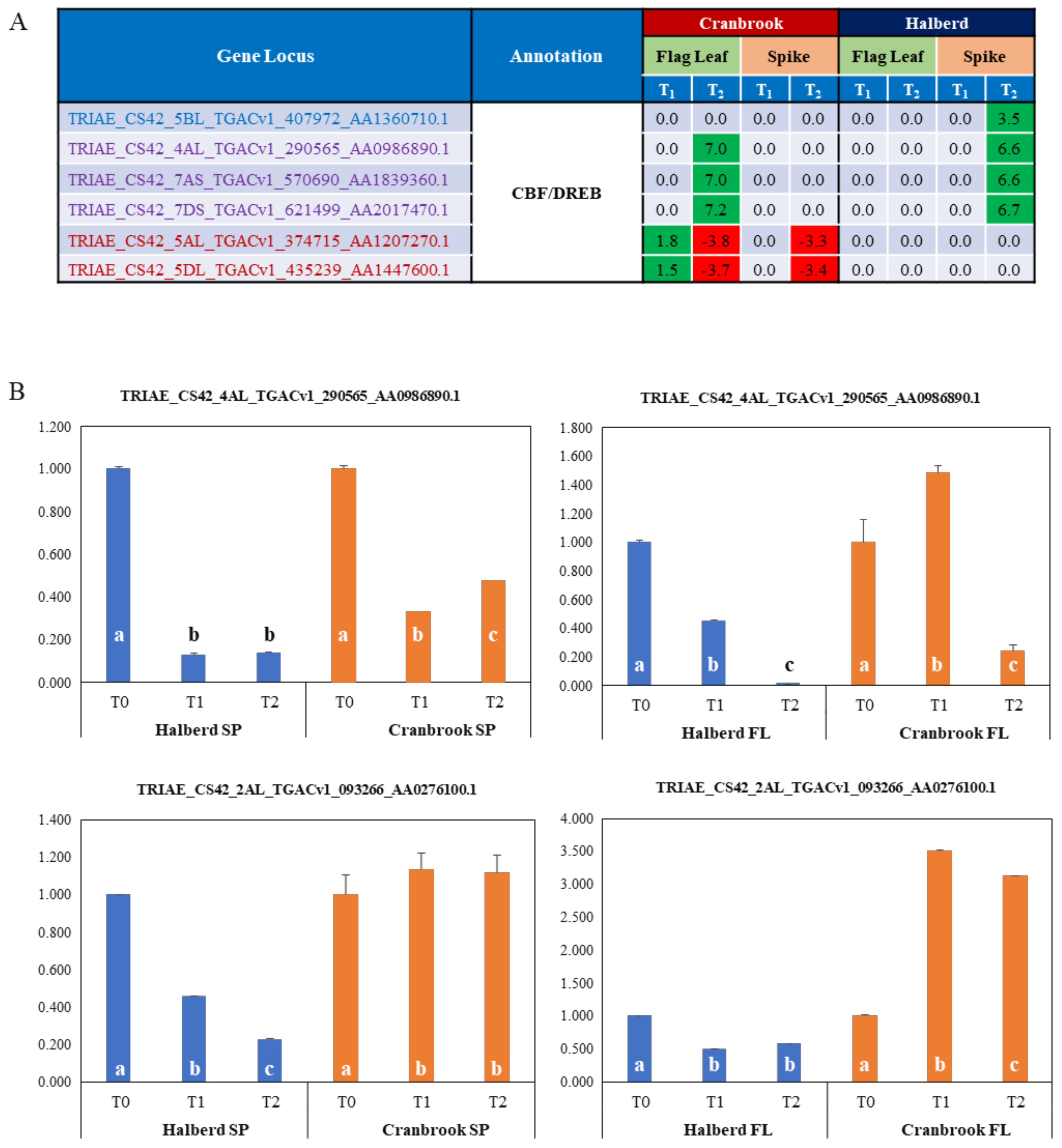
3.6. Identification of Drought Responsive CBF/DREB Factors in Halberd and Cranbrook
3.7. Differential Expression of ABA-Related Genes in Drought-Stressed Cranbrook and Halberd
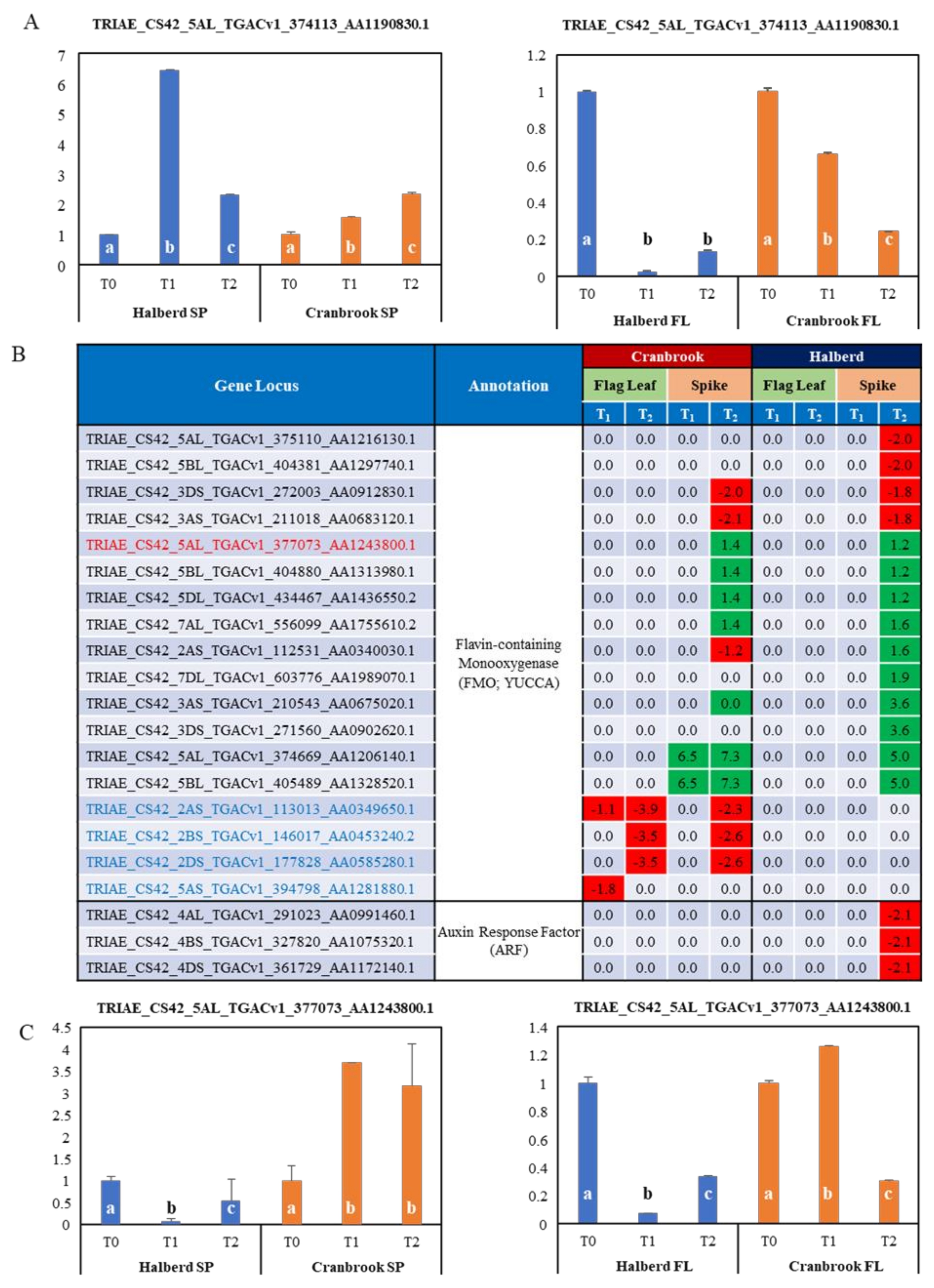
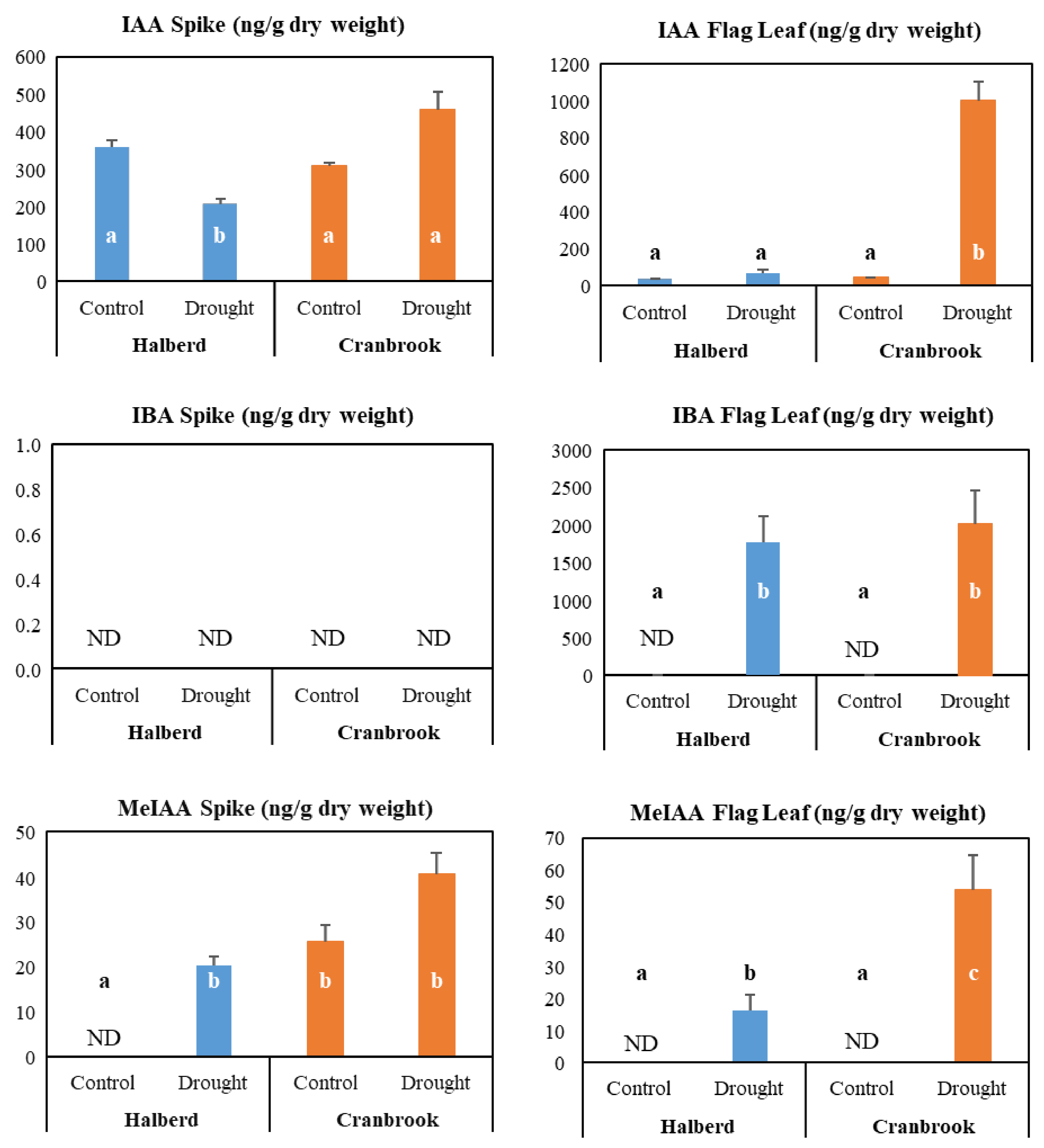
3.8. Potential Role of Auxin in the Drought Response of Halberd and Cranbrook
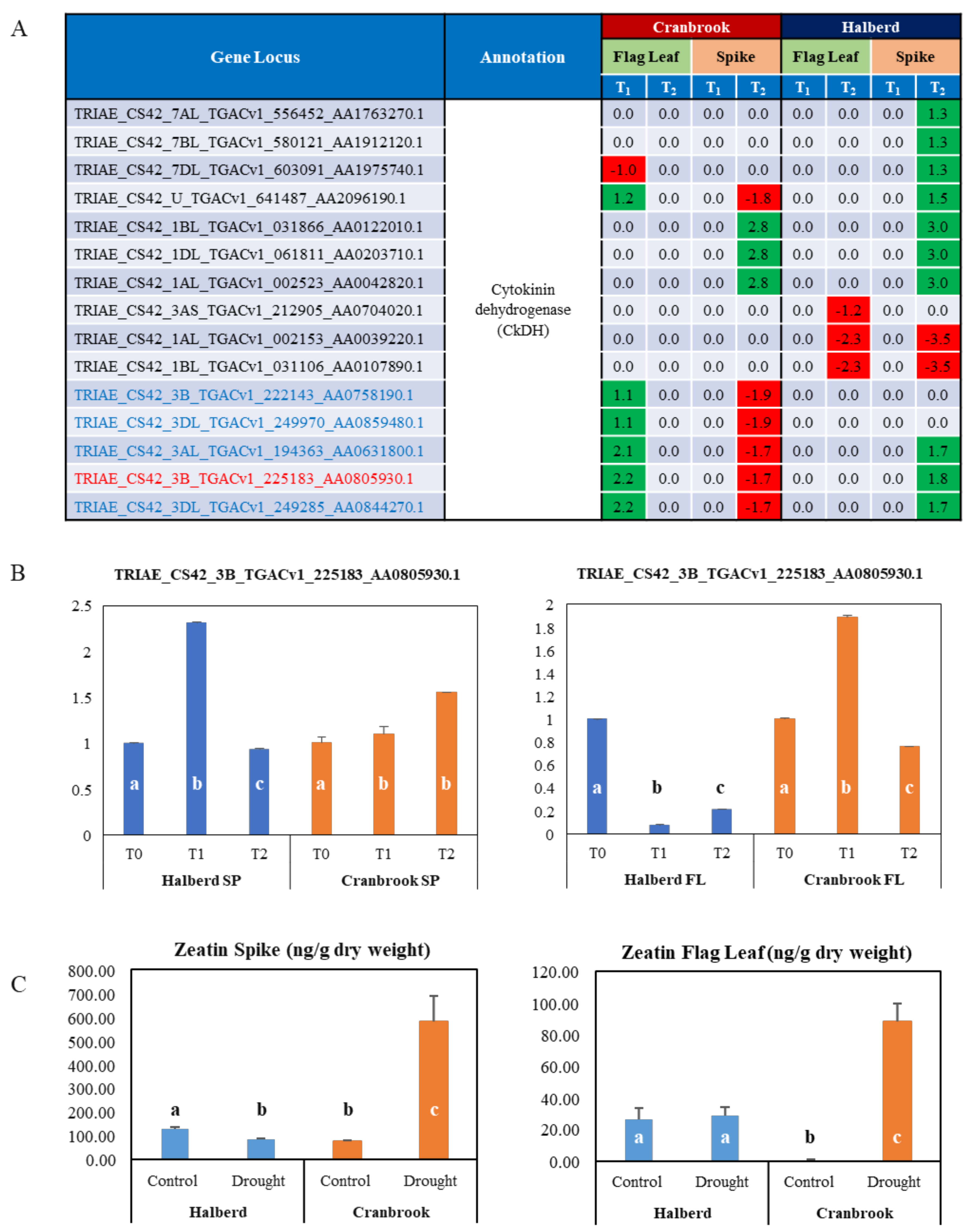
3.9. Role of Cytokinin in the Drought Response of Halberd and Cranbrook Spike and Flag Leaves
3.10. Effect of Drought on Ethylene Responsive Genes in Halberd and Cranbrook
3.11. The Drought Response at T1 in Halberd Flag Leaves
4. Discussion
4.1. Active and Passive Survival of Drought Conditions
4.2. The Role of ABA in Reproductive Stage Drought Tolerance
4.3. Halberd and Cranbrook Have a Different Response to Drought Stress
4.4. Drought Stress Differentially Affects Auxin and Cytokinin Metabolism and Signalling
4.5. Role of Ethylene in Controlling Reproductive Drought Tolerance
4.6. What Is Happening at T1 in Halberd Flag Leaves?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Semenov, M.A. Impacts of climate change on wheat in England and Wales. J. R. Soc. Interface 2009, 6, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.A.; Stratonovitch, P.; Alghabari, F.; Gooding, M.J. Adapting wheat in Europe for climate change. J. Cereal Sci. 2014, 59, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.J.; Araus, J.-L.; Park, R.; Calderini, D.; Miralles, D.; Shen, T.; Zhang, J.; Parry, M.A.J. Prospects of doubling global wheat yields. Food Energy Secur. 2013, 2, 34–48. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Watt, M.; Condon, A.G.; Spielmeyer, W.; Dolferus, R. Breeding for improved water productivity in temperate cereals: Phenotyping, quantitative trait loci, markers and the selection environment. Funct. Plant Biol. 2010, 37, 85–97. [Google Scholar] [CrossRef]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef] [Green Version]
- Collins, N.C.; Tardieu, F.; Tuberosa, R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef] [Green Version]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop. Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Blum, A. Towards a conceptual ABA ideotype in plant breeding for water limited environments. Funct. Plant Biol. 2015, 42, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Charra-Vaskou, K.; Badel, E.; Burlett, R.; Cochard, H.; Delzon, S.; Mayr, S. Hydraulic efficiency and safety of vascular and non-vascular components in Pinus pinaster leaves. Tree Physiol. 2012, 32, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Brodersen, C.; Jansen, S.; Choat, B.; Rico, C.; Pittermann, J. Cavitation resistance in seedless vascular plants: The structure and function of interconduit pit membranes. Plant Physiol. 2014, 165, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passioura, J.B. Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Funct. Plant Biol. 2012, 39, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Senapati, N.; Stratonovitch, P.; Paul, M.J.; Semenov, M.A. Drought tolerance during reproductive development is important for increasing wheat yield potential under climate change in Europe. J. Exp. Bot. 2018, 70, 2549–2560. [Google Scholar] [CrossRef] [Green Version]
- Considine, M.J.; Considine, J.A. On the language and physiology of dormancy and quiescence in plants. J. Exp. Bot. 2016, 67, 3189–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Siddique, K.H.M. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Yu, S.; Sun, M.; Yang, Z.; Gao, Z.-Q. Development of drought-tolerant transgenic wheat: Achievements and limitations. Int. J. Mol. Sci. 2019, 20, 3350. [Google Scholar] [CrossRef] [Green Version]
- Mullet, J. Traits and Genes for Plant Drought Tolerance. In Molecular Genetic Approaches to Maize Improvement; Kriz, A.L., Brian, A., Larkins, B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 63, pp. 55–64. [Google Scholar]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Martignago, D.; Rico-Medina, A.; Blasco-Escámez, D.; Fontanet-Manzaneque, J.B.; Caño-Delgado, A.I. Drought resistance by engineering plant tissue-specific responses. Front. Plant Sci. 2020, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A.; Hunt, J.R.; Kirkegaard, J.A.; Passioura, J.B. Yield improvement and adaptation of wheat to water-limited environments in Australia—A case study. Crop. Pasture Sci. 2014, 65, 676–689. [Google Scholar] [CrossRef]
- Hall, A.J.; Richards, R.A. Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crop. Res. 2013, 143, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Bechtold, U. Plant life in extreme environments: How do you improve drought tolerance? Front. Plant Sci. 2018, 9, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amtmann, A.; Bohnert, H.J.; Bressan, R.A. Abiotic stress and plant genome evolution. Search for new models. Plant Physiol. 2005, 138, 127–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberdi, M.; Bravo León, A.; Gutiérrez, A.; Gidekel, M.; Corcuera Luis, J. Ecophysiology of Antarctic vascular plants. Physiol. Plant. 2002, 115, 479–486. [Google Scholar] [CrossRef]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.-L.; Mathesius, U. Root-to-shoot signalling: Integration of diverse molecules, pathways and functions. Funct. Plant Biol. 2016, 43, 87–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelfsema, M.R.; Hedrich, R. In the light of stomatal opening: New insights into ‘the watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Pittermann, J.; Brodersen, C.; Watkins, J.E., Jr. The physiological resilience of fern sporophytes and gametophytes: Advances in water relations offer new insights into an old lineage. Front. Plant Sci. 2013, 4, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodribb, T.J.; McAdam, S.A.; Carins Murphy, M.R. Xylem and stomata, coordinated through time and space. Plant Cell Environ. 2017, 40, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M. Evolution of the stomatal regulation of plant water content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/anisohydry: A plant-environment interaction rather than a simple hydraulic trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Berger-Landefeldt, U. Das Wasserhaushalt der Alpenpflanzen; Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1936. [Google Scholar]
- Nahar, K.; Mirza, H.; Fujita, M. Roles of osmolytes in plant adaptation to drought and salinity. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 37–68. [Google Scholar]
- Argiolas, A.; Puleo, G.L.; Sinibaldi, E.; Mazzolai, B. Osmolyte cooperation affects turgor dynamics in plants. Sci. Rep. 2016, 6, 30139. [Google Scholar] [CrossRef] [Green Version]
- Negin, B.; Moshelion, M. The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Sci. 2016, 251, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Galle, A.; Csiszar, J.; Benyo, D.; Laskay, G.; Leviczky, T.; Erdei, L.; Tari, I. Isohydric and anisohydric strategies of wheat genotypes under osmotic stress: Biosynthesis and function of ABA in stress responses. J. Plant Physiol. 2013, 170, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chater, C.; Gray, J.E.; Beerling, D.J. Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr. Opin. Plant Biol. 2013, 16, 638–646. [Google Scholar] [CrossRef]
- Hartung, W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010, 37, 806–812. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Dong, B.; Shiran, B.; Talbot, M.J.; Edlington, J.E.; Hughes, T.; White, R.G.; Gubler, F.; Dolferus, R. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 2011, 156, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C. Hormonal interactions and stomatal responses. J. Plant Growth Regul. 2003, 22, 32–46. [Google Scholar] [CrossRef]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic acid—Enemy or savior in the response of cereals to abiotic and biotic stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.N.; Van Dongen, J.T.; Alfred, S.C.; Mamun, E.A.; Zhao, X.; Saini, H.S.; Fernandes, S.F.; Blanchard, C.L.; Sutton, B.G.; Geigenberger, P.; et al. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ. 2005, 28, 1534–1551. [Google Scholar] [CrossRef]
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 2007, 48, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammholz, S.J.; Campbell, A.W.; Sutherland, M.W.; Hollamby, G.J.; Martin, P.J.; Eastwood, R.F.; Barclay, I.; Wilson, R.E.; Brennan, P.S.; Sheppard, J.A. Establishment and characterisation of wheat genetic mapping populations. Aust. J. Agric. Res. 2001, 52, 1079–1088. [Google Scholar] [CrossRef]
- Dolferus, R.; Thavamanikumar, S.; Sangma, H.; Kleven, S.; Wallace, X.; Forrest, K.; Rebetzke, G.; Hayden, M.; Borg, L.; Smith, A.; et al. Determining the genetic architecture of reproductive stage drought tolerance in wheat using a correlated trait and correlated marker effect model. G3 Genes Genomes Genet. 2019, 9, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolferus, R.; Powell, N.; Ji, X.; Ravash, R.; Edlington, J.; Oliver, S.; Van Dongen, J.T.; Shiran, B. The physiology of reproductive-stage abiotic stress tolerance in cereals. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 193–216. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexa, A.; Rahnenfuhrer, J. TopGo: Enrichment Analysis for Gene Ontology, R Package Version 2.38.1. 2019. Available online: https://bioconductor.org/packages/release/bioc/html/topGO.html (accessed on 20 June 2021).
- Clavijo, B.J.; Venturini, L.; Schudoma, C.; Accinelli, G.G.; Kaithakottil, G.; Wright, J.; Borrill, P.; Kettleborough, G.; Heavens, D.; Chapman, H.; et al. An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Res. 2017, 27, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.-H.; Endo, A.; Zhou, L.; Penney, J.; Chen, H.-C.; Arroyo, A.; Leon, P.; Nambara, E.; Asami, T.; Seo, M.; et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 2002, 14, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Bhaskara, G.B.; Nguyen, T.T.; Verslues, P.E. Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol. 2012, 160, 379–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, M.; Hernández-García, J.; Pollmann, S.; Samodelov, S.L.; Kolb, M.; Friml, J.; Hammes, U.Z.; Zurbriggen, M.D.; Blázquez, M.A.; Alabadí, D. Auxin methylation is required for differential growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 6864–6869. [Google Scholar] [PubMed] [Green Version]
- Wellman, C.H.; Gray, J. The microfossil record of early land plants. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Kenrick, P.; Crane, P.R. The origin and early evolution of plants on land. Nature 1997, 389, 33–39. [Google Scholar] [CrossRef]
- Oliver, M.J.; Velten, J.; Mishler, B.D. Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 2005, 45, 788–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol. 2013, 40, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ruszala, E.; Beerling, D.; Franks, P.; Chater, C.; Casson, S.; Gray, J.; Hetherington, A. Land plants acquired active stomatal control early in their evolutionary history. Curr. Biol. 2011, 21, 1030–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperry, J.S. Evolution of water transport and xylem structure. Int. J. Plant Sci. 2003, 164, S115–S127. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A.; Edwards, D. Roots: Evolutionary origins and biogeochemical significance. J. Exp. Bot. 2001, 52, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Sussmilch, F.C.; Brodribb, T.J.; McAdam, S.A. What are the evolutionary origins of stomatal responses to abscisic acid in land plants? J. Integr. Plant Biol. 2017, 59, 240–260. [Google Scholar] [CrossRef] [Green Version]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [Green Version]
- Saradadevi, R.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Stomatal behaviour under terminal drought affects post-anthesis water use in wheat. Funct. Plant Biol. 2017, 44, 279–289. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Parent, B.; Tardieu, F. Can current crop models be used in the phenotyping era for predicting the genetic variability of yield of plants subjected to drought or high temperature? J. Exp. Bot. 2014, 21, 6179–6189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepstein, S.; Glick, B.R. Strategies to ameliorate abiotic stress-induced plant senescence. Plant Mol. Biol. 2013, 82, 623–633. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Blum, A.; Zhang, J.; Nguyen, H.T. Consistent differences among wheat cultivars in osmotic adjustment and their relationship to plant production. Field Crop. Res. 1999, 64, 287–291. [Google Scholar] [CrossRef]
- Morgan, J.M.; Hare, R.A.; Fletcher, R.J. Genetic variation in osmoregulation in bread and durum wheats and its relationship to grain yield in a range of field environments. Aust. J. Agric. Res. 1986, 37, 449–457. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, R.H.; Tarin, T.; Santini, N.S.; McAdam, S.A.M.; Ruman, R.; Eamus, D. Differences in osmotic adjustment, foliar abscisic acid dynamics, and stomatal regulation between an isohydric and anisohydric woody angiosperm during drought. Plant Cell Environ. 2017, 40, 3122–3134. [Google Scholar] [CrossRef]
- El Habti, A.; Fleury, D.; Jewell, N.; Garnett, T.; Tricker, P.J. Tolerance of combined drought and heat stress is associated with transpiration maintenance and water-soluble carbohydrates in wheat grains. Front. Plant Sci. 2020, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. In Survival Strategies in Extreme Cold and Desiccation; Iwaya-Inoue, M., Sakurai, M., Uemura, M., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1081, pp. 215–232. [Google Scholar]
- Rook, F.; Corke, F.; Baier, M.; Holman, R.; May, A.G.; Bevan, M.W. Impaired sucrose induction 1 encodes a conserved plant-specific protein that couples carbohydrate availability to gene expression and plant growth. Plant J. 2006, 46, 1045–1058. [Google Scholar] [CrossRef]
- Gazzarrini, S.; McCourt, P. Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr. Opin. Plant Biol. 2001, 4, 387–391. [Google Scholar] [CrossRef]
- Yamburenko, M.V.; Zubo, Y.O.; Borner, T. Abscisic acid affects transcription of chloroplast genes via protein phosphatase 2C-dependent activation of nuclear genes: Repression by guanosine-3′-5′-bisdiphosphate and activation by sigma factor 5. Plant J. 2015, 82, 1030–1041. [Google Scholar] [CrossRef]
- Tholen, D.; Pons, T.L.; Voesenek, L.A.; Poorter, H. Ethylene insensitivity results in down-regulation of rubisco expression and photosynthetic capacity in tobacco. Plant Physiol. 2007, 144, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.; Koshiba, T. Transport of ABA from the site of biosynthesis to the site of action. J. Plant Res. 2011, 124, 501–507. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Perez, I.B.; Brown, P.J. The role of ROS signaling in cross-tolerance: From model to crop. Front. Plant Sci. 2014, 5, 754. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Komatsuda, T.; Ma, J.F.; Li, C.; Yamaji, N.; Nevo, E. A functional cutin matrix is required for plant protection against water loss. Plant Signal. Behav. 2011, 6, 1297–1299. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chen, G.; Mishina, K.; Yamaji, N.; Ma, J.F.; Yukuhiro, F.; Tagiri, A.; Liu, C.; Pourkheirandish, M.; Anwar, N.; et al. A GDSL-motif esterase/acyltransferase/lipase is responsible for leaf water retention in barley. Plant Direct 2017, 1, e00025. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Kovalchuk, N.; Langridge, P.; Tricker, P.J.; Lopato, S.; Borisjuk, N. The impact of drought on wheat leaf cuticle properties. BMC Plant Biol. 2017, 17, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, C.N.; Jordan, G.J.; Jansen, S.; McAdam, S.A.M. A permeable cuticle, not open stomata, is the primary source of water loss from expanding leaves. Front. Plant Sci. 2020, 11, 774. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xiong, L.; Li, W.; Zhu, J.K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1971–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. APp2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Akhtar, M.; Jaiswal, A.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF transcription factors: Their structure, function and role in abiotic stress tolerance in plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef]
- Kasuga, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. A combination of the Arabidopsis DREB1a gene and stress-inducible rd29a promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol. 2004, 45, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.G.; Kim, J.; Kim, B.; Jeong, H.; Choi, S.H.; Kim, E.K.; Lee, H.Y.; Lim, P.O. Overexpression of FTL1/DDF1, an AP2 transcription factor, enhances tolerance to cold, drought, and heat stresses in Arabidopsis thaliana. Plant Sci. 2011, 180, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, Y.; Wu, Y.; Chen, H.; Chen, F.; Chu, C. Overexpression of a rice OsDREB1f gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 2008, 67, 589–602. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wu, Z.; Jia, Y.; Wang, H.; Sun, S.; Mao, C.; Wang, X. Abscisic acid regulates auxin homeostasis in rice root tips to promote root hair elongation. Front. Plant Sci. 2017, 8, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 family member, OSGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, L.; Nakurte, I.; Jalakas, P.; Kunga-Jegere, L.; Brosché, M.; Rostoks, N. Arabidopsis mutant dnd2 exhibits increased auxin and abscisic acid content and reduced stomatal conductance. Plant Physiol. Biochem. 2019, 140, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, J.; Song, J.; Jameson, P.E. Cytokinin dehydrogenase: A genetic target for yield improvement in wheat. Plant Biotechnol. J. 2020, 18, 614–630. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [Green Version]
- Ljung, K. Auxin metabolism and homeostasis during plant development. Development 2013, 140, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Hai, N.N.; Chuong, N.N.; Tu, N.H.C.; Kisiala, A.; Hoang, X.L.T.; Thao, N.P. Role and regulation of cytokinins in plant response to drought stress. Plants 2020, 9, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, A.; Ma, W.; Zhao, X.; Hu, M.; He, X.; Teng, W.; Li, H.; Tong, Y. The auxin biosynthetic tryptophan aminotransferase related TaTAR2.1-3A increases grain yield of wheat. Plant Physiol. 2017, 174, 2274–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fu, X.; Zhao, M.; Zhang, W.; Li, B.; An, D.; Li, J.; Zhang, A.; Liu, R.; Liu, X. A genome-wide view of transcriptome dynamics during early spike development in bread wheat. Sci. Rep. 2018, 8, 15338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheol Park, H.; Cha, J.Y.; Yun, D.J. Roles of yuccas in auxin biosynthesis and drought stress responses in plants. Plant Signal. Behav. 2013, 8, e24495. [Google Scholar] [CrossRef] [Green Version]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Stevenson, T.T.; Cleland, R.E. Osmoregulation in the Avena coleoptile in relation to auxin and growth. Plant Physiol. 1981, 67, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, T.T.; Cleland, R.E. Osmoregulation in the Avena coleoptile: Control of solute uptake in peeled sections. Plant Physiol. 1982, 69, 292–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutschera, U.; Niklas, K.J. Cell division and turgor-driven stem elongation in juvenile plants: A synthesis. Plant Sci. 2013, 207, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Rayle, D.L.; Cleland, R.E. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [Green Version]
- Kazama, H.; Katsumi, M. The role of the osmotic potential of the cell in auxin-induced cell elongation. Plant Cell Physiol. 1978, 19, 1145–1150. [Google Scholar]
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Park, M.Y.; Spartz, A.K.; Wong, J.H.; Gray, W.M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 2018, 14, e1007455. [Google Scholar] [CrossRef]
- Sakata, T.; Oshino, T.; Miura, S.; Tomabechi, M.; Tsunaga, Y.; Higashitani, N.; Miyazawa, Y.; Takahashi, H.; Watanabe, M.; Higashitani, A. Auxins reverse plant male sterility caused by high temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 8569–8574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashitani, A. High temperature injury and auxin biosynthesis in microsporogenesis. Front. Plant Sci. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, S.; Bi, Y.M.; Zhu, T.; Rothstein, S.J. SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009, 151, 691–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Koyama, T. The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Front. Plant Sci. 2014, 5, 650. [Google Scholar] [CrossRef]
- Fedoroff, N.V. Cross-talk in abscisic acid signaling. Sci. STKE 2002, 140, re10. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Parry, G.; Graham, N.; Allen, T.; Bennett, M. Auxin cross-talk: Integration of signalling pathways to control plant development. Plant Mol. Biol. 2002, 49, 411–426. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Malefo, M.B.; Mathibela, E.O.; Crampton, B.G.; Makgopa, M.E. Investigating the role of Bowman-Birk serine protease inhibitor in Arabidopsis plants under drought stress. Plant Physiol. Biochem. 2020, 149, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Li, C.; Chen, F.; Zhao, S.; Xia, G. A Bowman-Birk type protease inhibitor is involved in the tolerance to salt stress in wheat. Plant Cell Environ. 2008, 31, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Grant, J.J.; Seki, M.; Shinozaki, K.; Loake, G.J. Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J. 2004, 38, 810–822. [Google Scholar] [CrossRef] [PubMed]

| Halberd Flag Leaf BP GO Enrichment | Cranbrook Flag Leaf BP GO Enrichment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GO.ID | Term | Annotated | Significant | Expected | Classic | GO.ID | Term | Annotated | Significant | Expected | Classic |
| GO:0050794 | regulation of cellular process | 383 | 46 | 24.36 | 5.70 × 10−6 | GO:0019538 | protein metabolic process | 514 | 316 | 248.09 | 2.00 × 10−11 |
| GO:0050789 | regulation of biological process | 400 | 46 | 25.44 | 1.90 × 10−5 | GO:0010467 | gene expression | 413 | 273 | 199.34 | 2.10 × 10−15 |
| GO:0006355 | regulation of transcription, DNA-templat… | 264 | 38 | 16.79 | 5.20 × 10−7 | GO:0006796 | phosphate-containing compound metabolic … | 413 | 257 | 199.34 | 4.90 × 10−10 |
| GO:1903506 | regulation of nucleic acid-templated tra… | 264 | 38 | 16.79 | 5.20 × 10−7 | GO:0016070 | RNA metabolic process | 337 | 212 | 162.66 | 6.20 × 10−9 |
| GO:2000112 | regulation of cellular macromolecule bio… | 272 | 38 | 17.3 | 1.10 × 10−6 | GO:0006468 | protein phosphorylation | 298 | 182 | 143.84 | 1.80 × 10−6 |
| GO:0009790 | embryo development | 15 | 7 | 0.95 | 1.60 × 10−5 | GO:0006355 | regulation of transcription, DNA-templat… | 264 | 167 | 127.43 | 1.90 × 10−7 |
| GO:0006032 | chitin catabolic process | 22 | 7 | 1.4 | 0.00028 | GO:0043043 | peptide biosynthetic process | 100 | 76 | 48.27 | 7.70 × 10−9 |
| GO:0006869 | lipid transport | 20 | 6 | 1.27 | 0.00111 | GO:0006633 | fatty acid biosynthetic process | 85 | 49 | 41.03 | 0.0497 |
| GO:0019684 | photosynthesis, light reaction | 5 | 5 | 0.32 | 9.90× 10−7 | GO:0015672 | monovalent inorganic cation transport | 31 | 24 | 14.96 | 0.00084 |
| GO:0006086 | acetyl-CoA biosynthetic process from pyr… | 3 | 3 | 0.19 | 0.00025 | GO:0006559 | L-phenylalanine catabolic process | 32 | 24 | 15.45 | 0.00182 |
| GO:0006094 | gluconeogenesis | 3 | 3 | 0.19 | 0.00025 | GO:0072528 | pyrimidine-containing compound biosynthe… | 19 | 14 | 9.17 | 0.02206 |
| GO:0006099 | tricarboxylic acid cycle | 3 | 3 | 0.19 | 0.00025 | GO:0046856 | phosphatidylinositol dephosphorylation | 13 | 12 | 6.27 | 0.00113 |
| GO:0006561 | proline biosynthetic process | 3 | 3 | 0.19 | 0.00025 | GO:0016192 | vesicle-mediated transport | 17 | 12 | 8.21 | 0.05353 |
| GO:0006816 | calcium ion transport | 4 | 3 | 0.25 | 0.00097 | GO:0006563 | L-serine metabolic process | 15 | 11 | 7.24 | 0.04451 |
| GO:0006415 | translational termination | 5 | 3 | 0.32 | 0.0023 | GO:0006081 | cellular aldehyde metabolic process | 8 | 8 | 3.86 | 0.00291 |
| GO:0009269 | response to desiccation | 6 | 3 | 0.38 | 0.00438 | GO:0006544 | glycine metabolic process | 8 | 7 | 3.86 | 0.02803 |
| GO:0006183 | GTP biosynthetic process | 8 | 3 | 0.51 | 0.01116 | GO:0006656 | phosphatidylcholine biosynthetic process | 6 | 6 | 2.9 | 0.01257 |
| GO:0006228 | UTP biosynthetic process | 8 | 3 | 0.51 | 0.01116 | GO:0009107 | lipoate biosynthetic process | 6 | 6 | 2.9 | 0.01257 |
| GO:0015696 | ammonium transport | 8 | 3 | 0.51 | 0.01116 | GO:0009269 | response to desiccation | 6 | 6 | 2.9 | 0.01257 |
| GO:0006536 | glutamate metabolic process | 11 | 3 | 0.7 | 0.02855 | GO:0042128 | nitrate assimilation | 6 | 6 | 2.9 | 0.01257 |
| GO:0006813 | potassium ion transport | 13 | 3 | 0.83 | 0.04507 | GO:0006006 | glucose metabolic process | 5 | 5 | 2.41 | 0.0261 |
| GO:0015743 | malate transport | 3 | 2 | 0.19 | 0.01156 | GO:0006012 | galactose metabolic process | 5 | 5 | 2.41 | 0.0261 |
| GO:0006730 | one-carbon metabolic process | 6 | 2 | 0.38 | 0.05091 | GO:0007205 | protein kinase C-activating G protein-co… | 5 | 5 | 2.41 | 0.0261 |
| GO:0009101 | glycoprotein biosynthetic process | 5 | 5 | 2.41 | 0.0261 | ||||||
| GO:0006353 | DNA-templated transcription, termination | 4 | 4 | 1.93 | 0.05415 | ||||||
| GO:0006680 | glucosylceramide catabolic process | 4 | 4 | 1.93 | 0.05415 | ||||||
| GO:0015914 | phospholipid transport | 4 | 4 | 1.93 | 0.05415 | ||||||
| GO:0032957 | inositol trisphosphate metabolic process | 4 | 4 | 1.93 | 0.05415 | ||||||
| GO:0042372 | phylloquinone biosynthetic process | 4 | 4 | 1.93 | 0.05415 | ||||||
| GO:0046168 | glycerol-3-phosphate catabolic process | 4 | 4 | 1.93 | 0.05415 | ||||||
| Halberd Spike BP GO Enrichment | Cranbrook Spike BP GO Enrichment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GO.ID | Term | Annotated | Significant | Expected | Classic | GO.ID | Term | Annotated | Significant | Expected | Classic |
| GO:0050896 | response to stimulus | 372 | 221 | 167.73 | 1.90 × 10−9 | GO:0051179 | localization | 451 | 260 | 230.41 | 0.00137 |
| GO:0005975 | carbohydrate metabolic process | 185 | 107 | 83.41 | 0.00021 | GO:0051234 | establishment of localization | 449 | 258 | 229.39 | 0.00186 |
| GO:0008610 | lipid biosynthetic process | 99 | 59 | 44.64 | 0.00222 | GO:0006810 | transport | 449 | 258 | 229.39 | 0.00186 |
| GO:0007017 | microtubule-based process | 60 | 49 | 27.05 | 4.50 × 10−9 | GO:0015979 | photosynthesis | 55 | 52 | 28.1 | 1.50 × 10−12 |
| GO:0007018 | microtubule-based movement | 59 | 48 | 26.6 | 8.30 × 10−9 | GO:0042592 | homeostatic process | 39 | 31 | 19.92 | 0.00022 |
| GO:0009057 | macromolecule catabolic process | 65 | 47 | 29.31 | 6.40 × 10−6 | GO:0006820 | anion transport | 40 | 28 | 20.44 | 0.01146 |
| GO:0009733 | response to auxin | 58 | 41 | 26.15 | 6.10 × 10−5 | GO:0045454 | cell redox homeostasis | 34 | 26 | 17.37 | 0.00208 |
| GO:0006073 | cellular glucan metabolic process | 51 | 35 | 22.99 | 0.00053 | GO:0033014 | tetrapyrrole biosynthetic process | 23 | 23 | 11.75 | 1.80 × 10−7 |
| GO:0007275 | multicellular organism development | 45 | 33 | 20.29 | 0.0001 | GO:0006032 | chitin catabolic process | 22 | 19 | 11.24 | 0.00058 |
| GO:0042546 | cell wall biogenesis | 46 | 27 | 20.74 | 0.04294 | GO:0006418 | tRNA aminoacylation for protein translat… | 26 | 19 | 13.28 | 0.01862 |
| GO:0034655 | nucleobase-containing compound catabol… | 25 | 18 | 11.27 | 0.00579 | GO:0046148 | pigment biosynthetic process | 23 | 18 | 11.75 | 0.00692 |
| GO:0006032 | chitin catabolic process | 22 | 17 | 9.92 | 0.00213 | GO:0006814 | sodium ion transport | 16 | 16 | 8.17 | 2.10 × 10−5 |
| GO:0006260 | DNA replication | 23 | 17 | 10.37 | 0.00474 | GO:0006364 | rRNA processing | 15 | 14 | 7.66 | 0.00063 |
| GO:0000160 | phosphorelay signal transduction system | 19 | 13 | 8.57 | 0.03435 | GO:0046856 | phosphatidylinositol dephosphorylation | 13 | 12 | 6.64 | 0.00213 |
| GO:0007049 | cell cycle | 12 | 12 | 5.41 | 6.90 × 10−5 | GO:0016226 | iron-sulfur cluster assembly | 12 | 11 | 6.13 | 0.00389 |
| GO:0009690 | cytokinin metabolic process | 15 | 12 | 6.76 | 0.00633 | GO:0009082 | branched-chain amino acid biosynthetic p… | 10 | 10 | 5.11 | 0.00119 |
| GO:0006308 | DNA catabolic process | 16 | 12 | 7.21 | 0.01497 | GO:0006096 | glycolytic process | 7 | 7 | 3.58 | 0.00902 |
| GO:0033875 | ribonucleoside bisphosphate metabolic pr… | 11 | 11 | 4.96 | 0.00015 | GO:1901663 | quinone biosynthetic process | 6 | 6 | 3.07 | 0.01769 |
| GO:0034032 | purine nucleoside bisphosphate metabolic… | 11 | 11 | 4.96 | 0.00015 | GO:0006006 | glucose metabolic process | 5 | 5 | 2.55 | 0.03469 |
| GO:0009269 | response to desiccation | 6 | 6 | 2.71 | 0.00835 | GO:0006817 | phosphate ion transport | 5 | 5 | 2.55 | 0.03469 |
| GO:0015969 | guanosine tetraphosphate metabolic proce… | 6 | 6 | 2.71 | 0.00835 | GO:0007205 | protein kinase C-activating G protein-co… | 5 | 5 | 2.55 | 0.03469 |
| GO:0006002 | fructose 6-phosphate metabolic process | 5 | 5 | 2.25 | 0.01855 | GO:0046836 | glycolipid transport | 5 | 5 | 2.55 | 0.03469 |
| GO:0006275 | regulation of DNA replication | 5 | 5 | 2.25 | 0.01855 | ||||||
| GO:0007205 | protein kinase C-activating G protein-co… | 5 | 5 | 2.25 | 0.01855 | ||||||
| GO:0006635 | fatty acid beta-oxidation | 4 | 4 | 1.8 | 0.04122 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyemaobi, O.; Sangma, H.; Garg, G.; Wallace, X.; Kleven, S.; Suwanchaikasem, P.; Roessner, U.; Dolferus, R. Reproductive Stage Drought Tolerance in Wheat: Importance of Stomatal Conductance and Plant Growth Regulators. Genes 2021, 12, 1742. https://doi.org/10.3390/genes12111742
Onyemaobi O, Sangma H, Garg G, Wallace X, Kleven S, Suwanchaikasem P, Roessner U, Dolferus R. Reproductive Stage Drought Tolerance in Wheat: Importance of Stomatal Conductance and Plant Growth Regulators. Genes. 2021; 12(11):1742. https://doi.org/10.3390/genes12111742
Chicago/Turabian StyleOnyemaobi, Olive, Harriet Sangma, Gagan Garg, Xiaomei Wallace, Sue Kleven, Pipob Suwanchaikasem, Ute Roessner, and Rudy Dolferus. 2021. "Reproductive Stage Drought Tolerance in Wheat: Importance of Stomatal Conductance and Plant Growth Regulators" Genes 12, no. 11: 1742. https://doi.org/10.3390/genes12111742
APA StyleOnyemaobi, O., Sangma, H., Garg, G., Wallace, X., Kleven, S., Suwanchaikasem, P., Roessner, U., & Dolferus, R. (2021). Reproductive Stage Drought Tolerance in Wheat: Importance of Stomatal Conductance and Plant Growth Regulators. Genes, 12(11), 1742. https://doi.org/10.3390/genes12111742







